Abstract
The essential oil obtained from fresh leaves of Eucalyptus teretecornis (family Myrtaceae) was analysed by gas chromatography/mass spectrometry (GC/MS). Twenty eight compounds were identified and β-pinene (22.55%), α-pinene (22.50%), 1,8-cineole (19.84%), limonene (5.62%), β-fenchol (3.10%), α-phellandrene (2.90%), α-eudesmol (2.66%) and 4-(2-methylcyclohex-1-enyl)-but-2-enal (2.34%) were the main components. The antifungal activity of the essential oil was assayed against Alternaria alternata using bioautography. Two main bioactive components namely a1 (R f = 0.27) and a2 (R f = 0.33) were observed that produced inhibition zone of 4 mm and 8 mm in diameter respectively. The minimum inhibitory amount (MIA) of a1 and a2 against A. alternata was determined as 28 μg and 10 μg, respectively using bioautography assay. Components corresponding to a1 and a2 were determined as β-fenchol (oxygenated monoterpene) and α-eudesmol (oxygenated sesquiterpene) respectively using GC/MS analysis. The antioxidant activity of the essential oil and its bioactive fraction was evaluated by DPPH radical scavenging assay, β-carotene/linoleic acid bleaching assay, reducing power assay and metal chelating assay. In addition fraction of the essential oil that showed antioxidant activity was analyzed using GC/MS and α-fenchol, 4-terpineol and carvacrol were the main components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interest in medicinal plants and their biologically active secondary metabolites has increased in recent years, in relation to the possible development of novel antioxidant molecules and potential biocides (Hedberg 1993). The antimicrobial properties of volatile oils have been recognized since antiquity. The use of essential oils is important not only in preservation of foods but also in control of human and plant diseases of microbial origin (Baratta et al. 1998).
Fungal diseases are responsible for heavy toll of field crops every year and the micro-organisms have developed resistance to many synthetic fungicides due to their indiscriminate use. This has forced the researchers to search for new antimicrobial substances from various sources including medicinal plants (De Leo et al. 2004).
The role of free radicals and active oxygen species is becoming increasingly recognized in the pathogenesis of many human diseases including cancer, aging, and atherosclerosis (Perry et al. 2000). Free radicals can also cause lipid peroxidation in foods that leads to their deterioration. Although there are some synthetic antioxidants such as BHT and BHA but, these compounds are suspected to cause side effects (Ito et al. 1983). Therefore, the investigations on determination of natural sources of antioxidants and biocides from plants are important.
The genus Eucalyptus (family: Myrtaceae) comprises plants of 800 species (Louppe et al. 2008). Although most of the plants are native to Australia, numerous species have been introduced to other parts of the world including north-western Himalaya (India). Its leaves contain oil glands which produce oils of different composition (Grieve 1992), which mainly contain 1,8-cineole, monoterpenes, sesquiterpenes, aldehydes and ketones (Newall et al. 1996). Eucalyptus oil is used for industrial and medicinal purposes around the world for food, flavouring, nasal and cough drops. Also essential oils containing cineole had shown antimicrobial and nematicidal activity (Gundidza et al. 1993).
Eucalyptus teretecornis is the most commonly planted species in India (Singh et al. 2009). It is planted for its heartwood which is used for timber and railway sleepers (Boland et al. 1991). There are reports in literature on the antioxidant and antimicrobial activities of crude essential oil from E. teretecornis (Singh et al. 2009; Batish et al. 2008). However, to the best of our knowledge there is no information on isolation and identification of bioactive molecules from E. teretecornis essential oil having free radical scavenging and antifungal activity.
The level of interest in antioxidant and antimicrobial properties of volatile oils is just one aspect of the practical potential such oils have in various protective roles. There also appears to be a revival of traditional approaches to livestock welfare and food preservation in which essential oils play a part (Thomann et al. 1997). It is intended here to examine the antioxidant and antimicrobial activity of E. teretecornis essential oil to assess its potential application to human health care, food preservation and plant protection.
Materials and methods
Plant material and isolation of essential oil
Fresh leaves from E. teretecornis were collected locally in November, 2009. They were identified at Herbal Garden and Herbarium Research Institute in ISM, Joginder Nagar, District Mandi (HP), India and a voucher specimen was deposited at the herbarium of the institute. They were subjected to hydro-distillation for 2 h using a Clevenger apparatus, followed by exhaustive extraction of the distillate with petroleum ether. The resulting extract was dried over anhydrous sodium sulphate.
GC/MS analysis
Analysis of the oil was carried out at Indian Institute of Integrative Medicine (CSIR, India), Canal Road, Jammu, India. A GC-MS 4000 (Varian, USA) system with a varian CP-SIL 8CB column (30 m × 0.32 mm i.d., 1 μm film thickness). Injector temperature was 230°C. Oven temperature programme used was holding at 60°C for 5 min, heating to 250°C at 3°C/min and keeping the temperature constant at 250°C for 10 min. Helium was used as a carrier gas at a constant flow of 1.0 ml/min and an injection volume of 0.20 μl was employed. The MS scan parameters included electron impact ionization voltage of 70 eV, a mass range of 40–500 m/z. The identification of components of the essential oil was based on comparison of their mass spectra with those of NIST05 (version 2.0) library.
Bioautography
This technique was used to determine the active constituents of the essential oils (Marston et al. 1997). Aliquotes of 25–50 ml of inoculum spray solution (ca. 3 × 105 conidia/ml) were prepared for test fungus (A. alternata) with liquid potato dextrose (potato 200 g, dextrose 20 g, and water to make total volume of 1 l). Using a 100 ml chromatographic sprayer plate was sprayed lightly (to a damp appearance) three times with spore suspension and incubated for 4 d in a dark moist chamber at 25°C. Fungal growth inhibition appeared as clear zones against a dark background. The experiment was repeated thrice.
Isolation of antifungal constituents
After identification of the inhibition zones on the TLC plate, PTLC was performed by loading the essential oil onto a pre-activated silica gel 60 F254 coated glass plate (20 × 20 cm, 500 μm thickness) which was developed in n-hexane/ethyl acetate (9:1, v/v) solvent system (Sridhar et al. 2003). The separated compounds were visualized under UV light (365 and 254 nm) or by spraying with vanillin/sulphuric acid spray reagent. The isolation was carried out by scrapping off the detected zones corresponding to the antifungal constituents a1 (R f = 0.27) and a2 (R f = 0.33) and transferring them into percolator. The substances were then set free from silica gel by elution with dichloromethane.
Antifungal activity of bioactive molecules isolated from E. teretecornis
Different amounts of a1 and a2 were loaded onto the TLC plate and bioautography was performed as described earlier using A. alternata as test pathogen. Antifungal activity was determined as MIA of active compounds required for the inhibition of fungal growth on TLC plate.
Free radical scavenging activity on TLC
Hydrogen or electron donating ability of the essential oil was evaluated from the bleaching of the purple coloured solution of stable free radical DPPH. Five microlitres of 1:10 dilution of essential oil in dichloromethane were applied to the TLC plate (aluminium sheets covered with silica gel 60 F254, Merck) and hexane-ethyl acetate (9:1) mixture was used as mobile phase. The plate was sprayed with a 0.2% DPPH• reagent in methanol and left at room temperature for 20 min. Yellow spots formed from the bleaching of DPPH• were evaluated as positive free radical scavenging activity (Mimica-Dukic et al. 2003).
Isolation of antioxidant fraction
For the isolation of the antioxidant compounds, 100 mg of the crude oil were subjected to preparative TLC (silica gel 60 G254, Merck) in n-hexane/ethyl acetate (9:1,v/v) solvent system (Sridhar et al. 2003). The antioxidant fraction (F1) was detected by spraying the TLC plate with 0.2% DPPH• reagent in methanol (Mimica-Dukic et al. 2003). The silica gel corresponding to F1 was scratched and washed with dichloromethane to remove the bioactive compounds (3.7 mg).
DPPH radical scavenging assay
The radical scavenging activity was determined using DPPH• method (Bozin et al. 2006). 1 ml of different concentrations of the essential oil or bioactive fraction were mixed with 1 ml of a 90 μM DPPH• solution in methanol, and final volume was made to 4 ml with methanol. The mixtures were well shaken and kept at 25°C in the dark for 1 h. The absorbance was measured at 517 nm. Inhibition of DPPH radical in percent was calculated as:
Where Ablank is the absorbance of the control (containing all the reagents except the test compound), and Asample is the absorbance of the test sample. Oil concentration providing 50% inhibition (IC50) was calculated from the graph by plotting inhibition% against oil concentration. BHT was used as reference.
β-carotene bleaching inhibition assay
The antioxidant activity of essential oil/fraction was evaluated using β-carotene-linoleic acid model system (Kabouche et al. 2007). β-carotene (0.5 mg) in one ml of chloroform was added to 25 μl of linoleic acid, and 200 mg of Tween 40 emulsifier mixture. Chloroform was evaporated at 40°C by a rotary vacuum evaporator. Then, 100 ml of distilled water saturated with oxygen were slowly added to the residue and the solution was vigorously agitated to form a stable emulsion. Four thousand microlitres of this mixture were transferred into test tubes containing 0.2 ml portion of the essential oil/fraction prepared in methanol at different concentrations. As soon as the emulsion was added to each tube, zero time absorbance was measured at 470 nm using a spectrophotometer. The emulsion system was incubated for 120 min at 50°C. A blank, devoid of β-carotene, was used for background subtraction. Antioxidant activity was calculated as percent of inhibition (I%) relative to the control using the following equation:
Where As(0) was the initial absorbance of the sample at 0 min, As(120) was the absorbance of the sample at 120 min, Ac(0) was the initial absorbance of the negative control at 0 min, and Ac(120) was the absorbance of the negative control at 120 min. The essential oil/fraction concentration providing 50% antioxidant activity (IC50) was calculated from the graph of antioxidant activity percentage against essential oil/fraction concentration. BHT was used as standard antioxidant.
Reducing power assay
The reducing power of essential oil/fraction was determined by the method as described previously (Oyaizu 1986). Different concentrations of essential oil/fraction were mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of potassium ferricyanide [K3Fe(CN)6](1%). The mixture was incubated at 50°C for 20 min. Aliquots (2.5 ml) of 10% trichloroacetic acid were added to the mixture. The above mixture was then centrifuged for 10 min at 1,036 g. The upper layer of the solution (2.5 ml) was mixed with 2.5 ml of distilled water and 2.5 ml of 1% ferric chloride solution. The absorbance was measured at 700 nm in a double beam UV-VIS spectrophotometer. Increased absorbance of the reaction mixture indicated increased reducing power. The essential oil/fraction concentration providing 0.5 of absorbance (IC50) was calculated from the graph of absorbance at 700 nm against essential oil/fraction concentration and compared with those of standard antioxidant.
Chelating capacity assay
The chelating effect on ferrous ions of E. teretecornis essential oil/fraction was estimated by the method followed by Chua et al. 2008. Briefly, 200 μl of different concentrations of essential oil/fraction and 740 μl of methanol were added to 20 μl of 2 mM FeCl2. The reaction was initiated by the addition of 40 μl of 5 mM ferrozine into the mixture, which was then left at room temperature for 10 min before determining the absorbance of the mixture at 562 nm. The ratio of inhibition of ferrozine-Fe2+ complex formation was calculated using the equation: \( \% \;{\text{inhibition}} = \left[ {\left( {{\text{absorbance}}\;{\text{of}}\;{\text{control}} - {\text{absorbance}}\;{\text{of}}\;{\text{test}}\;{\text{sample}}} \right)/\left. {{\text{absorbance}}\;{\text{of}}\;{\text{control}}} \right)} \right] \times {1}00 \).
Identification of bioactive compounds
The isolated bioactive compounds corresponding to a1 and a2 and antioxidant fraction were determined using GC/MS analysis described earlier.
Statistical analysis
All experiments were carried out in triplicate. To determine whether there is a statistically significant difference among the results obtained for antioxidant activity assays, variance analyses were carried out using the SPSS 14.0 software package. Values of p < 0.05 were considered to be statistically different.
Results and discussion
Hydro-distillation of the fresh leaves from E. teretecornis grown in the north-western Himalaya gave yellowish oil with a yield of 1.35%. The oil was analyzed by GC/MS to determine its main constituents. It was further investigated for antifungal and antioxidant activity. The GC/MS analysis led to the identification of 28 components eluted between 10 and 47 min (Table 1), accounting for 91.34% of the total components present in the crude essential oil of E. teretecornis. The major constituents of the volatile oil were β-pinene (22.55%), α-pinene (22.50%), 1,8-cineole (19.84%), limonene (5.62%), β-fenchol (3.10%), α-phellandrene (2.90%), α-eudesmol (2.66%) and 4-(2-methylcyclohex-1-enyl)-but-2-enal (2.34%). The oil contained 10 monoterpenes (57.58%), 28.46% oxygenated monoterpenes, 1.09% sesquiterpene hydrocarbons and 2 oxygenated sesquiterpenes (4.21%). In general, the oil was rich in monoterpenoids and the presence of 1,8-cineole and α-pinene as the major constituents in the fresh leaf oil of E. teretecornis was in agreement with earlier studies (Singh et al. 2009; Pino et al. 2002). However, we have also observed higher level of β-pinene in essential oil of E. teretecornis grown in north-western Himalaya (Table 1).
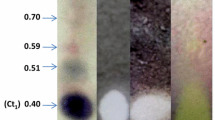
Antifungal potential of E. teretecornis essential oil was examined using direct bioautographic procedure and A. alternata as test organism. Bioautography is a simple procedure for evaluating plant extracts and essential oils against human and plant pathogens (Marston et al. 1997). This technique helps in activity guided tracking of bioactive compounds on the TLC plate. Application of E. teretecornis essential oil in the bioautography system mentioned above showed the presence of two distinct inhibition zones (Fig. 1b) on the TLC plate with diameters of 4 mm (R f = 0.27) and 8 mm (R f = 0.33) corresponding to a1 and a2 respectively (Table 2, Fig. 1c). As the essential oil presented antifungal activity in the bioautography test, it was further subjected to PTLC for isolation of pure molecules corresponding to the inhibition zones. PTLC of the essential oil yielded 3.3 mg of a1 and 4.8 mg of a2 and the MIA of bioactive molecules a1 and a2 was determined against A. alternata using bioautography test. The above study revealed stronger inhibition of A. alternata spores by a2 (MIA = 10 μg) than a1 (MIA = 28 μg) (Table 3). Furthermore, the antifungal compounds corresponding to a1 and a2 were determined as oxygenated terpenoids β-fenchol and α-eudesmol respectively using GC/MS analysis (Fig. 2). The above results are in agreement with earlier report that fungicidal effect of the essential oils would be due to oxygenated compounds (Griffin et al. 1999). The antifungal activity of essential oils extracted from other Eucalyptus species such as E. salgina and E. camaldulensis has been shown on Candida albicans and Phaeoramularia angolensis (Oyedeji et al. 1999; Dongmo et al. 2008).
TLC of essential oil from Eucalyptus teretecornis leaves. a detection of free radical scavenging activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical (0.5 mM); b bioautography with Alternaria alternata spores; c detection with vaniline/sulphuric acid reagent. a1 and a2 correspond to the components of the essential oil having antifungal activity
E. teretecornis essential oil also exhibited free radical scavenging activity in TLC based DPPH• assay (Fig. 1a). The capacity of E. teretecornis essential oil to scavenge DPPH free radical, β-carotene bleaching inhibition, reducing power and metal ion chelating capacity was quantified using spectrophotometer based assays and the results obtained are presented in Table 4. Though the oil showed striking antioxidant activity in TLC based antioxidant assay (Fig. 1a), it exhibited low scavenging activity (IC50, 5.17 ± 0.26 mg/ml) as compared to the positive control BHT (IC50 value of 0.023 ± 0.001 mg/ml) in the spectrophotometric assay (Table 4). Moderate DPPH radical scavenging activity (IC50, 1.54 ± 0.04 mg/ml) has been reported in essential oil from Eucalyptus oleosa leaves (Marzoug et al. 2011). Similarly, β-carotene bleaching inhibition (IC50 value of 6.89 ± 0.32 mg/ml) and reducing power (IC50 value of 14.51 ± 0.63 mg/ml) of E. teretecornis essential oil was also low as compared to BHT (IC50 value of 0.025 ± 0.002 mg/ml and 0.15 ± 0.004 mg/ml, respectively). Furthermore, the metal ion chelating activity was not detected in the essential oil at test concentration of 0.5 mg/ml.
The bioactive fraction (F1) corresponding to the spots showing DPPH radical scavenging activity in TLC assay was eluted using PTLC and tested for antioxidant activity. F1 exhibited significant (p < 0.05) radical scavenging activity (IC50 value of 0.53 ± 0.02 mg/ml), β-carotene bleaching inhibition (IC50 value of 0.81 ± 0.034 mg/ml) and reducing power (IC50 value of 1.98 ± 0.076) as compared to the essential oil (Table 4). Intriguingly, F1 showed good (54.67 ± 1.92%) metal ion chelating capacity at the test concentration (0.5 mg/ml). The major components of F1 were analyzed using GC/MS analysis and determined as α-fenchol (16.84%), 4-terpineol (17.41%) and carvacrol (26.19%). Antioxidant activity of essential oil of E. teretecornis leaves can be attributed to the presence of these components, although the amounts of these compounds are relatively low in oil (Table 1). Phenolic compounds such as thymol and carvacrol and essential oils rich in phenolic compounds show potent antioxidant and DPPH radical scavenging activities (Alma et al. 2003). Carvacrol was detected in low amount in essential oil of E. teretecornis (Table 1). Antioxidant activities of volatile components from other Eucalyptus species such as E. polyanthemos, E. globulus and E. perriniana have also been reported (Lee and Shibamoto 2001).
In the present study we investigated the antifungal and antioxidant activity of E. teretecornis essential oil. To the best of our knowledge this is the first report on isolation and identification of the bioactive constituents responsible for antifungal and antioxidant activity from the essential oil of E. teretecornis grown in north-western Himalaya. The results conclusively demonstrate the antifungal potential of bioactive molecules namely β-fenchol and α-eudesmol from E. teretecornis essential oil against A. alternata a pathogen of agricultural importance. In addition, though fraction from E. teretecornis essential oil showed significant antioxidant activity, the antioxidant compounds were present at low level in the essential oil. Future research is warranted on studying the seasonal variation in the level of bioactive molecules from E. teretecornis essential oil for determining its commercial value.
Abbreviations
- BHT:
-
Butylated hydroxyl toluene
- BHA:
-
Butylated hydroxyl anisole
- GC/MS:
-
Gas chromatography/mass spectrometry
- TLC:
-
Thin layer chromatography
- PTLC:
-
Preparative thin layer chromatography
- MIA:
-
Minimum inhibitory amount
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- ISM:
-
Indian system of medicine
References
Alma MH, Mavi A, Yildirim A, Digrak M, Hirata T (2003) Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biochem Pharm Bull 26:1725–1729
Baratta MT, Dorman HJ, Deans SG, Figueiredo AC, Barroso JG, Ruberto G (1998) Antimicrobial and antioxidant properties of some commercial essential oils. Flav Fragr J 13:235–244
Batish DR, Singh HP, Kohli RK, Kaur S (2008) Eucalyptus essential oil as a natural pesticide. For Ecol Manage 256:2166–2174
Boland DJ, Brophy JJ, House APN (1991) Eucalyptus leaf oils: use, chemistry, distillation and marketing. ACIAR/CSIRO, Inkata Press, Melbourne
Bozin B, Mimica-Dukic M, Simin N, Anackov G (2006) Characterization of the volatile composition of essential oils of some lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem 54:1822–1828
Chua MT, Tung YT, Chang ST (2008) Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour Technol 99:1918–1925
De Leo M, Braca A, De Tomasi N, Norscia I, Morelli I, Battinelli L, Mazzanti G (2004) Phenolic compounds from Baseonema acuminatum leaves: isolation and antimicrobial activity. Planta Med 70:841–846
Dongmo PMJ, Ngoune LT, Dongmo BN, Kuate J, Zollo PHA, Menut C (2008) Antifungal potential of Eucalyptus saligna and Eucalyptus camaldulensis essential oils from Cameroon against Phaeoramularia angolensis. Eur J Sci Res 24:348–357
Grieve M (1992) A modern herbal. Tiger Books International, London
Griffin SG, Wyllie G, Markham JL, Leach DN (1999) The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flav Fragr J 14:322–332
Gundidza M, Deans SG, Kennedy AI, Mavi S, Wanten-nan PG, Gray AI (1993) The essential oil from Heteropyxis natalensis Harv. Its antimicrobial activities and phytoconstituents. J Sci Food Agric 63:361–364
Hedberg I (1993) Botanical methods in ethnopharmacology and the need for conservation of medicinal plants. J Ethanopharmacol 38:121–128
Ito N, Fukushima S, Hassegawa A, Shibata M, Ogiso T (1983) Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst 70:343–352
Kabouche A, Kabouche Z, Oztürk M, Kolak U, Topçu G (2007) Antioxidant abieetane diterpenoids from Salvia barrelieri. Food Chem 102:1281–1287
Lee KG, Shibamoto T (2001) Antioxidant activities of volatile components isolated from Eucalyptus species. J Sci Food Agric 81:1573–1579
Louppe D, Oteng-Amoako AA, Brink M (2008) Ressources végétales de l’Afrique tropicale 7 (1). Fondation PROTA, Wageningen
Marston A, Maillard M, Hostettmann K (1997) The role of TLC in the investigations of medicinal plants of Africa, South America and other tropical regions. GIT Laboratory Journal, GIT Verlag Publishing Ltd. Rosslerstrasse, Darmstadt, pp 36–39
Marzoug HNB, Romdhane M, Lebrihi A, Mathieu F, Couderc F, Abderraba M, Khouja ML, Bouajila J (2011) Eucalyptus oleosa essential oils: chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 16:1695–1709
Mimica-Dukic N, Bozin B, Sokovic M, Mihajlovic B, Matavulj B (2003) Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med 69:413–419
Newall CA, Anderson LA, Phillipson JD (1996) Herbal medicines: a guide for health care professionals. Pharmaceutical Press, London
Oyaizu M (1986) Studies on product of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Oyedeji A, Ekundayo O, Olawore O, Adeniyi BA, Koenig W (1999) Antimicrobial activity of the essential oils of five Eucalyptus species growing in Nigeria. Fitoterapia 70:526–528
Perry RJ, Watson P, Hodges JR (2000) The nature and staging of attentional dysfunction in early (mininal and mild) Alzheimer’s disease: relationship to episodic and semantic memory impairment. Neuropsychologia 38:252–271
Pino J, Marbot R, Quert R, Garcia H (2002) Study of essential oils of Eucalyptus resinifera Smith, E. tereticornis Smith and Corymbia maculata (Hook.) K.D. Hill & L.A.S. Johnson, grown in Cuba. Flav Fragr J 17:1–4
Singh HP, Mittal S, Kaur S, Batish DR, Kohli RK (2009) Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J Agric Food Chem 57:6962–6966
Sridhar SR, Rajagopal RV, Rajavel R, Masilamani S, Narasimhan S (2003) Antifungal activity of some essential oils. J Agric Food Chem 51:7596–7599
Thomann R, Bauermann U, Hagemann L (1997) Essential oils and plant substances: an alternative to synthetic growth enhancers in animal feeding. In: Baser KHC (ed) Proc. 28th Internat. Symp. Ess. Oils, Eskischir, Turkey, September 1997, p 68
Acknowledgement
The authors thank Mr. S.K. Sharma, Botanist, Herbal Garden and Herbarium Research Institute in ISM, Joginder Nagar, District Mandi, H.P., India for the identification of plant material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guleria, S., Tiku, A.K., Gupta, S. et al. Chemical composition, antioxidant activity and inhibitory effects of essential oil of Eucalyptus teretecornis grown in north-western Himalaya against Alternaria alternata . J. Plant Biochem. Biotechnol. 21, 44–50 (2012). https://doi.org/10.1007/s13562-011-0073-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-011-0073-2