Abstract
The binary system of 4-octyl-4′-cyanobiphenyl (8CB) and 4-octyloxy-4′-cyanobiphenyl (8OCB) has been studied by means of differential scanning calorimetry (DSC) and ultraviolet absorption spectrophotometry (UV). The phase-transition temperatures, enthalpies, and entropies have been determined by using calorimetric methods on DSC. The results indicate clearly the existence of three-phase regions across the crystalline-to-smectic A, smectic A-to-nematic, and nematic-to-isotropic transitions in the 8CB/8OCB mixtures. The obtained phase-transition temperatures of the 8CB/8OCB mixtures are between the data for 8CB and 8OCB. A few of the phase transitions cannot be observed at high heating rates. The phase-transition temperatures of the 8CB/8OCB mixtures rise with the heating rate between 2°C/min and 15°C/min. The activation energies were calculated by the Ozawa method for the phase transitions of 25% 8CB and 75% 8OCB liquid crystal mixtures. UV experiments were carried out to characterize the absorptivity constants of liquid crystal and their mixtures. The molar absorptivity and maximum absorption wavelengths were measured in chloroform solution by UV spectrophotometry. The maximum absorption wavelength of the 8CB/8OCB mixtures increases with decreasing percent weight of 8CB in 8OCB, a result associated with the different lengths of the alkyl chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Liquid crystals, a phase of matter with properties between those of conventional liquids and of crystalline solids [1, 2], have been known for more than a century. In recent years, their electro-optic and thermal properties, which resulted in a variety of potential applications ranging from flat panel displays to laser beam steering and optical switching for telecommunications [3–6], have been studied in certain nematic liquid crystal mixtures. The thermotropic liquid crystals, well known and widely studied, are of interest from the standpoint of basic research and also from the perspective of applications in electro-optic displays and temperature and pressure sensors [7]. Most applications rely on the specific nature of the electro-optical response functions combined with the controllable thermophysical properties.
The present work is part of a research project aimed at developing liquid crystal mixtures. The design of prototypical devices and, even more important, the up-scaling from laboratory experiments to industrial processes call for detailed knowledge of the properties of the mixtures. This paper focuses on the mixtures of the nematogenic homologous p-(n-alkyl)-p′-cyanobiphenyl (nCB) and p-(n-alkoxy)-p′-cyanobiphenyl (nOCB), where n is the number of carbons in the n-alkyl or n-alkyloxy tails. In particular, 4-octyl-4′-cyanobiphenyl (8CB) and 4-octyloxy-4′-cyanobiphenyl (8OCB) are two of the best-known liquid crystalline substances. 8CB and 8OCB, as well as other members of the nCB and nOCB homologous series, are important for the technological applications due to the convenient temperature range of the nematic phase and good chemical stability [8]. Important characteristics, such as the melting and clearing temperatures and their enthalpies [9], can be computed from a single data run. Measurements for 8CB and 8OCB and other members of the homologous series were carried out by Oweimreen et al. [10] using an adiabatic scanning calorimeter, and the electro-optical properties of these liquid crystals were reported by BDH Chemicals [11]. Information on the phase-transition temperature and transition enthalpy of a pure liquid crystal allows one to estimate the effect of the mixture ratio (in wt.%) on the emergence of a region in which the nematic and isotropic phases coexist [12]. Structural investigations and the identification of phase transitions in liquid crystals are essentially based on thermal analysis, optical microscopy, and X-ray measurements. In the present work, we have investigated the structure and phase transition of nematic liquid crystals 8CB and 8OCB and their mixtures by differential scanning calorimetry (DSC).
The UV traces guided the choice of adequate wavelengths and the corresponding molar absorptivities (ε) for the liquid crystal (LC) blend assays. In order to investigate the effect of alkyl chain length on the LC blends in chloroform solution, the spectral behaviors of 8CB and 8OCB and their blends were independently measured by UV spectrometry. Although the spectra of the blends and of the pure LCs were similar, several distinctive spectral characteristics were found in the former. To compare the spectra, we found it convenient to determine the chain length for the pure LCs beforehand since the wavelength of maximum absorption (λ max) depends on the chain lengths. In particular, a maximum at the smallest wavelengths denotes the presence of short alkyl chains with aggregated hydrogen.
2 Experimental
2.1 Materials
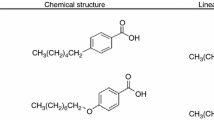
The LC materials 8CB and 8OCB were purchased from Sigma-Aldrich Corporation and were used without further purification; their phase-transition temperatures were in substantial agreement with the data given in the catalogue. The structural formulas of the 8CB and 8OCB nematic LCs in this study are shown in Fig. 1.
2.2 Thermal Analysis
The thermal properties of the LCs and their mixtures were measured in a Perkin-Elmer DSC-7, equipped with a data acquisition and analysis station. The samples were continuously heated in pure argon atmosphere. Indium (99.999 wt.% pure In) was used to calibrate the DSC unit for both temperature and enthalpy of transition. The experiments were carried out in the 15–90°C range with scanning rates of 2°C, 5°C, 10°C, and 15°C/min.
The LC-mixture samples were stirred with an injector needle at temperatures below T AN (smectic A to nematic) and T NI (nematic to isotropic) and above T NI by heating on a hot stage for about 30 min. The process was repeated, again for 30 min. After that, DSC samples were prepared in a thin aluminum pan, which was then placed on the stage of a piston-like sample crimper. The DSC was initially cooled to 20°C, and each sample was left in the sample pan for 2 min to ensure that thermal equilibrium was reached. The temperature was then ramped from 20°C to 90°C―the reported clearing point [11]―and held at 90° for about 2–3 min to ensure complete transformation to the isotropic phase.
2.3 UV Spectroscopy
While the 8CB liquid crystal is a somewhat viscous liquid, 8OCB is solid at room temperature. Solutions of mixtures within the concentration range 1.7 × 10−5 and 4.1 × 10−5 M were prepared by weighting and dissolving in chloroform. The following compositions (w/w) were prepared: 25% 8CB and 75% 8OCB, 50% 8CB and 50% 8OCB, and 75% 8CB and 25% 8OCB. In order to ensure the good solubility of the LCs in chloroform at the studied concentrations, we made it possible to prepare uniform samples just by magnetic mixing at a temperature slightly above T CrA. Absorption measurements were carried out in real time, in the wavelength interval between 200 and 400 nm, using a Perkin-Elmer Lambda 45 UV/vis spectrophotometer. The spectra were recorded at room temperature in a 1-cm quartz cuvette. The samples were separately placed in the spectrometer, with another quartz cuvette as reference for transmission measurements. Before starting the transmission measurements, auto-zero count was taken using two cuvettes.
3 Results and Discussion
Liquid crystal mixtures are used to modify the viscosity and electro-optical properties of the material in LCDs. Accurate measurements of transition temperatures and enthalpies of liquid crystal mixtures are needed to assess the effectiveness of blends for LCD technology. With this in mind, we applied DSC heating cycles to the 8CB and 8OCB LCs and to different mixtures. Oweimreen and Morsy report the following transition temperatures for pure LC 8CB: crystalline–smectic A transition temperature, T CrA = 19.74°C; smectic A–nematic transition temperature, T AN = 29.07°C; and nematic–isotropic transition temperature, T NI = 39.07°C. For pure LC 8OCB, the same authors have measured the following transition temperatures: crystalline–smectic A transition temperature, T CrA = 52.86°C; smectic A–nematic transition temperature, T AN = 66.65°C; and nematic–isotropic transition temperature, T NI = 79.10°C [10]. The good agreement between the values in the literature [10–13] and the sharp transition temperatures in our measurements attests to the high purity of our LCs. As Fig. 2 indicates, the DSC was unsuitable to measure temperatures below 20°C. For this reason, we were unable to measure the T CrA for the 8CB liquid crystal. For the 8OCB liquid crystal, the DSC traces show the spectrum from temperatures below the crystalline-to-smectic A transition to temperatures above the nematic-to-isotropic transition. The DSC spectra for three 8CB/8OCB mixtures obtained upon heating from the smectic A-to-nematic transition temperature to the nematic-to-isotropic transition temperature are shown in Fig. 2.
The DSC traces of the LC mixtures exhibit three endothermic peaks, indicating that the structural transformation to the isotropic phase takes places in three steps. It is evident that the phase-transition temperatures, T AN and T NI, depend on the ratio of mixtures (in wt.%). The transition temperatures increase from 40.91°C to 56.58°C and from 50.37°C to 68.50°C, respectively, as the wt.% of 8OCB grows from 25% to 75%. As Fig. 2 suggests, the phase transitions of the LC mixtures are similar to those of pure 8CB and 8OCB. As functions of the mixture ratio, the phase-transition temperatures of the 8CB/8OCB mixtures interpolate linearly between the transition temperatures of pure liquid crystal 8CB and 8OCB. And the spectra at the transition temperatures in Fig. 2 display two-phase regions consistent with a first- and a second-order transition at T AN and T NI, respectively.
The phase-transition temperatures (T CrA, T AN, and T NI) are listed in Table 1. The peak temperatures extracted from the DSC spectra are in good agreement with those reported in the literature for similar experiments [10, 14]. Since the entropy jump is related to the latent heat, i.e., ΔH = T ΔS, where T is the transition temperature and ΔS is the entropic change, the calorimetric measurements determine the order of each phase transition. Table 1 lists the ΔH and ΔS calculated from the experimental data. The tabulated values show that the phase transition from crystalline solid to smectic A and from nematic to isotropic liquid is of first order, while the phase transition from smectic A to nematic is of second order, in good agreement with the findings by Sied et al. [14]. The ΔH and ΔS in our experiment are similar to the values in literatures [10, 14–16].
Figure 3 shows the dependence of phase-transition temperature on the heating rate for the 25% 8CB and 75% 8OCB LC mixtures. We can apply the Ozawa procedure [17] to the data, to estimate the phase-transition activation energies. The Ozawa method describes the evolution of the order parameter from one phase to another, the configurations in the two phases being separated by an activation energy. If the barrier is sufficiently high, the evolution of the order parameter is temperature dependent, i.e., the molecules rotate faster at higher temperatures. Accordingly, the transition is expected to become sharper as the heating rate is reduced and the molecules have time to respond to the gradual changes in temperature, a trend that is evident in Fig. 3. Giving mathematical substance to this notion, Ozawa’s procedure extracts the activation energy from the dependence of the peak temperature on the heating rate.
From the plots in Fig. 3, Ozawa’s procedure yields the following activation energies: 1,302.17, 217.69, and 261.50 kJ/mol for the phase-transition peak temperatures T CrA, T AN, and T NI, respectively, of the 25% 8CB and 75% 8OCB LC mixtures. The peak phase-transition temperatures in Fig. 3 and Table 2 grow as the heating rate rise from 2°C/min to 15°C/min. While the peak heights decrease at the crystalline-to-smectic A and the smectic A-to-nematic transitions, the peak heights increase at the nematic-to-isotropic transition as the heating rate rises. The crystalline-to-smectic A and the smectic A-to-nematic transitions are much smaller than the nematic-to-isotropic transitions. In fact, the smectic A-to-nematic transitions are sometimes hard to detect, because at high heating rates they may overlap the crystalline-to-smectic A and the nematic-to-isotropic transitions. Likewise, we were unable to observe the T CrA phase transition at 10°C/min and 15°C/min heating rates.
The heating-rate dependences for the crystalline-to smectic A and the smectic A-to-nematic transition are in good agreement, but the nematic-to-isotropic transitions are not in good agreement with the data reported by Neuenfeld and Schick [18]. Lower heating rates may be required to make the dependence linear. The heating-rate dependence of the peak shape of the liquid crystal mixture transitions is very complex and not well understood. A more complex model is necessary to describe the shape of maxima such as the ones displayed in Fig. 3. More detailed investigations of phase-transition curves recorded by highly sensitive calorimeters are called for.
The UV absorption spectra of 8CB and 8OCB and their mixtures in chloroform solutions were measured between 200 and 400 nm. Figure 4 presents the resulting molar ε spectra in the 225 to 400-nm range. The 8CB and 8OCB spectra display λ max wavelengths at 276 and 297 nm, respectively, in agreement with the wavelength of λ max in the literature [19], as well as with the E7 liquid crystal. The spectra of LC mixtures also exhibit strong absorption bands between 276 and 297 nm, much like pure 8CB and 8OCB. The λ max increases from 281 to 291 nm. The resulting ε, calculated according to Beer’s law [20], decreased from 40.1 × 103 M−1 cm–1 to 27.1 × 103 M−1 cm–1, respectively, as the wt.% of 8OCB increased from 25% to 75%, due to the oxygen atom and phenyl ring. The measured ε and λ max are listed in Table 3.
The UV spectra in Fig. 4 show negligible absorptivity at wavelengths above 340 nm, the wavelength of either the pulsed laser beam used in the liquid crystal deposition or the UV light used for curing sealants in liquid crystal devices [21]. Irradiation at 340 nm causes a decrease of the π→π* band intensity, an indication that the isomerization is taking place. We decided to consider the process complete when we could not observe any further change in the intensities of consecutive UV-visible spectra. All these features of 8CB/8OCB mixtures are characteristic of LC molecules, due to π→π* transition as found in single liquid crystals. Thus, these results in chloroform media seem to confirm the reported stability of mixtures to photodegradation [22]. One can choose the adequate wavelength and the corresponding molar ε for the LC mixture assays.
4 Conclusions
DSC curves recorded to determine the transition temperatures, enthalpies, and entropies of the phase transitions showed different transition peaks in liquid crystal 8CB/8OCB mixtures, the shape of the maxima depending on the heating rate. We employed Ozawa’s procedure to determine the activation energies. The heating-rate dependence of the peak shape still defies our understanding and calls for additional investigations with more sensitive calorimetry of the phase transitions.
Parts of this study overlapped earlier work on pure LC 8CB or 8OCB. In such instances, the 8CB/8OCB mixtures were assessed to be very similar to the pure liquid crystals, simply because the measured DSC curves of the 8CB/8OCB mixtures lay between the data for 8CB and 8OCB. The UV measurements showed that the properties of the 8CB/8OCB mixtures to be characteristic of LC molecules in chloroform. In particular, the wavelength of λ max for the 8CB/8OCB mixtures increase with decreasing wt.% of 8CB in 8OCB due to the alkyl chain length with H-aggregation and oxygen atom subsistence.
References
E.B. Priestley, P.J. Wojtowicz, P. Sheng, Introduction to liquid crystals (Plenum Press, London, 1974). 1
K. Hans, H. Rolf, Handbook of liquid crystals (Verlag Chemie, Weinheim, 1980)
J.W. Doane, in Liquid crystals—applications and uses, vol.1, ed. by B. Bahadur (World Scientific, Singapure, 1990), p. 361
H.S. Kitzerow, Liq. Cryst. 16, 1 (1994)
M. Schadt, Annu. Rev. Mater. Sci. 27, 305 (1997)
K. Ichimura, Chem. Rev. 100, 1847 (2000)
P.G. De Gennes, J. Prost, The physics of liquid crystals, 2nd edn. (Oxford Science Publications, Oxford, 1993)
J. Czub, S. Urban, A. Würflinger, Liq. Cryst. 33(1), 85 (2006)
G.W. Gray, K.J. Harrison, J.A. Nash, Electron. Lett. 9, 130 (1973)
G.A. Oweimreen, M.A. Morsy, Thermochimica Acta 346, 37 (2000)
M/s. Liquid Crystals Catalogue BDH Chemicals Limited Company, Poole, BH12 4NN, England (2000).
G.A. Oweimreen, D.E. Martire, J. Phys. Chem. 72, 2500 (1980)
H. Marynissen, J. Thoen, W. Van Dael, Mol. Cryst. Liq. Cryst. 97, 149 (1983)
M.B. Sied, D.O. Lόpez, J.L. Tamarit, M. Barrio, Liq. Cryst. 29(1), 57 (2002)
H. Suzuki, A. Inaba, J. Krawczyk, M. Massalska-Arodz, J. Chem. Thermodynamics 40, 1232 (2008)
J. Jadzyn, G. Czechowski, S. Laskowski, Acta Physica Polonica A 109, 723 (2006)
T. Ozawa, J. Therm. Anal. 2, 301 (1970)
S. Neuenfeld, C. Schick, Thermochimica Acta 446, 55 (2006)
S.T. Wu, J. Appl. Phys. 69(4), 2080 (1991)
J.D.J. Ingle, S.R. Crouch, Spectrochemical Analysis (Prentice Hall, Englewood Cliffs, 1988), p. 372
J. Gonzalo, P.E. Dyer, M. Hird, Appl. Phys. Lett. 71(19), 2752 (1997)
P.T. Lin, S.T. Wu, C.Y. Shang, C.S. Hsu, Mol. Cryst. Liq. Cryst. 411, 243 (2004)
Acknowledgements
The work has been supported by Kahramanmaras Sutcu Imam University Scientific Research Projects Coordination Department under Project Number: 2008/4-25 M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özgan, Ş., Okumuş, M. Thermal and Spectrophotometric Analysis of Liquid Crystal 8CB/8OCB Mixtures. Braz J Phys 41, 118–122 (2011). https://doi.org/10.1007/s13538-011-0034-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13538-011-0034-1