Abstract
The present study aimed to investigate the association of insulin resistance (IR) with inflammatory gene expression levels, metabolic health, lipid profile, and body composition in the apparently healthy obese. In this cross-sectional study, 88 apparently healthy obese subjects were recruited and divided into insulin-resistant and non-insulin-resistant (NIR) groups. Fasting blood samples were taken to determine serum metabolic features. mRNA expression of inflammatory genes were assessed in freshly isolated peripheral blood mononuclear cells (PBMCs), using quantitative real-time PCR (qPCR). Bioelectrical impedance analysis (BIA) was used to describe body composition. Among inflammatory genes, toll-like receptor 4 (TLR4) mRNA revealed significant upregulation in PBMCs of IR group compared with NIR individuals (p = 0.035). High-density lipoprotein cholesterol (HDL-C, p = 0.04), low-density lipoprotein cholesterol (LDL-C, p < 0.001), waist circumference (p = 0.025), and waist to hip ratio (p = 0.013) were significantly different between the two groups. A significant but weak correlation of HDL-C was observed with TLR4 (r = − 0.305; p = 0.011) and myeloid differentiation factor 88 (MyD88, r = − 0.27; p = 0.024) expression level. Also, LDL-C was found to be correlated with TLR4 (r = 0.302; p = 0.012) and MyD88 (r = 0.267; p = 0.027) expression levels. There was also a significant correlation between HOMA-IR and HDL-C (r = − 0.25; p = 0.019). The results of this study indicated the possible link between IR and TLR4. Also, there was a significant correlation between HDL-C and LDL-C as well as between TLR4 and MyD88. Some inflammatory genes and metabolic parameters were also significantly correlated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity as a serious concern to public health is reaching epidemic rates worldwide. The major health complications associated with the growing prevalence of obesity are type 2 diabetes mellitus (T2DM), fatty liver disease, cardiovascular disease, and obesity-related cancers [1]. Obesity is also associated with abnormal inflammatory responses that are a risk for obesity-related insulin resistance (IR) [2].
IR is linked to various chronic diseases such as hypertension, hyperlipidemia, and atherosclerosis, the hallmarks of metabolic syndrome (MetS) [3]. The dyslipidemia of IR seems to be triggered by features of IR than obesity. However, not all subjects with diminished insulin sensitivity develop dyslipidemia [4]. In fact, adipocyte size is proposed as a key factor for determining the contributing degree of adipose tissue to dyslipidemia. Enlargement of adipocytes, enhanced in lipolysis, can lead to more circulating free fatty acids and their delivery to the liver to increase triglyceride synthesis, exacerbate IR, and promote dyslipidemia [5]. The study of Veilleux et al. [6] reported the association between enlargement of visceral adipocytes and dyslipidemia independent of body composition in obese subjects (181). In another study [7], serum triglyceride and high-density lipoprotein cholesterol (HDL-C) levels were directly correlated with IR and visceral fat. A research [8] on two groups with normal BMI showed that individuals were severely IR. Previous studies propose that the distribution of fat, particularly visceral obesity, may be a more important determinant of IR than overall obesity [9,10,11]. The key role of adipose tissue in the development of IR by releasing a wide range of proinflammatory cytokines and chemokines is clarified, but molecular basis for the link between obesity, IR, and metabolic state is not thoroughly identified.
There is considerable evidence that activation of membrane receptors such as toll-like receptors (TLRs) plays a significant role in the initiation and development of IR [2, 12, 13]. Glucose and saturated fatty acids can contribute to TLR expression and activation in human mononuclear cells and induce inflammatory cytokine production [14]. TLR4 recognizes free fatty acids which contribute to the pathogenesis of IR [15, 16]. Animal studies remarkably show that lack of TLR4 can protect mice from diet-induced obesity [13, 17, 18]. Furthermore, inhibition of TLR2 improved insulin sensitivity and signaling in muscles and white adipose tissue of mice [19]. Hardy et al. [20] reported increased TLR2 and TLR4 expression in adolescents with MetS compared to BMI-matched controls. However, studies between metabolically healthy and unhealthy obese individuals showed no significant differences in TLR2 and TLR4 gene expression levels [21, 22].
TLRs act via two downstream molecules, myeloid differentiation factor 88 (MyD88) and TIR domain-containing adaptor-inducing interferon-β (TRIF), which have connections with insulin homeostasis [2, 14]. An animal survey indicated that mice lacking MyD88 have decreased β cell mass compared to wild-type controls and they have normal glucose tolerance [23]. Also, another study revealed that TRIF deficiency induces decreased glucose tolerance and β-cell dysfunction [24].
In the study of Jialal et al. [25], there was a positive correlation between HOMA-IR and increased levels of TLR2 and TLR4 in patients with MetS. However, they did not report any downstream signaling proteins. While these important findings imply that TLR2 and TLR4 activation is important in the pathogenesis of IR, the association of IR and inflammatory genes is rarely studied. Therefore, the purpose of this study was to test the association of IR with TLR2 and TLR4 and downstream signaling in peripheral blood mononuclear cells (PBMCs) isolated from apparently healthy obese persons. Also, the association of IR with lipid profile, metabolic health, and body composition was studied.
Materials and methods
Study participants
In this cross-sectional study, 88 apparently healthy obese persons aged 29–43 years were recruited from numerous clinics in the northwest of Iran from June 15th to November 6th, 2015. All of the participants were classified as abdominally obese (waist circumference (WC) ≥ 95 cm) based on the Iranian National Committee of Obesity [26]. Informed consent was obtained from each participant and the study was approved by regional ethics committee of Tabriz University of Medical Sciences, Tabriz, Iran. The whole investigation was conducted according to the principles of the Declaration of Helsinki (ethical code: TBZMED.REC.1394.1191). Metabolic health status was defined using Meigs et al.’s [27] criteria in which the presence of less than three of the following components was considered as metabolically healthy state: high WC (≥ 95 cm), high serum triglyceride (TG) concentration (≥ 150 mg/dl), low serum high-density lipoprotein cholesterol (HDL-C) (< 40 mg/dl for men and < 50 mg/dl for women), elevated blood pressure (BP) (≥ 130/85 mmHg), and fasting blood sugar (FBS) (≥ 100 mg/dl).
The exclusion criteria were pregnancy and lactation, postmenopausal, recent change or misreport in energy intake (i.e., < 800 or ≥ 4200 kcal/d), chronic high-intensity exercise (> 100 min/week), smoking, alcohol consumption, individuals with serum TG (> 400 mg/dl), malabsorption, irritable bowel syndrome, recent gastrointestinal surgery in the past 1 year, and diarrhea for 3 consecutive days in the past 3 months. Patients with diabetes mellitus, acute or chronic infectious or inflammatory disease, thyroid disease, cardiovascular disease, abnormal complete blood count, malignant disease, kidney disease, and mental disorders were excluded from the study. Individuals receiving medications/therapies including anticoagulant therapy, anti-obesity drugs, steroid therapy, anti-inflammatory drugs, antibiotics, beta blockers, corticosteroids, oral contraceptives, and dietary supplements in the past 2 months were also excluded. Demographic data, medical history, and physical history questionnaires were obtained. Anthropometric indices were measured according to standard measurement protocols as described in our previous study [28]. Bioelectrical impedance analysis (BIA: BC-418MA, Tanita, Japan) was used to define the fat percentage, fat mass (FM), and fat-free mass (FFM).
Laboratory assays
After a 12-h overnight fast, 5 cm3 blood was obtained. Instantly, after centrifugation at 3000 rpm for 5 min, metabolic parameters were examined. FBS, total cholesterol (TC), TG, and HDL-C were assayed, using the standard enzymatic methods via Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran) and a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, Netherlands). Inter- and intra-assay coefficient of variation (CV) was < 5% for all analyses. Low-density lipoprotein cholesterol (LDL-C) was calculated with the Friedewald formula: LDL-C (mg/dl) = [TC] − [HDL-C] − [TG] ∕ 5.0. Insulin was assayed using ELISA Monobind kit.
The homeostasis model of insulin resistance (HOMA-IR) was calculated as
Quantitative insulin sensitivity check index (QUICKI), an indicator of insulin sensitivity, was calculated as
The patients were divided into insulin-resistant (IR) and non-insulin-resistant (NIR) groups.
Peripheral blood mononuclear cell isolation
PBMCs were isolated using Ficoll-Hypaque gradient density centrifugation (Baharafshan, Tehran, Iran).
Real-time PCR
RNA was extracted from PBMCs, using Accusol (Bioneer Pacific, USA). cDNA from total RNA was synthesized, using the Revert Aid First Strand cDNA Synthesis kit (Fermentas, Thermo fisher Scientific, USA). Real-Time PCR was performed using primers specific for TLR2, TLR4, MyD88, TRIF, and NFĸB (Invitro Gen), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as normalizer (Invitro Gen). Reactions were performed in triplicate, using a light cycler 96 real-time PCR instrument (Roche, Switzerland). Data were calculated via the 2−ΔΔCT method [31, 32].
Statistical analysis
All analyses were performed using SPSS software version 17.0 for Windows (PASW Statistics; SPSS Inc., Chicago, IL, USA). Normality of the data was checked, using Kolmogorov-Smirnov test. Mean ± standard deviation (SD) was used for parametric data and median (25th, 75th) for nonparametric variables. Standard statistical tests for comparison of the means of the two groups were independent sample t test for normal values and Mann-Whitney U test for abnormal data. Correlation between gene expression levels and other quantitative variables was assessed, using the Spearman correlation test. A p value less than 0.05 was considered significant. The sample size was estimated to be 67 persons, according to a previous study [33] based on serum HDL-C level, with 80% power and an α-error of 5%. Considering a drop-out rate of 30%, the total sample size required was 88.
Results
The general characteristics and anthropometric measurements of the participants are given in Table 1. Despite similar age (36.11 vs. 36.83 years) and BMI (30.66 vs. 31.77 kg/m2), the waist circumference (WC) (p = 0.025) and the waist/hip ratio (WHR) (p = 0.013) were higher in the IR group than the NIR group. There was no significant difference in body fat mass (FM) and fat-free mass (FFM) measured by bioelectric impedance between the two groups. The IR and NIR groups had no significant difference in terms of systolic and diastolic blood pressure.
The IR group had significantly lower HDL-C (p = 0.04) and higher low-density lipoprotein cholesterol (LDL-C) (p = 0.02) compared with the NIR group; however, there were no significant differences in other components of lipid profile (Table 2). Fasting insulin concentration and HOMA-IR were significantly higher (p < 0.001) and QUICKI was significantly lower (p < 0.001) in the IR compared to the NIR group (Table 2). There was a significant correlation between HOMA-IR and HDL-C (r = − 0.25; p = 0.019) (Fig. 1).
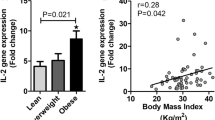
TLR4 mRNA revealed significant upregulation in PBMCs of the IR group compared with the NIR individuals (p = 0.035). However, TLR2 mRNA expression revealed no significant difference between the two groups. There was also no significant difference in mRNA expression of downstream signaling proteins of TLRs including MyD88, TRIF, and NFĸB between the two groups (Table 3). But there was a significant correlation between HDL-C and TLR4 (r = − 0.305; p = 0.011) and MyD88 (r = − 0.27; p = 0.024) expression level in all the persons (Fig. 2). A significant correlation of LDL-C was also observed with TLR4 (r = 0.302; p = 0.012) as well as with MyD88 (r = 0.267; p = 0.027) expression level (Fig. 3). Gene mRNA expression revealed no significant difference between the IR groups compared with the NIR individuals when analyzed based on gender (Table 4).
Discussion
The present study investigated the relationship of IR with inflammatory gene expression, lipid profile, metabolic health, and body composition in apparently healthy obese subjects. We found that TLR4 mRNA expression was significantly different in PBMCs of the IR group compared with the NIR individuals. Though Myd88 and NFĸB gene expression were increased in the IR group compared with the NIR group, it did not reach the significant level. However, there was a significant link of HDL-C and LDL-C with TLR4 and Myd88 expression. Approximately 70% of the participants were insulin-resistant. According to prior studies, high-risk abdominally obese patients are characterized by the cluster of metabolic abnormalities particularly high fasting insulin concentration [3]. Furthermore, it is proved that IR in population is related more to abdominal obesity than to general adiposity [8, 34]. Hence, there is a cause and effect relationship between them.
In the present study, we hypothesized that TLR4 signaling might be activated in obese condition which may mediate IR. We observed that mRNA expression of TLR4 was significantly increased in PBMCs of the IR group compared with the NIR group. In line with our study, the research conducted by Kim et al. [35] showed that TLR4 is a mediating key factor in the development of vascular inflammation and IR in diet-induced obesity via upregulation of transcriptional factors such as NFĸB. Also, it was recently proved that free fatty acids (FFAs) can activate inflammatory pathways by activating TLR4 signaling in different cells like adipocytes and macrophages. In fact, in the absence of TLR4, initiation of inflammatory signaling pathways cannot occur [13].
The research conducted by Dasu et al. [36] on subjects with T2DM indicated that TLR4 expression was significantly increased compared to control subjects. It also had a positive correlation with HOMA-IR. Moreover, Reyna et al. [37] in a study on skeletal muscle of insulin-resistant subjects (n = 22) reported abnormal TLR4 expression with little information on TLR4-MyD88 signaling pathway and its correlation to IR. In addition, Creely et al. [38] showed increased TLR2 expression in the adipose tissue of T2DM patients with strong correlates to endotoxin levels and with no change in TLR4 expression.
We failed to find a significant difference in the mRNA expression of TLR2 and downstream signaling proteins of TLRs including MyD88, TRIF, and NFĸB between the two groups, though significant correlations were observed between expression levels of inflammatory genes. Likewise, Telle-Hansen et al. [22] reported no differences in the expression level of TLR2 and TLR4 in the PBMCs of metabolically unhealthy obese (MUO) subjects compared with metabolically healthy obese (MHO) persons, although in MUO group, HOMA-IR was significantly higher than MHO group. In a study conducted by Gomez-Ambrosi et al. [21], no significant difference was noticed in the expression level of TLR4 and TNF-α between MHO and MUO groups with similar IR status. Although some studies [21, 22] showed that metabolic state of obese persons can be an important factor in determining inflammatory gene expression, our smaller sample size might have contributed to such results.
Our study also showed that among lipid profile, the levels of HDL-C and LDL-C were significantly different between the two groups, a finding which is consistent with prior reports [39,40,41,42,43]. Additionally, HDL-C levels inversely and LDL-C levels positively correlated with TLR4 and Myd88 expression levels. Although these correlations were significant, they were not strong, possibly due to lower sample size of our study. In line with our results, in a microarray study on obese subjects, TLR4 and Myd88 were inversely associated with plasma HDL-C levels [44]. Also, another study revealed that HDL-C causes MyD88-specific downregulation of TLR4 expression and signaling [45]. Moreover, several researchers [12, 46, 47] have shown that accumulation of LDL-C can trigger TLR2 and TLR4 signaling. Therefore, there is a strong link between HDL-C and LDL-C with TLR4 signaling and its relation with metabolic disorders like IR.
As the present work showed, there was a significant correlation of HOMA-IR with HDL-C and FBS. Similar to our finding, in a study [48] on 2283 patients with coronary heart disease (CHD), HDL-C was correlated with HOMA-IR levels. Another research on people with T2DM or MetS indicated significant positive correlations between the HOMA-IR and TC/HDL and between HOMA-IR and TG/HDL in MetS and T2DM patients [49]. Thus, low HDL-C may contribute to the high prevalence of IR; also, the combination of HOMA-IR and HDL-C as available and economic markers would help in identifying high-risk patients in clinical practice.
In this research, the mean WC and WHR were higher in the IR than those in the NIR patients. In line with this result, a study [50] from Quebec Heart Institute revealed that high WC is powerfully related to plasma insulin levels. Besides, Reaven et al. [51] showed that WC is a better predictor than BMI of insulin-mediated glucose uptake. Therefore, WC not only contributes to IR, but also can be used as a powerful predictor of clinical outcomes linked to IR.
Overall, to the best of our knowledge, this study is the first to be conducted on Tabriz population and to investigate the association of IR in relation to inflammatory gene expression levels, metabolic health, lipid profile, and body composition in apparently healthy obese individuals. However, some limitations of the study need to be considered, first, the small sample size and second, the case control nature of the study the cause of which could not be assessed through it.
Conclusion
The results of this study indicated the possible link between IR with TLR4. Also, there was significant correlation between HDL-C and LDL-C with TLR4 and MyD88. Some inflammatory genes and metabolic parameters were also significantly correlated.
Abbreviations
- BP:
-
Blood pressure
- BMI:
-
Body mass index
- FBS:
-
Fasting blood sugar
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model of insulin resistance
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- MetS:
-
Metabolic syndrome
- MHO:
-
Metabolically healthy obese
- MUO:
-
Metabolically unhealthy obese
- MyD88:
-
Myeloid differentiation factor 88
- NFĸB:
-
Nuclear factor kappa B
- NIR:
-
Non-insulin resistance
- PBMCs:
-
Peripheral blood mononuclear cells
- QUICKI:
-
Quantitative insulin sensitivity check index
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TRIF:
-
TIR-domain containing adaptor-inducing interferon-β
- TLR2:
-
Toll-like receptor 2
- TLR4:
-
Toll-like receptor 4
- WC:
-
Waist circumference
- WHR:
-
Waist/hip ratio
References
Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–9.
Könner AC, Brüning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22(1):16–23.
Seidell JC. Obesity, insulin resistance and diabetes—a worldwide epidemic. Br J Nutr. 2000;83(S1):S5–8.
Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;84(1):28–32.
Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl Ser. 1988;19:26–9.
Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60(5):1504–11.
Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84(1):137–44.
Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86(11):5366–71.
Lundgren H, Bengtsson C, Blohme G, Lapidus L, Sjöström L. Adiposity and adipose tissue distribution in relation to incidence of diabetes in women: results from a prospective population study in Gothenburg, Sweden. Int J Obes. 1989;13(4):413–23.
Ohlson L-O, Larsson B, Svärdsudd K, Welin L, Eriksson H, Wilhelmsen L, et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34(10):1055–8.
Banerji M, Lebowitz J, Chaiken RL, Gordon D, Kral JG, Lebovitz HE. Relationship of visceral adipose tissue and glucose disposal is independent of sex in black NIDDM subjects. Am J Physiol Endocrinol Metab. 1997;273(2):E425–E32.
Curtiss LK, Tobias PS. Emerging role of toll-like receptors in atherosclerosis. J Lipid Res. 2009;50(Supplement):S340–S5.
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid–induced insulin resistance. J Clin Investig. 2006;116(11):3015–25.
Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99(1):39–48.
Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–7.
Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7.
Poggi M, Bastelica D, Gual P, Iglesias M, Gremeaux T, Knauf C, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50(6):1267–76.
Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, et al. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56(8):1986–98.
Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, Carvalheira JB, et al. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol. 2008;199(3):399–406.
Hardy OT, Kim A, Ciccarelli C, Hayman LL, Wiecha J. Increased toll-like receptor (TLR) mRNA expression in monocytes is a feature of metabolic syndrome in adolescents. Pediatr Obes. 2013;8(1):e19–23.
Gómez-Ambrosi J, Catalán V, Rodríguez A, Andrada P, Ramírez B, Ibáñez P, et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37(10):2813–21.
Telle-Hansen V, Halvorsen B, Dalen K, Narverud I, Wesseltoft-Rao N, Granlund L, et al. Altered expression of genes involved in lipid metabolism in obese subjects with unfavourable phenotype. Genes Nutr. 2013;8(4):425–34.
Bollyky PL, Bice JB, Sweet IR, Falk BA, Gebe JA, Clark AE, et al. The toll-like receptor signaling molecule Myd88 contributes to pancreatic beta-cell homeostasis in response to injury. PLoS One. 2009;4(4):e5063.
Hutton MJ, Soukhatcheva G, Johnson JD, Verchere CB. Role of the TLR signaling molecule TRIF in β-cell function and glucose homeostasis. Islets. 2010;2(2):104–11.
Jialal I, Huet BA, Kaur H, Chien A, Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012;35(4):900–4.
Mirmiran P, Esmaillzadeh A, Azizi F. Detection of cardiovascular risk factors by anthropometric measures in Tehranian adults: receiver operating characteristic (ROC) curve analysis. Eur J Clin Nutr. 2004;58(8):1110–8.
Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906–12.
Saghafi-Asl M, Amiri P, Naghizadeh M, Ghavami SM, Karamzad N. Association of endotoxaemia with serum free fatty acids in metabolically healthy and unhealthy abdominally obese individuals: a case-control study in northwest of Iran. BMJ Open. 2017;7(5):e015910.
Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24(2):362–5.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10.
Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93(2):578–83.
Dasu MRDS, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–8.
Faam B, Zarkesh M, Daneshpour MS, Azizi F, Hedayati M. The association between inflammatory markers and obesity-related factors in Tehranian adults: Tehran lipid and glucose study. Iran J Basic Med Sci. 2014;17(8):577.
Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34(8):1617–21.
Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100(11):1589–96.
Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33(4):861–8.
Reyna SM, Ghosh S, Tantiwong P, Meka CR, Eagan P, Jenkinson CP, et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57(10):2595–602.
Creely SJ, McTernan PG, Kusminski CM, Da Silva N, Khanolkar M, Evans M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–E7.
Burchfiel CM, HAMMAN RF, MARSHALL JA, BAXTER J, KAHN LB, AMIRANI JJ. Cardiovascular risk factors and impaired glucose tolerance: the San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131(1):57–70.
Manolio TA, Savage PJ, Burke GL, Liu K, Wagenknecht LE, Sidney S, et al. Association of fasting insulin with blood pressure and lipids in young adults. The CARDIA study. Arterioscler Thromb Vasc Biol. 1990;10(3):430–6.
McKeigue PM, Miller G, Marmot M. Coronary heart disease in south Asians overseas: a review. J Clin Epidemiol. 1989;42(7):597–609.
Modan M, Halkin H, Lusky A, Segal P, Fuchs Z, Chetrit A. Hyperinsulinemia is characterized by jointly disturbed plasma VLDL, LDL, and HDL levels. A population-based study. Arterioscler Thromb Vasc Biol. 1988;8(3):227–36.
Haffner SM, Fong D, Hazuda HP, Pugh JA, Patterson JK. Hyperinsulinemia, upper body adiposity, and cardiovascular risk factors in non-diabetics. Metabolism. 1988;37(4):338–45.
Wolfs MG, Rensen SS, Bruin-Van Dijk EJ, Verdam FJ, Greve J-W, Sanjabi B, et al. Co-expressed immune and metabolic genes in visceral and subcutaneous adipose tissue from severely obese individuals are associated with plasma HDL and glucose levels: a microarray study. BMC Med Genet. 2010;3(1):34.
Van Linthout S, Spillmann F, Graiani G, Miteva K, Peng J, Van Craeyveld E, et al. Down-regulation of endothelial TLR4 signalling after apo AI gene transfer contributes to improved survival in an experimental model of lipopolysaccharide-induced inflammation. J Mol Med. 2011;89(2):151–60.
Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–22.
Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467–82.
Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, et al. Insulin resistance and cardiovascular events with low HDL cholesterol. Diabetes Care. 2003;26(5):1513–7.
Shaikh AZ, Badade ZG, Thomas SS. Homa-Ir and Ratios of Tg/Hdl and Tc/Hdl as indicators of insulin resistance in patient with metabolic syndrome and type 2 diabetes mellitus. Indian J Appl Res. 2015;5(8):668–91.
Lemieux I, Pascot A, Couillard C, Lamarche BT, Tchernof A, Alméras N, et al. Hypertriglyceridemic waist. Circulation. 2000;102(2):179–84.
Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res. 2005;2(3):105–12.
Acknowledgments
Thanks are due to all who participated in the study.
Funding
This study was financially supported by the Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. The results of this paper were extracted from MSc. thesis of Parichehr Amiri, registered at Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MSA and PA wrote the study protocol and study design. BB and DS helped with qPCR. MSA and PA analyzed and interpreted the data. PA, MN, and SM helped with the sampling. PA and MSA were involved in drafting the manuscript or revising it critically for content. All authors have given final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Amiri, P., Naghizadeh, M., Baradaran, B. et al. Insulin resistance in relation to inflammatory gene expression and metabolic features in apparently healthy obese individuals. Int J Diabetes Dev Ctries 39, 66–73 (2019). https://doi.org/10.1007/s13410-018-0626-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-018-0626-1