Abstract
Objective
The purpose of this study is to characterize the levels of particulate matter (PM10) and heavy metals (HMs) such as Cd and Pb, compare blood HMs (B-HMs) and high-sensitivity C-reactive protein (hs-CRP) levels and examine the prevalence of allergic diseases among general population living near the Gwangyang industrial complex (GIC).
Methods
A survey and environmental and biomonitoring sample collection were done as part of a study involving 300 residents who were 20 years of age and older. To compare, the participants were split into two groups: those with physician-diagnosed allergic diseases (PDAD) and without physician-diagnosed allergic diseases (non-PDAD). ICP/MS and GF/AAS were used to measure the HMs in PM10 and B-HMs. SPSS version v25.0 was used to analyze the data to identify risk factors between exposure and diseases.
Results
The levels of three air pollutants close to road transport (≤ 50 m) and industrial complex areas (≤ 5 km) were higher than those over 100 m and 15 km from each source. The GMs for the PDAD group were 0.97 μg/L in B-Cd and 1.04 μg/dL in serum hs-CRP, significantly higher than 0.86 μg/L and 0.71 μg/dL in the non-PDAD group. In the PDAD, the mean B-Pb in males was significantly higher than that in females. The mean B-Pb among locals close to the GIC area (≤ 5 km) was significantly higher than that over 15 km. We found several risk factors, including gender, daily average housing, and distance to road transport, were significantly related to the increased levels of B-HMs.
Conclusions
A significant association was found between higher levels of environmental exposure to PM10 and HMs, B-HMs and hs-CRP levels with an increased prevalence of PDAD. This study provided the information required to carry out a risk assessment and take measures to mitigate the potential health risk in the GIC area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to increased exposure to particulate matter (PM) like PM10 and PM2.5, environmental diseases, in particular respiratory and allergy diseases, are of growing concern in Korea [1]. The prevalence of allergic diseases, such as rhinitis and atopic dermatitis, is increasing among the general population [2]. It has been discovered that various environmental variables are linked to allergy disorders [3]. There may be a connection between allergy symptoms and air pollution levels, as evidenced by the increased frequency of allergic disorders and diagnosis rates in industrial complex areas with high levels of emissions [4, 5].
Gwangyang is a research location with a significant environmental exposure to air pollutants because of the volume of chemicals that the GIC produces and releases into the environment. Some of the sources of PM [6, 7] and HMs [8] include power plants, industrial facilities, fuel combustion, car exhaust gas, and rubbish incineration. The three most common particle sizes in the region are PM10, PM2.5, and PM1.0, which can be deposited in the lungs [9]. Exposure to environmental PM is linked to a number of harmful health outcomes, including lung cancer, allergic rhinitis, poor pulmonary function, conjunctivitis, hypertension, and atopy [10, 11].
Vulnerable groups may experience possible health impacts from high levels of heavy metal buildup in the body [12]. Lead (Pb) can enter the bloodstream [13] and travel to organs like the brain, liver, and kidney, disrupting the digestive, neurological, respiratory, and reproductive systems [14]. Pb is a metal that is produced by the burning of fossil fuels, mining, and industrial processes [15]. The most harmful contaminant is cadmium (Cd), which can harm the bones, kidneys, neurological system, and respiratory system [16, 17]. Cd is a potent systemic inflammatory trigger that can lead to acute or chronic inflammation in a number of organs [18]. The body absorbs and processes HMs in different ways depending on the individual. In order to determine health risks and potential health impacts, it is essential to evaluate the level of exposure using biological markers [19]. The body processes HMs differently, and evaluating exposure using biological markers is crucial to assess health risks [19]. Accumulation can cause immunological and inflammatory reactions. Likewise, allergic diseases can also be exacerbated when the inflammatory reactions occur in certain sites [20]. A great volume of PM containing HMs such as Pb and Cd is emitted from metal processing in refineries and petroleum combustion in GIC. A previous study indicates that Onsan, Noksan, Changwon, Ulsan, Pohang, and Shihwa industrial areas are heavily polluted with Cu, Zn, Cd, and Pb [21].
Systemic inflammation is one of the major characteristics of respiratory allergic diseases and hs-CRP is an inflammatory marker. Atopic dermatitis (AD), allergic conjunctivitis (AC), allergic rhinitis (AR), and bronchial asthma (BA) are common allergic disorders [22]. Physicians claim that C-reactive protein tests are non-specific and can be elevated due to inflammatory conditions, with the American Heart Association recommending a high risk cutpoint of 3.0 mg/L. In general, the low-concentration measurement limit of conventional hs-CRP tests used to detect acute inflammatory reactions is 3–5 mg/L, while the tests used to predict the risk of cardiovascular disease have clinical decisions of 1 mg/L to 3 mg/L [23]. The levels of hs-CRP in the patients with allergic rhinitis were higher than those in the control group [24]. It was also reported that the risk of atopic dermatitis was approximately twofold higher in the groups with hs-CRP greater than 3 mg/L compared to the other groups with hs-CRP less than 1 mg/L [25]. Studies identify a causal relationship between allergic diseases [3, 4] and environmental exposure to air pollutants, including HMs [21, 26] and PM [27, 28] using hs-CRP. However, most studies have mainly focused on the general population [21], but few studies have been conducted to investigate the association between environmental air pollution and allergic diseases among older adults living near industrial complexes.
The goal of this study is to characterize the levels of environmental air pollutants like particulate matter (PM10) and HMs (Cd and Pb), collect biomonitoring samples to identify B-HMs and serum hs-CRP levels, and compare the prevalence of allergic diseases among older adults living in the GIC area.
Materials and methods
Study area and subjects
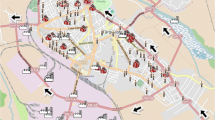
Figure 1 depicts the research area, which includes a 5 km area within 5 km of the industrial complex and a control area between 5 and 15 km. The proximity of individual residences to sources was presumed to link exposure-control to information on residence locations: the closer the subject's residence approaches within 50 m of the traffic road, within 5 km of the industrial complex area, and within a control area of 5–15 km, the higher the possibility of pollution and allergic diseases.
At the start of the study in July 2019, 300 volunteers aged 20 and up who had lived in Gwangyang for more than 5 years were chosen. The average age of the 300 participants was 65 years. As allergies grew beyond the age of 65 in the industrial complex area, cases were divided into two age groups to compare the differences between B-HMs: older (≥ 65 years) and younger (< 65 years) populations. The study participants were further divided into two groups: those with physician-diagnosed allergic disorders (PDAD) and those without (non-PDAD), based on whether or not allergic rhinitis, atopic dermatitis, or allergic eye disease had ever been diagnosed by a physician.
Questionnaire survey
The questionnaire contains several questions about personal information and residential characteristics, lifestyle factors, time activity pattern and individual health conditions (history and types of diagnosed diseases, etc.).
Environmental sampling and analysis
Using mini-volume air samplers (Air Metrics, USA), PM10 was detected in outside residential dwellings for 8 h. 5 L/min was the absorption rate. Before and after sampling, the Pallflex membrane filter (47 mm, Pall Corp., USA) was kept at the constant temperature and humidity device for at least 48 h. A microbalance (CP2P-F, Sartorius, Germany) was used to repeat three times in order to calculate the weight concentration.
HMs in PM10 were extracted using a heated plate, and the extraction solvent was 65% high-purity nitric acid (HNO3). Following the placement of the filter in a Teflon jar, 7 mL of HNO3 and 2 mL of hydrofluoric acid (HF) were added for pre-treatment. The materials were degraded for 8 h at a temperature of 180 °C. The Teflon jar was then filled with 15 mL of distilled water, and the acid was volatilized after breakdown. This procedure was carried out three times. Finally, tertiary distilled water was used to reduce the final amount to 20 mL. Following pre-treatment, heavy metal concentrations were measured using an inductively coupled plasma/mass spectrometer (ICP/MS, PerkinElmer, USA) and adjusted for blank sample values.
Collection of biomonitoring samples (B-HMs and serum hs-CRP)
A total of 5 mL of blood, including samples for long-term storage, were collected in a K2-EDTA vacutainer, agitated to prevent hardening, and frozen (− 20 °C). After combining whole blood 100 μL, 0.2% HNO3 100 μL, and diluted solution (2.5% NH4H2PO4 and 0.15% g (NO3)2 in 0.1% Triton X-100) 800 μL in a 5 mL tube, B-Cd was measured using GF/AAS (Atomic Absorption Spectrometer, Graphite Furnace, Thermo Fisher). After combining whole blood 50 L, 0.2% HNO3 500 L, and a diluted solution (0.2% NH4H2PO4/0.2% Triton X-100) 900 μL in a 5 mL tube, B-Pb was evaluated using GF-AAS (Graphite Furnace-Atomic Absorption Spectrometer, Thermo Fisher). Serum hs-CRP was tested by Roche/Hitachi Cobas C 701/702 after 15–20 min of centrifugation at 2500–3000 rpm.
Statistical analysis
The study used IBM SPSS v25.0 for statistical analyses, using a student's t-test to determine mean differences in environmental PM10 and HMs concentrations. Mann–Whitney tests were used to compare the geometric mean (GM) of B-Cd, B-Pb, and serum hs-CRP levels. A reference value of 3.0 g/dL was used to identify a relationship between serum hs-CRP and B-HMs [29]. Spearman's correlation coefficients were calculated to identify relationships between environmental exposure and biomarker concentrations. A multiple regression analysis was performed to determine risk factors affecting B-HM levels. A p-value of 0.05 or lower is generally considered statistically significant.
Results
Demographic characteristics of study subjects
Table 1 shows the demographic characteristics of study subjects obtained from the questionnaire survey. Of 300, 169 subjects were non-PDAD, and 131 were PDAD. In both groups, the proportion of females was higher than males, and the mean age was over 60 years old. The average period of residence was over 25 years. Subjects spent more than 19 h in their residential housings on average. About 90% of the study subjects were non-smokers in both groups. The proportion of subjects living near the road transport and GIC area was relatively high.
Mean levels of environmental PM10 and HMs
The average PM10 concentration was 10.95 μg/m3, whereas Cd and Pb values were 1.90 ng/m3 and 24.92 ng/m3, respectively (Table 2). They did not surpass the exposure limits when compared to the Korean environmental exposure limits of 50 μg/m3 for PM10 and 500 ng/m3 for Pb on an annual basis. The average outdoor PM10, Cd, and Pb values in residential housings near 50 m of a road were 14.24 μg/m3, 2.00 ng/m3, and 27.42 ng/m3, respectively. They were greater than PM10 4.35 μg/m3, Cd 1.68 ng/m3, and Pb 19.92 ng/m3 in residential housings located more than 100 m from road transport. The average of PM10, Cd, and Pb concentrations within 5 km of the GIC area was 12.36 μg/m3, 2.45 ng/m3, and 31.69 ng/m3, respectively, which are significantly higher than PM10 concentrations of 8.12 μg/m3, 0.79 ng/m3 for Cd, and 11.39 ng/m3 for Pb near residential housing.
Prevalence of physician-diagnosed allergic diseases (PDAD)
The prevalence of PDAD was high in the order of allergic rhinitis (N = 92), atopic dermatitis (N = 64), and allergic eye disease (N = 24), and the prevalent allergic diseases were also high among the residents living close to the road transport and industrial complex area (Table 3).
B-HMs and a marker of systemic inflammation (serum hs-CRP)
Table 4 lists the GM and GSD for many biomarkers, including serum hs-CRP and B-HMs. The GMs of non-PDAD were 0.86 μg/L for B-Cd, 1.32 μg/dL for B-Pb, and 0.71 mg/dL for serum hs-CRP, whereas the GMs of B-Cd and B-Pb, and serum hs-CRP among PDAD were 0.97 μg/L, 1.29 μg/dL, and 1.04 mg/dL, respectively. The only hs-CRP level was significantly different between non-PDAD and PDAD (p < 0.05).
Table 4 also illustrated the mean concentrations of B-HMs and serum hs-CRP for non-PDAD and PDAD groups by questionnaire survey. The GMs of B-Cd and B-Pb in the non-PDAD group were 0.65 μg/L and 1.35 μg/dL for males, and 1.08 μg/L and 1.30 μg/dL for females, respectively. On the other hand, the GMs of B-HMs in the PDAD group were 0.85 μg/L and 1.43 μg/dL for males, and 1.07 μg/L and 1.20 μg/dL for females.
B-Cd levels were significantly different between age groups: ≥ 65 years (0.95 μg/L) and < 65 years (0.79 μg/L) in the non-PDAD group (p < 0.05), but not in the PDAD group. However, younger populations had higher B-Pb levels in both non-PDAD and PDAD groups.
B-Pb levels in the younger (< 65 years) were higher than those of the older (≥ 65 years) subjects. A group of subjects residing within a distance of 50 m from the road transport had significantly higher B-Cd, 0.94 μg/L for non-PDAD and 1.05 μg/L for PDAD, than those for other subjects residing over 100 m, 0.74 μg/L and 0.82 μg/L, respectively (p < 0.05). The GMs of B-Pb within 50 m, 1.35 μg/dL for non-PDAD and 1.39 μg/dL for PDAD, were also higher than those over 100 m, 1.27 μg/dL and 1.10 μg/dL.
A group of subjects residing within a distance of 5 km from the industrial complex area had higher means of B-Cd, 0.90 μg/L for non-PDAD and 1.03 μg/L for PDAD, than those for other subjects residing over 15 km, 0.79 μg/L and 0.85 μg/L, respectively, but not statistically significant. The B-Pb levels within 5 km, 1.39 μg/dL for non-PDAD and 1.37 μg/dL for PDAD, were also higher than those over 15 km, 1.21 μg/dL and 1.14 μg/dL.
Furthermore, the GM of serum hs-CRP in the non-PDAD group was 0.95 μg/dL for females, which is higher than that of males, 0.85 μg/dL. As for the PDAD group, the GMs of serum hs-CRP levels were 1.07 μg/dL for males, higher than that of females, 1.02 μg/dL. Overall, there appeared significant difference in the means of serum hs-CRP between non-PDAD (0.71 μg/dL) and PDAD (1.04 μg/dL) groups (p < 0.05), but not significantly differed by each characteristic.
Table 5 shows the GMs of B-HMs by a reference value of 3.0 mg/dL for serum hs-CRP by characteristic. Overall, the GMs of B-Cd with a level of ≥ 3.0 mg/dL of hs-CRP were higher than those with a level of < 3.0 mg/dL in both non-PDAD and PDAD groups. However, the B-Cd mean in the PDAD group was 1.03 μg/L, which is higher than that in the non-PDAD group, 0.96 μg/L, when hs-CRP level is ≥ 3.0 mg/dL. The mean B-Pb levels in both non-PDAD and PDAD groups, however, were not significantly different between two categories, < 3.0 mg/dL and ≥ 3.0 mg/dL of hs-CRP. When a level of ≥ 3.0 mg/dL of hs-CRP, the mean B-Cd level was 1.13 μg/L for females, higher than 0.91 μg/L for males. In the non-PDAD group, the mean B-Cd level among a group of < 65 years old was 1.18 μg/L for a level of ≥ 3.0 mg/dL of hs-CRP, significantly higher than that for a level of < 3.0 mg/dL, 0.77 μg/L (p < 0.05).
Table 6 shows Spearman’s correlation coefficients between environmental air pollutants (outdoor PM10 and HMs) and biomarkers (B-Cd, B-Pb, and serum hs-CRP) for the study subjects (N = 60) who voluntarily agreed and participated in the questionnaire surveys. The levels of outdoor PM10 were significantly correlated with outdoor Cd (r2 = 0.34, p < 0.01) and Pb (r2 = 0.28, p < 0.05), respectively. However, the rest of environmental air pollutants and biomarkers were not significantly correlated.
The results of multiple regression analysis are shown in Table 7. Among the non-PDAD group, risk factors significantly affecting the B-Cd levels among the residents were gender for female (β = 0.44, p < 0.05), average daily housing hours (β = 0.25, p < 0.05), and a distance to the road transport (≤ 50 m) (β = 0.25, p < 0.05) with a power of explanation, r2 = 0.27. Among the PDAD group, however, two risk factors, such as gender, especially for female (β = 0.19, p < 0.05), and a distance to the road transport (≤ 50 m) (β = − 0.27, p < 0.05) with a power of explanation, r2 = 0.14, were significantly associated with the increased B-Cd levels. The only factor, gender for male (β = − 0.20, p < 0.05), was significantly associated with the increased B-Pb levels.
Discussion
PM10, Cd, and Pb concentrations were substantially greater near the industrial complex region than in the residential area [30]. Another investigation on the steel industrial complex in China found Cd and Pb concentrations of 4.0 ng/m3 and 195.2 ng/m3, respectively, which were higher than in the surrounding areas [6]. Chae et al. found a heavy metal enrichment factor of 10 or greater in the atmosphere around the GIC region, indicating significant pollution from manmade sources [31]. We discovered that PM10, Cd, and Pb concentrations in the GIC were much higher than previously reported.
PM10 and heavy metal levels may be influenced by exhaust from public transportation, induced by running tires and road friction, and by the re-dispersion of dust held on highways [32]. Road transport and industrial operations contribute 25% of urban ambient PM2.5, while industrial activities account for 15% globally [33]. This suggests that the high concentrations of environmental contamination surrounding residential dwellings are caused by their closeness to vehicle transit.
A recent study found that 15.9% of elementary school students in Korea have atopic dermatitis, which is higher than in suburban, coastal, and urban areas [4]. The prevalence of allergic rhinitis and allergic eye disease is also high in residential areas near national industrial complexes [34]. Park et al. found a significant increase in outpatient allergic rhinitis due to rising PM10 and PM2.5 concentrations [1]. Oh et al. reported a high prevalence of allergic rhinitis and allergic eye disease in the GIC area [35]. The high prevalence of allergic diseases is strongly associated with traffic-related pollutants, including NO2 and PM2.5 [36, 37]. In this study, we discovered that transport roads had high concentrations of PM10 and HMs as well as a high prevalence of allergy symptoms nearby. Further research is needed to identify the association between environmental pollutants and allergic diseases.
The Korean general population over the age of 20 had GMs of 0.77 μg/L in B-Cd and 1.48 μg/dL in B-Pb [38]. The GMs of B-Cd and Pb in the USA were 0.30 μg/L and 0.92 μg/dL, respectively [39]. The GM of B-Cd aged 3–79 in Canada was 0.28 μg/L and 0.93 μg/dL [40]. Both Cd and Pb are mostly exposed in the human body through nutrition [41], and the concentration of Cd in the Korean population was much higher due to dietary patterns [42].
The study area near the GIC, which emits high levels of mercury (HMs), has a significantly higher mean B-Cd level among study subjects compared to the Korean general population. Pb exposure in the industrial complex area has a lower tendency than the general population [43] but a higher tendency than other countries. Long-term exposure to Cd and Pb can negatively impact the nervous system, reproductive system, respiratory system, and cancer [17]. Further observation of health effects among residents is necessary. Previous studies in Ulsan, Korea, found higher concentrations of urinary Cd for allergic patients [12] and cord B-Cd among schoolchildren diagnosed with atopic dermatitis [26].
The mean serum hs-CRP level was 3.38 mg/dL among children diagnosed with allergic conjunctivitis, which was higher than 3.27 mg/dL for a control group [27]. Similarly, the mean level of serum hs-CRP for allergic rhinitis patients was 3.64 mg/dL, significantly higher than that of the control group with a level of 2.13 mg/dL [24]. All the results showed consistency with our study findings that allergic diseases might be related to high levels of B-HMs and hs-CRP, an indicator of systemic inflammation. More research is needed to determine the relationship between long-term exposure to low levels of HMs and a rise in the systemic inflammatory marker.
In both PDAD and non-PDAD groups, the GM of B-Cd levels was higher in females, while B-Pb was high in males. In a previous study using the Korean National Health and Nutrition Survey data, the mean level of B-Cd in females was 1.31 μg/L, significantly higher than that in males, 0.89 μg/L, but the average B-Pb level was higher in males, 2.59 μg/dL, than that in females, 2.08 μg/dL [44], similar to our study findings.
Joo et al. reported that B-Cd levels can reflect the most recent exposure thus explain significant differences by period or route, unlike urine Cd levels representing the cumulative mean exposure levels [45, 46]. Our study showed similar results, with the difference between the mean B-Cd levels of two age categories (≥ 65 vs. < 65 years) depending on the period and route of exposure. Furthermore, it is well-known that the internal concentration and distribution patterns of B-Pb increase with age but decline after certain age groups, from 50 to 60 years old, by its half-life [47]. Similarly, we found that the mean levels of B-Pb in the younger population were higher than those in the older population.
Residents in industrial complexes in South Chungcheong Province are exposed to higher levels of B-HMs than those living over 1 km [19], with those living within 3 km of smelters having the most noticeable effects on B-Cd and B-Pb levels [48].
Further in-depth data on health issues, lifestyle choices, and environmental influences on poor health outcomes must be gathered through more research. Serum hs-CRP can assess low inflammatory responses [21] and detect symptoms and associated conditions in locals, including asthma, allergies, metabolic syndrome, and cardiovascular disease [23, 46, 49, 50]. Characterizing sources and routes of exposure, forecasting longitudinal patterns of agent-specific cumulative exposure, and establishing the relationship between environmental exposure and chronic diseases using the serum hs-CRP marker all require additional study.
The study's strength lies in its ability to identify potential confounders and provide valuable insights into the link between exposure from industrial sources and likely health issues in the GIC. High B-HMs levels prominently increase serum hs-CRP concentration, suggesting a link between high B-HMs body burden and the development of various allergic diseases. However, there were some limitations: First, data on HMs concentrations in PM10 may not always reflect individual exposures and specific allergic diseases. Second, while total serum hs-CRP measurement is not specific to all allergic disorders, various secondary predictors (IgE, IL, FeNO, eosinophils, etc.) as well as hs-CRP will be predictive biomarkers for connecting systemic allergic endpoints. Finally, the result does not explain to what extent other air pollutants such as ozone and nitrogen dioxide could affect allergic endpoints.
Conclusions
Higher levels of environmental exposure to outdoor PM10 and HMs, B-Cd and B-Pb, and serum hs-CRP levels were significantly associated with the increased rates of prevalence of PDAD among the adult and elderly population residing in the housings near the road transport. Several risk factors, such as sex, average daily housing hours, and distance to road transport, were significantly associated with the increased levels of B-HMs among both non-PDAD and PDAD groups. Furthermore, longitudinal studies are needed to characterize long-term exposure to environmental pollutants, gather health conditions and lifestyle factors, collect biomonitoring samples, and identify the association between environmental exposure and chronic diseases in Korea’s urban and industrial complex areas. This study provides data to manage public health concerns and inform policymakers and the general public about air pollutant exposure, management strategies at the national industrial complexes.
References
Park JH, Park YY, Lee E et al (2020) Analysis on the effects of particular matter distribution on the number of outpatient visits for allergic rhinitis. Korean J Health Policy Manag 30(1):50–61
Ha J, Lee SW, Yon DK (2020) 10-year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008–2017. Clin Exp Pediatr 63(7):278–283
Lee J, Oh I, Kim M et al (2015) Correlation between allergic rhinitis prevalence and immune responses of children in Ulsan: a case-control study. Korean J Environ Health Sci 41(4):249–258
Lee J, Yun S, Oh I et al (2020) Impact of environmental factors on the prevalence changes of allergic diseases in elementary school students in Ulsan, Korea: a longitudinal study. Int J Environ Res Public Health 17(23):8831
Choi BK, Lim HS, Chung YS (2015) Prevalence of allergic rhinitis between urban and rural residents in a local community. J Agric Med Community Health 40(3):148–157
Dai QL, Bi XH, Wu JH et al (2015) Characterization and source identification of heavy metals in ambient PM10 and PM2.5 in an integrated iron and steel industry zone compared with a background site. Aerosol Air Qual Res. 15(3):875–887
Basith S, Manavalan B, Shin TH et al (2022) The impact of fine particulate matter 2.5 on the cardiovascular system: a review of the invisible killer. Nanomaterials (Basel) 12(15):2656
Kang BW, Kim MJ, Baek KM et al (2018) A study on the Concentration Distribution of Airborne HMs in Major Industrial Complexes in Korea. J Korean Soc Atmos Environ 34(2):269–280
Jung HJ, Han DH (2023) Effects of Particulate matter on allergic rhinitis and possible mechanisms. Korean J Otorhinolaryngol-Head Neck Surg 66(2):75–84
Kyung SY, Jeong SH (2017) Adverse health effects of particulate matter. J Korean Med Assoc 60(5):391–398
Hong E, Lee S, Kim GB et al (2018) Effects of environmental air pollution on pulmonary function level of residents in Korean industrial complexes. Int J Environ Res Public Health 15(5):834
Kim A, Hong YS, Bang JH et al (2016) The relationship between the prevalence of allergic diseases and urinary Cd concentrations among school-age children in two regions in Ulsan metropolitan city. Korean J Environ Health Sci 42(6):396–408
Olufemi AC, Mji A, Mukhola MS (2022) Potential health risks of lead exposure from early life through later life: implications for public health education. Int J Environ Res Public Health 19(23):16006
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8(2):55–64
Wan Mahiyuddin WR, Ismail R, Mohammad Sham N et al (2023) Cardiovascular and respiratory health effects of fine particulate matters (PM2.5): a review on time series studies. Atmosphere 14(5):856
Mijal RS, Holzman CB (2010) Blood Cd levels in women of childbearing age vary by race/ethnicity. Environ Res 110(5):505–512
Rehman K, Fatima F, Waheed I et al (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119:157–184
Markiewicz-Górka I, Chowaniec M, Martynowicz H et al (2022) Cd body burden and inflammatory arthritis: a pilot study in patients from lower Silesia, Poland. Int J Environ Res Public Health 19(5):3099
Joo Y, Roh S (2016) Exposure assessment of heavy metals using exposure biomarkers among residents living near a Chungcheongnam-do province industrial complex area. Korean J Environ Health Sci 42(3):213–223
Arikan TA, Kelles M (2019) Plasma selenium and Cd levels in patients with chronic otitis media in a Turkish population and their relation to inflammation markers. Biol Trace Elem Res 189(1):55–63
Jeong H, Choi JY, Lim J et al (2020) Pollution caused by potentially toxic elements present in road dust from industrial areas in Korea. Atmosphere 11(12):1366
Ito Y, Kato T, Yoshida K et al (2023) Prevalence of allergic diseases across all ages in Japan: a nationwide cross-sectional study employing designated allergic disease medical hospital network. JMA J 6(2):165–174
Pearson TA, Mensah GA, Alexander RW et al (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation 107(3):499–511
Yıldırım YS, Apuhan T, Koçoğlu E et al (2011) High sensitivity C-reactive protein levels in chronic rhinosinusitis and allergic rhinitis. Kulak Burun Bogaz Ihtis Derg 21(5):266–269
Sinikumpu SP, Huilaja L, Auvinen J et al (2018) The association between low grade systemic inflammation and skin diseases: a cross-sectional survey in the northern Finland birth cohort 1966. Acta Derm Venereol 98(1):65–69
Kim JH, Jeong KS, Ha EH et al (2013) Association between prenatal exposure to Cd and atopic dermatitis in infancy. J Korean Med Sci 28(4):516–521
Kurtul BE, Kabatas EU, Boybeyi SD et al (2018) Increased red cell distribution width levels in children with seasonal allergic conjunctivitis. Int Ophthalmol 38(3):1079–1084
Pilz V, Wolf K, Breitner S et al (2018) C-reactive protein (CRP) and long-term air pollution with a focus on ultrafine particles. Int J Hyg Environ Health 221(3):510–518
Malik R, Aneni EC, Shahrayar S et al (2016) Elevated serum uric acid is associated with vascular inflammation but not coronary artery calcification in the healthy octogenarians: the Brazilian study on healthy aging. Aging Clin Exp Res 28(2):359–362
Ko JC, Yang JH (2018) The investigation of the particle size distribution and the content of metal components in particulate matter (PM) from industrial complex. J Korean Soc Environ Tech 19:263–273
Chae JS, Park KR, Jeon JM et al (2020) Characteristics of heavy metal pollution in the atmosphere in residential area near the Gwangyang industrial complex. J Korean Soc Urban Eng 20:25–35
Hong N, Guan Y, Yang B et al (2020) Quantitative source tracking of heavy metals contained in urban road deposited sediments. J Hazard Mater 393:122362
Karagulian F, Belis CA, Dora CFC et al (2015) Contributions to cities’ ambient particulate matter (PM): a systematic review of local source contributions at global level. Atmos Environ 120:475–483
Lim DP, Jeong HY (2015) An analysis on characteristics of spatial distribution of the atopic dermatitis patients: with an application of the moran indices. J Korean Geogr Soc 21(3):583–592
Oh YJ, Choi JH, Park HJ et al (2017) Relationship between PM10 and O3 concentration and allergy symptoms among residents in the Gwangyang area. J Odor Indoor Environ 16:277–286
Kang SY, Song WJ, Cho SH et al (2018) Time trends of the prevalence of allergic diseases in korea: a systemic literature review. Asia Pac Allergy 8(1):e8
Jung DY, Leem JH, Kim HC et al (2015) Effect of traffic-related air pollution on allergic disease: results of the children’s health and environmental research. Allergy Asthma Immunol Res 7(4):359–366
Moon CS (2022) Variation of the oral intake and exposure characteristics of Pb among young ages in Korea: data analysis of 2011–2017 KNHANES. J Korea Contents Assoc 22(4):706–713
Centers for Disease Control and Prevention (CDC) (2021) Fourth National Report on Human Exposure to Environmental Chemicals : updated tables, March 2021: Vol 3: analysis of pooled serum samples for select chemicals, NHANES 2005–2016. Centers for Disease Control and Prevention, Atlanta, U.S.A. 2021, March
Haines DA, Saravanabhavan G, Werry K et al (2017) An overview of human biomonitoring of environmental chemicals in the Canadian health measures survey: 2007–2019. Int J Hyg Environ Health 220:13–28
Oh E, Lee EI, Lim H et al (2006) Human multi-route exposure assessment of lead and cadmium for Korean volunteers. J Prev Med Public Health 39(1):53–58
Kim SH, Lee J, Oh I et al (2021) Allergic rhinitis is associated with atmospheric SO2: follow-up study of children from elementary schools in Ulsan. Korea PLoS One 16(3):e0248624
Heo JA, Kim HM, Lee JT (2012) A study of the relationships between proximity to an industrial complex and blood lead levels and urinary cd levels. Korean J Environ Health Sci 38(2):95–104
Seo JW, Kim BG, Kim YM et al (2018) Associations of blood lead and cd levels with hypertension using the Korea national health and nutrition examination survey III–VI. Korean J Environ Health Sci 44(4):380–390
Joo Y, Kwon M, Kim SY et al (2019) A study on HMs exposure and major sociodemographic influence factors among Korean adults-Korean national environmental health survey (2009–2017). Korean J Environ Health Sci 45(5):541–555
Yang AM, Lo K, Zheng TZ et al (2020) environmental heavy metals and cardiovascular diseases: status and future direction. Chronic Dis Transl Med 6(4):251–259
Park SW, Kim KY, Kim DW et al (2006) The relation between blood lead concentration, epidemiologic factors and body iron status. Korean J Environ Toxicol 21(2):153–163
Jo H, Kim G, Chang J et al (2021) Chronic Exposure to lead and cadmium in residents living near a zinc smelter. Int J Environ Res Public Health 18(4):1731
Voils SA, Cooper-DeHoff RM (2014) Association between high sensitivity c-reactive protein and metabolic syndrome in subjects completing the national health and nutrition examination survey (NHANES) 2009–10. Diabetes Metab Syndr 8(2):88–90
Wang A, Liu J, Li C et al (2017) Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of cardiovascular disease. J Am Heart Assoc 6(10):e005610
Acknowledgements
This research was supported by the Soonchunhyang University Brain Korea 21 and Korea Ministry of Environment (2016001360009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Sunghyeon Jung, Jae-Hyoun Kim, Seung-Woo Jeong, Jong-Wha Lee and Bu-Soon Son declare that they have no conflict of interest.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB approval code 1608-138-787).
Informed consent
Written informed consent was obtained from all participants in this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jung, S., Kim, JH., Jeong, SW. et al. Association between exposure to air pollutants and allergic diseases among residents near the Gwangyang industrial complex in Korea. Toxicol. Environ. Health Sci. 15, 425–435 (2023). https://doi.org/10.1007/s13530-023-00193-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-023-00193-6