Abstract
Endogenous levels of indole-3-acetic acid (IAA) were determined in the internodal explants of Indian tetraploid potato cultivars (cvs) viz., Kufri Sutlej (K.Sutlej) and Kufri Giriraj (K.Giriraj). Seven fold higher level of endogenous IAA was recorded for cv K.Sutlej over cv K.Giriraj. As a result, perhaps there was a callusing response from the cut end of the internodal explants of both the cvs in the MS basal medium. The extent of callusing was relatively higher in cv K.Sutlej when compared to that in K.Giriraj. The callusing response was inhibitory to shoot morphogenesis. Inclusion of an established anti-auxin, 2,3,5-tri-iodobenzoic acid (TIBA) in the regeneration medium facilitated a high frequency adventitious shoot regeneration response with lower cytokinin levels. Murashige and Skoog (MS) medium containing TIBA at 2.5 mg l−1 and 0.25 mg l−1 zeatin evoked a 100% regeneration response (4.5 shoot buds per explant) in cv K.Sutlej within 20–25 days. However, in cv K.Giriraj, which had lower levels of endogenous IAA, 80% regeneration response (1.4 shoot buds per explant) was recorded in an extended period of 40–45 days on a medium containing 0.5 mg l−1 TIBA and 0.1 mg l−1 zeatin. Although, TIBA and zeatin induced shoot bud formation, it failed to support sustained growth of the regenerated shoots in cv K.Giriraj. Hence, 0.01 mg l−1 α-naphthaleneacetic acid (NAA) with a relatively higher concentration of zeatin (1.0 mg l−1) were used for sustained shoot regeneration (3.3 shoot buds per explant) within 25 days. From our results, it is evident that there was a difference in the requirement of exogenous zeatin levels required to induce a regeneration response in two cultivars of potato and this is attributed to the variable levels of endogenous IAA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Genetic basis to plant regeneration in vitro and cultivar specificity in regeneration response is a common problem in the tissue culture of several important crop plants. Potato is no exception in this regard and previous reports clearly illustrated variable approaches followed to demonstrate a regeneration response in different cultivars (Visser et al. 1989; Ehsanpour and Jones 2000; Khokan et al. 2009; Afrasiab and Iqbal 2010). The differences among these published protocols mostly lie in the composition of the regeneration media and precise levels and/or types of auxins and cytokinins used for specific cultivars. Many authors (Dobranszki et al. 1999; Hansen et al. 1999; Asthma et al. 2001; Torabi et al. 2008; Elaleem et al. 2009; Cingel et al. 2010) have generally combined NAA with BA for adventitious shoot regeneration. Kiel et al. (1989) and Tavazza et al. (1988) used a one-step method where a single culture medium was used for shoot regeneration. A two step method, where callus initiation occurred on one medium and shoot formation and development on another was also reported (Webb et al. 1983). In the three-step method however, different media were used for each phase, viz., callus induction, adventitious shoot formation and shoot elongation (De Block 1988).
Zeatin riboside in combination with an auxin are often used in a one-step regeneration protocol for potato (Hansen et al. 1999; Kumar et al. 2010) wherein, zeatin riboside was found to be better than BA or Kinetin for shoot formation (Hoekema et al. 1989; Ovesna et al. 1993). Sheerman and Bevan (1988) emphasized that IAA and indole-3-acetyl aspartic acid are better than NAA in stimulating regeneration.
All previous reports indicated a high degree of cultivar specificity to a PGR regime, however, their study fall short to illustrate the basis of change in the levels or combinations of the PGR employed. In addition to cultivar specificity, herein we also report that there are differences in the endogenous IAA levels in internodal explants of two Indian tetraploid potato (Solanum tuberosum ssp. tuberosum L.) cvs K.Giriraj and K.Sutlej and that influences their competence for adventitious shoot regeneration. Based on these differences we have used TIBA to overcome the adverse impact of high endogenous IAA on the adventitious shoot regeneration from explants and also used lower levels of zeatin in our studies.
We propose that study of endogenous levels of IAA could facilitate optimization of regeneration protocols. Cultivars with higher IAA perhaps would require inclusion of TIBA with concomitant decrease in the level of zeatin. The results with the two Indian cvs K.Giriraj and K.Sutlej demonstrate to some extent this phenomenon.
Materials and methods
Plant material, culture media and growth conditions
In vitro shoot cultures of Solanum tuberosum L. ssp. tuberosum cvs Kufri Sutlej hitherto mentioned as K.Sutlej and Kufri Giriraj hitherto mentioned as K.Giriraj were obtained from Central Potato Research Institute (CPRI), Shimla (India). Shoot cultures were maintained on 100 ml of standardized medium in the study being reported comprised of basal MS medium (Murashige and Skoog, 1962) supplemented with 20.0 g l−1 sucrose, 1.32 mg l−1 silver thiosulfate (STS) and solidified with 8.0 g l−1 agar in 250 ml Erlenmeyer flasks (Borosil, Mumbai, India). Prior to the addition of agar, the pH of the medium was adjusted to 5.7 and autoclaved for 20 min at 121°C and 1.06 kg cm−2. The cultures were maintained under a 16-h photoperiod (70 μmol m−2 s−1 PPFD) at 25 ± 2°C and 50–60% relative humidity. Regular sub-culturing was done after 3–4 weeks.
IAA extraction and estimation
Endogenous IAA was measured in the internodal segments of above maintained cultures of both K.Sutlej and K.Giriraj using the modified method of Nagar (1995). Explants were crushed in liquid nitrogen and homogenized in 80% (v/v) chilled methanol (15 ml g−1 FW) containing 100 mg l−1 butylated hydroxytoluene (BHT) and kept overnight at 4°C. The supernatant obtained after centrifugation at 10,000 rpm (Sigma, Model-3 K30, Sigma, Labozentrfuzen, Germany) for 25 min at 6°C was concentrated in vacuo at 30°C, resuspended in 0.1 M potassium phosphate buffer (pH 8.0) and applied to a polyvinyl pyrrolidone (PVP) column (20 cm × 1.5 cm) which was eluted with 0.1 M potassium phosphate buffer (pH 8.0). The pH of the eluate was adjusted to 8.0 and neutral compounds were extracted five times with peroxide-free diethyl ether. The aqueous fraction was acidified to pH 3.0 with 1 N hydrochloric acid (HCl) and partitioned three times against peroxide-free diethyl ether. The ether phases (acidic indole fraction containing IAA) were combined, dried over anhydrous sodium sulfate (Na2SO4) and evaporated to dryness in vacuo (30°C). The concentrated extract was redissolved in 2 ml of High Performance Liquid Chromatography (HPLC) grade methanol and filtered through 0.45 μm syringe filters (Millipore, Bangalore, India) prior to use.
The analysis for the endogenous level of IAA was performed on a Shimadzu HPLC (Model LC-20AT pump, DGU-20A5 degasser, CT-10 AS column oven and SIL-20Ac autosampler) equipped with photodiode array detector (CBM-20A; Shimadzu, Kyoto, Japan) and a Phenomenex Luna C18 column (250 × 4.6 mm i.d., 5 μm). The temperature of the column was set at 25°C. Elution of the standard (IAA, Sigma St. Louis, USA) and the samples (20 μl) was performed with gradient elution program at a flow rate of 1.0 ml min−1 for 25 min. The mobile phase consisted of 1.54 g l−1 ammonium acetate in H2O (A) and methanol (B). The HPLC program was: 80% A to 20% A over the first 15 min, from 20% A to absolute methanol (0% A) between 15 and 18 min and finally back to 80% A between 18 and 25 min. The detection wavelength was set at 280 nm. Identification of IAA was performed based on the retention time and spectral matching with the reference standard. For the preparation of calibration curve, standard IAA stock solutions (1.0 g l−1) was prepared in methanol and appropriately diluted to obtain the desired concentrations in the quantification range.
Optimization of PGR concentrations for adventitious shoot regeneration
Initially, MS basal medium supplemented with different auxins [IAA (0.01–0.05 mg l−1) and NAA (0.05–0.5 mg l−1)] in combination with different concentrations of cytokinins [BA (0.1–0.5 mg l−1), Kinetin (0.25 mg l−1) and zeatin (3.0 mg l−1)] were attempted for adventitious shoot regeneration. However, the main regeneration experiment based on the measured levels of endogenous IAA consisted of two factors, i.e. TIBA and zeatin. Four levels of TIBA (0, 0.5, 1.25 and 2.5 mg l−1) and six levels of zeatin (0, 0.05, 0.1, 0.25, 0.5 and 3.0 mg l−1) were used in combination. For cv K.Giriraj as an additional step, low concentrations of NAA (0–0.05 mg l−1) and zeatin (0–3 mg l−1) were studied. TIBA and zeatin being thermolabile were filter-sterilized and added to the cooled (~45–50°C) MS medium in laminar hood. The internodal explants of 10–15 mm length, excised from in vitro shoot cultures and inoculated onto 90 mm Petri dishes (Hi-Media, Mumbai, India) containing 25 ml of regeneration media. The Petri dishes were sealed with Parafilm (American Can Co., New Orleans, USA) and incubated under similar culture conditions as stated above. The cut ends of the internodal explants towards the shoot apex were considered as apical end and that towards the root system as basal end. Percentage response represents the number of explant showing regeneration (at least one shoot bud) × 100/ total number of explants per Petri dish. Number of shoots per explant was calculated by dividing the total number of shoot buds regenerated in one Petri dish by total number of explants per Petri dish.
Acclimatization and greenhouse establishment
Regenerated shoots were excided and transplanted into 250 ml flasks containing 50 ml basal MS medium supplemented with 20.0 g l−1 sucrose. Rooted plants were carefully removed from the culture medium after 2 weeks of sub-culturing and washed with sterile distilled water to remove all traces of agar. Plantlets were first transferred to sand, sterilized by autoclaving at 121°C for 20 min. Sand trays were kept inside a humid chamber. After 2 weeks, acclimatized plants were transferred into potting mixture containing sand:soil:compost (1:1:1). Potted plants were watered regularly and survival percentage was determined after 3 weeks of their shifting in the greenhouse.
Statistical analysis
The experiment was conducted in a Completely Randomized Design (CRD), with three replications; each replication comprised a single Petri dish containing 19 explants. Data was analyzed by one-way analysis of variance (ANOVA) followed by separation of means by Duncan’s Multiple Range Test (DMRT). The term significant has been used to indicate difference at P ≤ 0.05.
Results
Responses of internodal explants to PGR
Based on previous reports on regeneration in potato experiments for morphogenesis, internodal explants of cv K.Sutlej and K.Giriraj were placed on media containing different concentrations of NAA and IAA in combination with cytokinins i.e. BA, kinetin and zeatin as is shown in Table 1. Invariably a friable callus response was evident. On MS basal medium without the inclusion of plant growth regulators, the degree of callus formation (represented by number of + signs) in the cv K.Sutlej was relatively more than that of cv K.Giriraj. A nodular callus response was demonstrated in all the concentrations and combinations of PGR.
Endogenous IAA estimation
Endogenous IAA content in K.Sutlej and K.Giriraj was 4.1 μg g−1 FW and 0.6 μg g−1 FW, respectively. K.Sutlej explants had shown approximately seven times higher endogenous IAA over K.Giriraj (Fig. 1).
Optimization of PGR concentrations for adventitious shoot regeneration
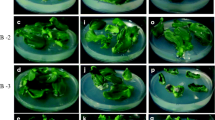
To overcome the response of profuse callusing, subsequent experiments were done using different concentrations of TIBA along with zeatin as is shown in Table 2. The callus formation was inhibited largely in all the combinations with TIBA and in general, regeneration response was observed even at lower concentrations of zeatin in both the cvs i.e., K.Sutlej and K.Giriraj (Table 2; Fig. 2). The cv K.Sutlej showed 100% regeneration response at higher level of zeatin (0.1–3.0 mg l−1) irrespective of the inclusion of TIBA. Although there was 100% regeneration response but number of shoot buds regenerated per explant was diverse ranging from 0.8–4.5. Maximum numbers of shoots were regenerated when zeatin was used in the range of 0.1–0.25 mg l−1 in combination with TIBA ranging between 1.25 and 2.5 mg l−1. Inclusion of TIBA was not necessary for direct regeneration of shoots for K.Sutlej at higher concentrations of zeatin (0.25–3.0 mg l−1).
The cv K.Giriraj, which had a relatively lower concentration of endogenous IAA, required relatively lower concentrations of TIBA for the induction of optimal number of shoot buds. The best response was observed at 0.1 mg l−1 zeatin in combination with 0.5 mg l−1 TIBA. At higher concentrations of both zeatin or TIBA, percentage regeneration response of the internodal explants was lower and also the number of shoot buds formed per explant was low. In cv K.Giriraj the potential regeneration response was not achieved in any of the TIBA:zeatin combinations and also all the adventitious shoot primordia induced did not grow into normal shoots. Hence higher concentrations of zeatin (1.0–3.0 mg l−1) in combination with NAA (0.01–0.02 mg l−1) induced 100% regeneration response with 3.3 number of shoot buds per explant (Table 3). In our experiments, a low concentration of the auxin NAA was found to be necessary for the sustained growth of induced shoot primordia for the cv K.Girairaj.
In general, the requirement of zeatin reduced on inclusion of TIBA into the regeneration media for inducing a regeneration response, in both the cvs. Comparatively, the frequency of adventitious shoot regeneration was higher in K.Sutlej (4.5 shoots per explant) than that observed in cv K.Giriraj (1.4 shoots per explant). The time taken to complete shoot formation for K.Sutlej was 25 days and that for K.Giriraj was 45 days with zeatin:TIBA combinations. However, for cv K.Giriraj this time to complete shoot formation could come down to 25 days with zeatin (3.0 mg l−1) and NAA (0.01 mg l−1) combination. An interesting feature of the regeneration response in both the cvs K.Sutlej and K.Giriraj, was that the shoots invariably regenerated towards the basal end of the internodal explants (Fig. 3a and b).
Acclimatization and greenhouse establishment
In both the cvs hardening and green house establishment was not a problem. Approximately 85–90% survival rate was achieved. The hardened plants grew well and attained maturity in the greenhouse.
Discussion
The present study on in vitro morphogenesis from internodal explants in two cvs of potato namely, K.Sutlej and K.Giriraj i.e., with high and low level of endogenous IAA, respectively, provide us a clue to harmonize application of exogenous PGR particularly zeatin and to rationalize the use of TIBA for the regeneration of shoot buds. High endogenous IAA level in explants is detrimental and often interferes with adventitious shoot regeneration (McCown 1989). One approach to inhibit or block the inhibitory effect of endogenous auxin on regeneration is the use of an anti-auxin. Several chemicals included in the medium can act in this fashion and one such compound is TIBA, an anti-auxin (Gaspar et al. 1996). It has been shown by Bronsema et al. (2001) that TIBA caused suppression of callus formation in maize. TIBA was found to be effective in regeneration studies of Capsicum annuum by Kumar et al. (2005), improved bud formation in herbaceous (Cassells 1979; Vinod et al. 2005) or woody plants (Belaizi et al. 1991). TIBA might also increase shoot proliferation by inhibiting the transport of IAA to the proliferation site leading to a more favorable balance between cytokinins and auxins (Cambecedes et al. 1991). Herein, our results with the inclusion of TIBA in combination with zeatin also show a positive regeneration response.
In the present study, besides suppressing callus formation, it was observed that TIBA significantly reduced the concentration of zeatin needed for the induction of adventitious regeneration. This reduction in the level of zeatin used was 5–10 fold. Addition of TIBA into regeneration medium probably inhibits the transport of IAA to the regeneration sites; thereby lowering the requirement of zeatin. This finding is important because zeatin is an expensive supplement of the media. There is a need to harmonize the levels of endogenous IAA in the explants with suitable concentration of TIBA which is grossly cheaper than zeatin. Results of our study on shoot regeneration response demonstrated that for the two cvs K.Giriraj and K.Sutlej, TIBA/Zeatin concentrations vary depending on the endogenous level of IAA.
Earlier studies on potato cvs (Russet Burbank, Desiree and Maris piper), zeatin has been used in the range of 0.5–3.0 mg l−1 (De Block 1988; Sheerman and Bevan 1988). Our results showed an optimal regeneration response by using zeatin 0.25 mg l−1 (K.Sutlej) and 0.1 mg l−1 (K.Giriraj) in combination with TIBA at 2.5 mg l−1 and 0.5 mg l−1, respectively. However, shoot regeneration in K.Sutlej was also observed in the absence of TIBA but with a higher level of zeatin (0.25–3.0 mg l−1) giving rise to a low number of shoots that are regenerated. In the case of K.Giriraj, where levels of endogenous auxin were lower, the requirement for TIBA was five times lower and zeatin concentration was reduced by less than half to that used for K.Sutlej. For K.Giriraj where the endogenous level of IAA is low, subculture to an auxin (NAA) containing medium was important for the sustained growth of differentiated shoot primordia. Sheerman and Bevan (1988) emphasized that IAA and indole-3-acetyl aspartic acid are better than NAA in stimulating regeneration. In contrast to it, NAA in comparison to IAA was an auxin of choice in the present study on K.Giriraj and this may be due to cv specificity for a particular auxin. Induction and growth of shoots does require a critical minimum level of auxin which was inhibited by TIBA in the case of K.Giriraj. A strict requirement for an optimal balance in the levels of PGR particularly the auxin:cytokinin ratio is well known (Skoog and Miller 1957) and is reiterated in subsequent studies on in vitro regeneration (Webb et al. 1983; De Block 1988; Hulme et al. 1992).
Considering the nature of hindrance to regeneration, it may be necessary to assess endogenous levels of abscisic acid so as to induce maturation of somatic embryos that enter into dormancy (Bhattacharya et al. 2002). Mercier et al. (2003) correlated the increase of endogenous N6-(2-isopentenyl) adenine (2iP) and IAA levels in apple leaf explants with shoot organogenesis process. However in the present study, since profuse callusing was a hindrance to shoot regeneration, endogenous levels of IAA were determined.
Published protocols for regeneration in potato have used low concentrations of auxin along with higher concentrations i.e. 3.0 mg l−1 of zeatin (Kumar et al. 2010) or 2.0 mg l−1 of BA (Cingel et al. 2010). However, our report shows that TIBA is effective in reducing zeatin concentration (0.125 mg l−1–0.25 mg l−1) required for effective adventitious shoot regeneration in vitro. The regeneration of shoot buds from the basal end of the internodal explants (Fig. 3) also reflects an element of polarity in endogenous IAA distribution. The present study suggests that there are distinct varietal differences in the endogenous levels of IAA in potato varieties and this critically determines the in vitro shoot regeneration.
Abbreviations
- BA:
-
N6-benzyladenine
- MS:
-
Murashige and Skoog
- NAA:
-
α-naphthaleneacetic acid
- PGR:
-
Plant growth regulator
- TIBA:
-
2,3,5-tri-iodobenzoic acid
References
Afrasiab H, Iqbal J (2010) In vitro techniques and mutagenesis for the genetic improvement of potato cvs Desiree and Diamant. Pak J Bot 42:1629–1637
Asthma R, Beenish A, Abbasi NA, Mussarat B, Azra Q (2001) Effect of growth regulators on in-vitro multiplication of potato. Int J Agr Biol 3:181–182
Belaizi M, Paul H, Sangwan RS, Sangwan-Norreel BS (1991) Direct organogenesis from internodal segments of in vitro grown shoots of apple cv Golden Delicious. Plant Cell Rep 9:471–474
Bhattacharya A, Nagar PK, Ahuja PS (2002) Seed development in Camellia sinensis (L.) O. Kuntze. Seed Sci Res 12:39–46
Bronsema FBF, Van Oostveen WJF, Van Lammeren AAM (2001) Influence of 2,4-D, TIBA and 3,5-D on the growth response of cultured maize embryos. Plant Cell Tissue and Organ Cult 65:45–56
Cambecedes J, Duron M, Decourtye L (1991) Adventitious bud regeneration from leaf explants of shrubby ornamental honysuckle Lonicera nitida Wills cv Maigrun: effects of thidiazuron and 2,3,5 tri-iodobenzoic acid. Plant Cell Rep 10:471–474
Cassells AC (1979) The effect of 2,3,5-triiodobenzoic acid on caulogenesis in callus cultures of tomato and pelargonium. Physiol Plant 46:159–164
Cingel A, Vinterhalte B, Vinterhalter D, Dragosavac DA, Smigocki A, Ninkovi S (2010) Agrobacterium-mediated transformation of two Serbian potato cultivars (Solanum tuberosum L. cv Draga_evka and cv Jelica). Afr J Biotechnol 9:4644–4650
De Block M (1988) Genotype-independent leaf disc transformation of potato (Solanum tuberosum) using Agrobacterium tumefaciens. Theor Appl Genet 76:767–779
Dobranszki J, Takacs HA, Magyar TK, Ferenczy A (1999) Effect of medium on the callus forming capacity of different potato genotypes. Acta Agron Hung 47:59–61
Ehsanpour AA, Jones MG (2000) Evaluation of direct shoot regeneration from stem explants of potato (Solanum tuberosum L.) cv. Delaware by thidiazuron TDZ. J Sci Technol Agri Natur Res 4:47–54
Elaleem AKG, Modawi RS, Khalafalla MM (2009) Effect of plant growth regulators on callus induction and plant regeneration in tuber segment culture of potato (Solanum tuberosum L.) cultivar Diamant. Afr J Biotechnol 8:2529–2534
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol Plant 32:272–289
Hansen J, Nielsen B, Nielsen SVS (1999) In vitro shoot regeneration of Solanum tuberosum cultivars: interactions of medium composition and leaf, leaflet and explant position. J Natur Sci Found Sri Lanka 27:17–28
Hoekema A, Huisman MJ, Molendijk L, Van den Elzen PJM, Cornelissen BJC (1989) The genetic engineering of two commercial potato cultivars for resistance to potato virus X. Bio/Tech 7:273–278
Hulme JS, Higgins ES, Shields R (1992) An efficient genotype-independent method for regeneration of potato plants from leaf tissue. Plant Cell Tissue Organ Cult 31:161–167
Khokan EH, Haydar A, Ara T, Alam MK, Sharma MD (2009) Enhancement of Agrobacterium-mediated transformation method for the production of heme-protein (ferritin protein) rich potato. Int J Sustain Crop Prod 4:17–22
Kiel M, Sanchez-Serrano JJ, Willmitzer L (1989) Both wound inducible and tuber specific expression mediated by the promoter of a single member of the protease inhibitor 11 gene families. EMBO J 8:1323–1330
Kumar M, Chimote V, Singh R, Mishra GP, Naik PS, Pandey SK, Chakrabarti SK (2010) Development of Bt transgenic potatoes for effective control of potato tuber moth by using cry1Ab gene regulated by GBSS promoter. Crop Prot 29:121–127
Kumar V, Gururaj HB, Narasimha BCP, Giridhar P, Ravishankar GA (2005) Direct shoot organogenesis on shoot apex from seedling explants of Capsicum annum. Sci Hortic 106:237–246
McCown BH (1989) Birch (Betula spp.). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry 5.Trees II. Springer-Verlag, Berlin-Heidelberg, pp 324–341
Mercier H, Souza BM, Kraus JE, Hamasaki RM, Sotta B (2003) Endogenous auxin and cytokinin contents associated with shoot formation in leaves of pineapple cultured in vitro. Braz J Plant Physiol 15(2):107–112
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagar PK (1995) Changes in abscisic acid, phenols and indoleacetic acid in bulbs of tuberose (Polianthes tuberosa L.) during dormancy and sprouting. Sci Hortic 63:77–82
Ovesna J, Burdova I, Krizkova L, Opatrny Z (1993) Modification of cultivar-specific regeneration capacity of potato explants by phytohormones and by Agrobacterium oncogenes. Biol Plant 35:199–208
Sheerman S, Bevan MW (1988) A rapid transformation method for Solanum tuberosum using binary Agrobacterium tumefaciens vectors. Plant Cell Rep 7:13–16
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissue cultured in vitro. In: Biological action of growth substances. Symp Soc Exp Biol 11:118–131
Tavazza R, Tavazza M, Ordas RJ, Ancora G, Benvenuto E (1988) Genetic transformation of potato (Solanum tuberosum): An efficient method to obtain transgenic plants. Plant Sci 49:175–181
Torabi F, Majad A, Ehsanpour AA (2008) Plant regeneration from cell suspension culture of potato (Solanum tuberosum L.). Pak J Biol Sci 11:778–782
Visser RGF, Jacobsen E, Hesselingmeinders A, Schans MJ, Witholt B, Feenstra WJ (1989) Transformation of homozygous diploid potato with an Agrobacterium tumefaciens vector system by adventitious shoot regeneration on leaf and stem segments. Plant Mol Biol 12:329–337
Webb KJ, Osifio EO, Henshaw GG (1983) Shoot regeneration from leaflet discs of six cultivars of potato. (S. tuberosum subsp. tuberosum). Plant Sci 63:79–85
Acknowledgements
The authors AK Pal and K Acharya gratefully acknowledge Council of Scientific and Industrial Research (CSIR), New Delhi, India for research fellowships and also to provide grant under project SIP 0003. The authors thank Mr. Upendra K Sharma for the HPLC analysis of IAA. This research was partially supported by the Department of Biotechnology, New Delhi, India. Acknowledgement has also been given to Central Potato Research Institute, Shimla, India for providing cultivars K.Sutlej and K.Giriraj.
Author information
Authors and Affiliations
Corresponding author
Additional information
IHBT Publication number 0891
A.K. Pal and K. Acharya with equal contributions
Rights and permissions
About this article
Cite this article
Pal, A.K., Acharya, K. & Ahuja, P.S. Endogenous auxin level is a critical determinant for in vitro adventitious shoot regeneration in potato (Solanum tuberosum L.). J. Plant Biochem. Biotechnol. 21, 205–212 (2012). https://doi.org/10.1007/s13562-011-0092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-011-0092-z