Abstract
We assessed the prognostic accuracy of urinary N-acetyl-β-D-glucosaminidase (NAG), an early proximal tubular damage marker for the onset of diabetic nephropathy. The study included 491 eligible participants with 76 healthy controls, 194 type 2 diabetes mellitus (T2DM) patients with 0–5, 5–10, 10–15, and 15–20 years of T2DM duration, 71 microalbuminuric patients, 100 diabetic nephropathy patients, and 50 non-diabetic nephropathy patients. Fasting glucose, serum fructosamine, HbA1C, urinary microalbumin, serum creatinine, estimated glomerular filtration rate (eGFR), serum NAG, and urinary NAG were estimated. We compared urinary NAG activity with other well-established markers of diabetic nephropathy like microalbuminuria, eGFR, and serum creatinine. Urinary NAG excretion was increased by 8 and 12 folds in T2DM patients of 10–15 and 15–20 years of diabetes duration (p < 0.0001), respectively, without the appearance of microalbuminuria. The urinary NAG activity increased 16 and 18 fold in moderately increased albuminuria and diabetic nephropathy patients, respectively (p < 0.0001), without any change in non-diabetic nephropathy patients. A cutoff value of 3 U/L of urinary NAG has demonstrated a sensitivity of 96.1 % and a specificity of 100 % discriminating healthy controls from patients with T2DM duration of 10–15 years (AUC 1.000) and 15–20 years (AUC 0.999); microalbuminuria (AUC 0.999), and diabetic nephropathy (AUC 1.000). Urinary NAG excretion gradually increases with the increase in duration of diabetes and appeared much before the microalbuminuria, decreased eGFR, and increased serum creatinine. Thus, the urinary NAG may be considered as a potential site-specific early tubular damage marker leading to diabetic nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy leads to structural alterations, including renal hypertrophy, thickening of basement membrane, and progressive glomerular accumulation of extracellular matrix components [1]. Ideally, urinary biomarkers of renal origin are derived from two sources: (i) glomerular damage or (ii) tubular damage [2]. Progression of diabetic nephropathy has a strong association with irreversible loss of renal function and mesangial expansion including the proximal tubule injury, which plays an important role in the pathogenesis of diabetic nephropathy [3, 4]. Evaluation of enzymuria has served as a sensitive and non-invasive method to measure the tubular integrity, and demonstrated its clinical application in the early detection of tubular injury [5]. The most initial tubular structural alterations are characterized by excessive accumulation of extracellular matrix at the glomerular basement membrane. Long-standing hyperglycemia and lack of insulin triggered these changes, especially in type 2 diabetes mellitus (T2DM) [6, 7]. About 40 % of T2DM patients develop evidence of diabetic nephropathy, diagnosed to have moderately increased albuminuria and severely increased albuminuria (albuminuria >300 mg/g creatinine). Microalbuminuria, decreased creatinine clearance, and raised serum creatinine levels are widely accepted as earliest clinical markers of diabetic nephropathy but with some limitations [2, 8, 9]. This condition demands further a more specific biomarker that can reflect the onset of diabetic nephropathy at an early reversible stage to prevent and worsen further.
Urinary enzymes are the valuable clinical tools for impaired renal function and help to assess the preclinical stage and monitor the progression of diabetic nephropathy [5]. Urinary N-acetyl-β-D-glucosaminidase (NAG) (E.C.3.2.1.30) is a hydrolytic enzyme of 150 KDa involved in mucopolysaccharide and glycoprotein metabolism of tubular basement membrane, and is localized exclusively in lysosomes of proximal tubules [2, 10, 11]. Normally, due to its high molecular weight, it is unable to cross the glomerular filtration barrier. Therefore, the increased excretion of urinary NAG may predict the early proximal tubular basement membrane damage in diabetic patients [12]. The increased excretion of urinary NAG and its efficiency has shown to detect tubular damage in T2DM individuals with normal kidney functioning [13].
Tubular damage markers are considered to be more sensitive and specific biomarkers for detecting early renal damage in various kidney diseases. Bazzi et al. found NAG as a promising marker for detecting early tubular toxicity in patients with idiopathic membranous nephropathy, minimal change disease, and primary focal segmental glomerulosclerosis [14]. The study carried out in acute kidney injury (AKI) patients demonstrated biomarkers like NAG, kidney injury molecule 1, and membrane metalloprotein 9, combined, could be useful to identify early proximal tubule injury [15]. Many clinical studies have supported the diagnostic and prognostic utility of such tubular markers in predicting microalbuminuria and diabetic nephropathy much before their appearance [16]. Tubular damage markers have gained the attention of many investigators, but modestly, patients with varying duration of diabetes are studied. Ouchi et al. showed a positive correlation of NAG with renal tubular damage in pre-diabetic patients [17]. Thus, our study intended to evaluate the efficacy of urinary NAG for early detection of proximal tubular damage leading to diabetic nephropathy in T2DM patients with varying duration of diabetes, and to compare it with other established markers of renal damage. The diagnostic accuracy and cutoff value of urinary NAG was calculated in susceptible T2DM patients prone to develop diabetic nephropathy.
Subjects
In our cross-sectional study, we analysed data of total 491 age-matched patients, attending OPD of Muljibhai Patel Urological Hospital, Nadiad, Gujarat, India. The cross-sectional study design was approved by the human ethical committee. The study comprised of total eight groups, where all T2DM patients were on oral hypoglycemic agents (OHA) like metformin or glibenclamide, on insulin or on both OHA along with insulin to maintain their glycemic index.
-
1.
Healthy controls (N = 76; female/male: 36/40)
(Mean age in years; mean ± SE: 58.4 ± 1.2)
-
2.
Normoalbuminuria with T2DM duration of 0–5 years (N = 81; female/male: 35/46)
(Mean age in years; mean ± SE: 56.9 ± 1.3)
(Therapeutic module: OHA 19.2 %, OHA + insulin 62.34 %, insulin 18.46 %)
-
3.
Normoalbuminuria with T2DM duration of 5–10 years (N = 38; female/male: 20/18)
(Mean age in years; mean ± SE: 59.1 ± 1.8)
(Therapeutic module: OHA 14.3 %, OHA + insulin 68.2 %, insulin 17.3 %)
-
4.
Normoalbuminuria with T2DM duration of 10–15 years (N = 45; female/male: 13/32)
(Mean age in years; mean ± SE: 56.8 ± 1.8)
(Therapeutic module: OHA 12.2 %, OHA + insulin 66.5 %, insulin 21.2 %)
-
5.
Normoalbuminuria with T2DM duration of 15–20 years (N = 30; female/male: 12/18)
(Mean age in years; mean ± SE: 57.6 ± 1.8)
(Therapeutic module: OHA 19.5 %, OHA + insulin 49.6 %, insulin 30.8 %)
-
6.
Microalbuminuria with T2DM duration of >15 years (N = 71; female/male: 38/33)
(Mean age in years; mean ± SE: 57.9 ± 1.1)
(Therapeutic module: OHA 22.5 %, OHA + insulin 41.9 %, insulin 35.6 %)
-
7.
Diabetic nephropathy with T2DM duration of >15 years (DN) (N = 100; female/male: 42/58)
(Mean age in years; mean ± SE: 57.4 ± 1.1)
(Therapeutic module: OHA 29.1 %, OHA + insulin 32.4 %, insulin 38.5 %)
-
8.
Non-diabetic nephropathy (N = 50; female/male: 29/21)
(Mean age in years; mean ± SE: 60.2 ± 1.4)
[IgA nephropathy (N = 22), focal segmental glomerulosclerosis (N = 12), minimal change disease (N = 9), nephrotic syndrome (N = 7)]
We distributed our study groups according to the duration of diabetes, microalbuminuria, and patients having proximal tubule damage as a primary consequence of nephropathy other than diabetes (non-diabetic nephropathy group). The patients were grouped to study earliest excretion of urinary NAG.
All eligible participants gave written and informed consent. Physicians at the hospital examined the patients. Study participants belonged to same economic status, food habits, and physical activity. Exclusion criteria consisted of malignant disease, infectious disease, liver disease, chronic renal failure, renal surgery, renal transplantation, urinary tract diseases, use of nephrotoxic drugs, and lactating and pregnant females. The study protocol was according the guidelines approved by the Local Human Research Ethics Committee. Fasting blood samples were collected in plain, as well as EDTA-coated pre-sterile Vacutainers obtained from BD-Biosciences. Clean midstream urine samples were collected during morning period in pre-sterile containers obtained from Tarson. The samples were transported to the laboratory at 4 °C. Serum/plasma was separated from whole blood at 4 °C at 3000 rpm and aliquots of urine samples (with 0.05 % sodium azide) were stored at −20 °C until further analysis.
Materials and methods
Serum and urinary creatinine were measured by Jaffe’s kinetic method using alkaline picrate reaction [18]. Glomerular filtration rate (eGFR) was calculated by Cockroft and Gaults equation [19]. The glycated hemoglobin was quantified as a long-term glycemic index by the method of Parker et.al [20]. Fasting blood glucose was estimated based on GOD-POD enzymatic reaction using Automatic Random Access Biochemistry Analyzer XL-300 (Erba Diagnostics, Mannheim).
The urinary and serum NAG activity was determined following the protocol of Horak et al., with some modifications [21]. Cellular debris and nucleic acid contamination were removed from the fresh urine samples (5 ml) by 5 min centrifugation process at 10,000×g, and clear supernatant was desalted using 3 KDa cutoff Millipore Amicon Ultra® centrifugal filters (Merck, Germany). Further, the supernatant was used for enzyme activity. 0.5 ml of urine sample/serum (1:100) working standard of freshly prepared 100 μmol/L p-nitrophenol in 0.15 mol/L NaCl was mixed with 0.5 ml of substrate (10 mmol/L p-nitrophenyl-N-acetyl-β-d-glucosaminide in 0.2 mmol/L citrate buffer pH 4.4) and incubated for 15 min at 37 °C. The enzymatic hydrolysis was terminated by adding 0.5 ml AMP buffer (2-amino-2-methyl-1-propanol hydrochloride, 0.75 mol/L, pH 10.25), and optical density was measured at 405 nM. The absorbance of NAG activity was proportional to the liberated p-nitrophenylate ion, after correction for absorbance of a urine/serum “blank” sample. One unit (U) of NAG activity was defined as amount of enzyme required to produce 1 μmole of p-nitrophenylate ion (substrate p-nitrophenyl-N-acetyl-β-d-glucosaminide) per minute under optimum test conditions (temperature 37 °C and pH 4.4).
Urinary microalbumin was estimated using ELISA-based method following the instructions manual of bovine albumin ELISA kit provided by Biovendor Research Diagnostics (European Union). 100 μl of bovine serum albumin (standard series of 0.78–50 ng/ml) or diluted urine samples (1:100–1:1000) were incubated in monoclonal anti-albumin antibody-coated 96-well plate to capture albumin with incubation of 1 h at 25 °C, 100 μl of HRP-conjugated anti-albumin antibody was added and incubated for 1 h. The remaining HRP complex was reacted with 100 μl of TMB for 30 min. 100 μl 1 M H2SO4 stopped the reaction, and absorbance was measured at 450 nm within 30 min. The microalbumin concentrations of unknown samples were determined using the standard curve plotted for standard series.
Statistical analysis
Mean ± standard error (SE) was used for data representation. Categorical data are expressed in percentages of total population for the particular study group. ANOVA followed by Kruskal-Wallis test following post hoc analysis by Dunn’s test analysed the differences between the groups. p < 0.05 was assumed statistically significant. Receiver operating characteristics curve (ROC) analysis was employed to calculate the area under curve, cutoff value, specificity, and sensitivity for urinary NAG activity, and significance was assumed at p < 0.0001. Multiple regression analysis was done to investigate the correlation of some independent variables with urinary NAG excretion as a dependent variable. Youden’s index was used for calculating cutoff value of urinary NAG and plotted via Youden’s plot taking into consideration the formula sensitivity + (1-specificity). All these analyses were carried out using GraphPad Prism version 6.00 software and SPSS version 11.5 software compatible with windows.
Results
Table 1 summarizes details of baseline parameters, serum and urinary biochemical and tubular activity parameters estimated in control and various test groups. Basal metabolic index (BMI) was found slightly higher but statistically non-significant in all groups of patients as compared to healthy controls. All T2DM patients showed higher fasting blood glucose level, glycated hemoglobin (the marker of a long-term glycemic index) as compared to healthy controls (p < 0.0001). Serum creatinine, used for assessing renal function, showed no significant changes in T2DM patients with diabetes duration of 0–5, 5–10, 10–15, and 15–20 years, whereas, elevated levels were observed in diabetic nephropathy (DN) and non-diabetic nephropathy (NDN) patients, which were at advanced stages of renal deterioration. eGFR commonly used to assess the renal efficiency was found normal in healthy control, whereas, the increased eGFR was observed in patients with 0–5, 5–10, and 10–15 years of diabetes duration which was further declined in T2DM patients with 15–20 years of duration, and moderately increased in albuminuria (MIA) group. The patients with DN (p < 0.0001) and NDN (p < 0.0001) showed further significant decline in eGFR. The patients with 0–5, 5–10, 10–15, and 15–20 years of diabetes duration showed the same urinary microalbumin excretion as that of healthy controls (normoalbuminuria), whereas, significantly increased microalbumin excretion was observed in MIA patients (albuminuria ranging in 30–299 mg/g creatinine). The DN and NDN patients showed severely increased albuminuria (with albuminuria >300 mg/g creatinine).
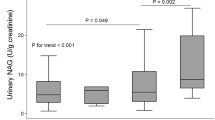
Urinary NAG activity showed a slight non-significant increase in early years of T2DM (0–5 and 5–10 years) which was significantly increased by 8 and 10 folds in 10–15 and 15–20 years of diabetes duration, respectively (p < 0.0001) (Fig. 1a), however, serum creatinine and microalbuminuria were still within the normal limits without decrease in eGFR during this period in T2DM patients (Table 1). Further, urinary NAG activity was gradually increased by 16 and 18 folds in patients with MIA and DN, respectively (p < 0.0001) (Fig. 1a). Furthermore, the serum NAG activity was not altered in any of the studied groups (Table 1). Therefore, the urine and serum ratio of NAG activity showed a similar pattern as that of urinary NAG activity with significant increase at 10–15 years onwards of diabetes duration (p < 0.0001) as shown in Table 1.
The cutoff value of urinary NAG activity was 3 U/L, as calculated by Youden’s index and plotted via Youden’s plot considering the formula sensitivity + (1-specificity) (Fig. 1b). This cutoff value has demonstrated a sensitivity of 96.1 % and a specificity of 100 % discriminating healthy controls from patients with T2DM duration of 10–15 years (AUC 1.000) and 15–20 years (AUC 0.999), MIA (AUC 0.999), and diabetic nephropathy (AUC 1.000). ROC curves were plotted to quantify the ability of cutoff value of urinary NAG to discriminate between healthy controls and T2DM patients with varying duration of diabetes with normoalbuminuria and normal kidney functioning as indicated by serum creatinine and eGFR. Specificity, sensitivity, and calculated cutoff value of NAG distinguished healthy controls from T2DM patients of 10–15 and 15–20 years of diabetes duration, MIA and DN with sensitivity 100 % and specificity 96.1 %, and AUC 1.000 (p < 0.0001) (Fig. 2a and Table 2). The cutoff value of NAG was unable to distinguish the healthy controls from T2DM patients with 0–5 years of diabetes duration (AUC 0.738) and 10–15 years of diabetes duration (AUC 0.678) (Fig. 2a). The cutoff value for NAG also discriminated T2DM patients with 0–5 and 5–10 years of diabetes with T2DM duration of 10–15 and 15–20 years, MIA, and DN (Figs. 2b, c and Table 2). Urinary NAG cutoff value also discriminated 10–15 years of T2DM duration with 15–20-year duration of T2DM, MIA, and DN (AUC 0.756, 0.855, and 0.946) (with sensitivity 43.3, 87.8, and 92 %; specificity 86.6, 86.6, and 86.6 %) (p < 0.0001) (Fig. 2d). Even the patients with 15–20 years of T2DM duration showed significant urinary NAG activity and was able to discriminate MIA group and DN group (AUC 0.806 and 0.916; with sensitivity 87.8 and 92 %; specificity 56.6 and 56.6 %) (Fig. 2e) (p < 0.0001). The urinary NAG excretion in NDN patients discriminated T2DM patients with 15–20 years of diabetes duration, MIA group, and DN group (AUC 0.951, 0.972, and 0.993; sensitivity 74, 85.3, and 100 %; specificity 96.6, 96, and 76 %) (Fig. 2e and Table 2) (Fig. 2f and Table 2) (p < 0.0001).
Multiple regression analysis (curves not shown) has indicated that urinary NAG activity was independent of factors like age, fasting blood glucose, serum creatinine, and eGFR (Table 3). Urinary NAG activity was dependent on duration of diabetes (p < 0.0001) and with varying degree of microalbuminuria (p < 0.0001) (Table 3).
Discussion
Hyperglycemia induces functional and structural changes like hyperfiltration-hyperperfusion, thickening of basement membrane, and mesangial layer expansion of nephrons. These alterations appear much before the onset of measurable clinical changes [1]. Amadori products and advanced glycation end products (AGE) play a pivotal role in developing renal deterioration [22]. Such functional and structural changes are reversible up to the certain extent and accelerated by uncontrolled hyperglycemia [3]. Hyperglycemia-induced hyperfiltration was indicated by elevated eGFR in our study groups of up to 10 years of T2DM without any complication, which was observed as the earliest clinical marker and significant risk factor in developing diabetic nephropathy [23]. The clinical manifestations like microalbuminuria, increased serum creatinine, decreased creatinine clearance, and urinary IgG were reported as markers of well-established renal damage as a consequence of hyperglycemia-induced glomerular damage [2, 24]. Recently, high urinary IgG creatinine ratio has shown a significant association with decreased eGFR possibly due to glomerulopathy and a threat for rapid progression of diabetic nephropathy [25]. The urinary IgG creatinine ratio was associated with faster deterioration of renal function in T2DM patients than urinary microalbumin creatinine ratio [26].
The tubular damage has a central role in the pathogenesis of diabetic nephropathy [4]. The excretion of renal cell origin enzymes in urine had shown to evaluate sensitively the tubular cell integrity, as they appeared much before the increased excretion of microalbumin and decreased creatinine clearance [2]. Elevated levels of enzymuria and low molecular weight proteinuria has reported due to deficient reabsorption of proteins by tubular epithelial cells or their increased urinary excretion by damaged or regenerating tubular epithelial cells [2, 27]. Urinary enzymes are high molecular weight and cannot cross the glomerular barrier, hence their excretion in urine indicates renal tubular damage [2]. Hence, in the study, we investigated the efficacy of urinary NAG as early tubular damage marker and established a cutoff value for validating its efficiency in differentiating diabetic patients who can develop diabetic nephropathy.
The present study has shown the gradual increase in NAG excretion with the duration of T2DM and MIA, indicating that the hyperglycemia-induced tubular damage eventually leads to glomerulopathy [12, 13, 28]. The altered urine/serum NAG ratio was the same as the changes observed in urinary NAG activity in different groups of the present study. This change was due to unaltered serum NAG activity in any of the studied groups, supporting the fact that the presence of urinary NAG activity was primarily due to tubulopathy. It can be depicted from Fig. 1 that NAG excretion was parallel with the degree of urinary albumin, which was also reported elsewhere [29]. Abbate et al. has reported that the tubulointerstitium damage was due to tubulotoxic effects of albumin and other proteins [30]. Furthermore, our study has indicated the NAG excretion in patients with normoalbuminuria and disagrees with the assumption made by Abbate et al. [30]. The urinary excretion of NAG could be due to early hyperglycemia-induced structural changes, which were due to T2DM rather than tubulotoxic effects of albumin. The tubular injury was also evident in patients with intact glomerular function. These results supported the fact that in pathogenesis of type 2 diabetic nephropathy, tubular injury precedes to glomerular damage [2, 31], whereas in the present study, the glomerular damage was predicted by elevation in microalbuminuria and serum creatinine and decreased creatinine clearance.
Many histological studies illustrated a “Classical” lesion of diabetic nephropathy predominantly found with an initial involvement of tubulointerstitial changes. The later involvements of glomerular basement membrane damage were the landmark of hyperglycemia-induced damage in renal tissue [32], whereas, this situation was not observed in NDN patients. Our study group of non-diabetic nephropathy includes patients with IgA nephropathy, focal segmental glomerulosclerosis, minimal change disease, and nephrotic syndrome to rule out the early involvement of proximal tubular damage in such NDNs with primary tubulopathy. In patients characterized with primary glomerular lesions followed by tubulointerstitial changes such as IgA nephropathy, the urinary NAG levels often lack to depict the morphologic changes due to tubulointerstitial injury. Regardless to this, Bazzi et al. showed that in patients with focal segmental glomerulosclerosis, minimal change disease and nephrotic syndrome urinary NAG can prove as a non-invasive repeatable early biomarker [14]. Our results show no alteration of urinary NAG level in patients with primary glomerulonephritis that may be due to our smaller study population. Urinary neutrophil gelatinase-associated lipocalin (NGAL) level holds promise as a non-invasive more sensitive and specific marker than NAG for early detection of submicroscopic tubulointerstitial injury of IgA nephropathy [33]. Unaltered NAG activity in NDN patients might be indicative of normal or undetected damage of proximal tubular injury. It may also indicate glomerular damage accompanied by proximal tubule damage in the later stage. In NDN, there was either activation of antibodies or various immune complexes, which binds directly to the mesangial layer of the glomerular region, activates cytokines, and initiates fibrotic growth that leads to mesangial layer degradation, instigate hematuria along with proteinuria [34–36].
In IgA nephropathy patients where tubulointerstitial changes follow primary glomerular lesions, the urinary NAG levels often lack the ability to detect morphologic changes due to tubulointerstitial injury [14]. The urinary neutrophil gelatinase-associated lipocalin (NGAL) level holds better promise as a non-invasive, sensitive, and specific marker than NAG for early detection of submicroscopic tubulointerstitial injury in IgA nephropathy [33]. Thus, the urinary NAG activity was not elevated in patients with non-diabetic nephropathy, indicated the normal or undetected damage of proximal tubular cell injury in patients of the presently studied group. In non-diabetic nephropathies, activation of antibodies or various immune complexes binding directly to the mesangial layer of the glomerular region. Furthermore, it activates cytokines and initiates fibrotic growth and mesangial layer degradation, instigate hematuria along with proteinuria [34–36].
In our cross-sectional study, we found a pathological link between urinary NAG and T2DM patients with varying duration of diabetes. Further, longitudinal study with number of patients can help us better understand the role of NAG in the pathology of long-standing diabetes leading to diabetic nephropathy. Such study would help in therapeutic interventions for such patients. Our results suggest that urinary NAG plays an important role in the early diagnosis and monitoring of renal disease in T2DM patients even before the onset of moderately increased albuminuria or decrease in eGFR. Urinary NAG might be useful as a non-invasive surrogate test of incipient diabetic nephropathy and in monitoring disease progression. Analyzing urinary NAG can enlighten the dark side of the clinical silence where the significant structural alterations take place.
In conclusion, our data suggested that increased excretion of urinary NAG be a sign of the site-specific early tubular manifestations of diabetic nephropathy in T2DM patients due to long-standing hyperglycemia. The urinary NAG with the cutoff value of 3 U/L may be taken into consideration as a promising prognostic marker of diabetic nephropathy in T2DM patients.
References
Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endorinol Metab. 2008;4:444–52.
Hong CY, Chia KS. Markers of diabetic nephropathy. J Diabetes Complicat. 1998;12:43–60.
Wolf G, Ziyadeh F. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999;56:393–405.
Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol. 2002;17:247–52.
Turecky L, Uhlikova E. Diagnostic significance of urinary enzymes in nephrology. Bratisl Lek Listy. 2003;104(1):27–31.
Fukui M, Nakamura T, Ebihara I, Shirato I, Tomino Y, Koide H. ECM gene expression and its modulation by insulin in diabetic rats. Diabetes. 1992;41:1520–7.
Steffes MW, Osterby R, Chavers B, Mauer M. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–81.
Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk is albumin excretion rate sufficient? Diabetes. 2000;49:1399–408.
Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–53.
Belfiore F, Napoli E, Vecchio LL. Serum N-acetyl-beta-glucosaminidase activity in diabetic patients. Diabetes. 1972;21(12):1168–72.
Price RG, Dance N. The cellular distribution of some rat-kidney glycosidases. Biochem J. 1967;105:877–83.
Karakani MA, Haghighi SA, Khansari MG, Hosseini R. Determination of urinary enzymes as a marker of early renal damage in diabetic patients. J Clin Lab Anal. 2007;21:413–7.
Piwowar A, Kordecka MK, Fus I, Warwas M. Urinary activities of cathepsin B, N-acetyl-beta-d-glucosaminidase, and albuminuria in patients with type 2 diabetes mellitus. Med Sci Monit. 2006;12(5):CR210–4.
Bazzi C, Petrini C, Rizza V, Arrigo G, Napodano P, et al. Urinary N-acetyl-b-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant. 2002;17:1890–6.
Han W, Waikar S, Johnson A, Betensky R, Dent C, Devarajan P, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–9.
Fu W, Xiong S, Fang Y, Wen S, Chen M, Deng R, et al. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine. 2012;41:82–8.
Ouchi M, Suzuki T, Hashimoto M, Motoyama M, Ohara M, Suzuki K, et al. Urinary N-acetyl-β-d-glucosaminidase levels are positively correlated with 2-h plasma glucose levels during oral glucose tolerance testing in prediabetes. J Clin Lab Anal. 2012;26:473–80.
Kroll MH, Chesler R, Hagengruber C, Blank DW, Kestner J, Rawe M. Automated determination of urinary creatinine without sample dilution: theory and practice. Clin Chem. 1986;32(3):446–52.
Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Parker KM, England JD, Da Costa J, Hess RL, Gloldstein DE. Improved colorimetric assay for glycated hemoglobin. Clin Chem. 1981;27(5):669–72.
Horak E, Hopfer SM, Sunderman Jr WF. Spectrophotometric assay for urinary N-acetyl-b-d-glucosaminidase. Clin Chem. 1981;27(7):1180–5.
Lehmann R, Schleicher ED. Molecular mechanism of diabetic nephropathy. Clin Chim Acta. 2000;297:135–44.
Fua WJ, Liang LB, Wang SB, Chen ML, Deng TR, Qin YC, et al. Changes of the tubular markers in type 2 diabetes mellitus with glomerular hyperfiltration. Diabetes Res Clin Pract. 2012;95:105–9.
Kalia K, Sharma S, Mistry K. Non-enzymatic glycosylation of immunoglobulins in diabetic nephropathy. Clin Chim Acta. 2004;347:169–76.
Mohan S, Kalia K, Mannari J. Diabetic nephropathy and associated risk factors for renal deterioration. Int J Diabetes Dev Ctries. 2012;32(1):52–9.
Mohan S, Kalia K, Mannari J. Urinary IgG is a pure strong indicator of diabetic nephropathy than microalbuminuria in type 2 diabetic patients. Int J Diabetes Dev Ctries. 2013;33(1):46–54.
Barratt J, Topham P. Urine proteomics: the present and future of measuring urinary protein components in disease. CMAJ. 2007;177(4):361–8.
Moresco RN, Sangoi MB, Carvalho J, Tatsch E, Bochi G. Diabetic nephropathy: traditional to proteomic markers. Clin Chim Acta. 2013;421:17–30.
Nauta FL, Boertien WE, Bakker SJ, Goor HV, Oeveren WV, Jong PE, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care. 2011;34:975–81.
Abate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–84.
Kalansooriya A, Jennings P, Haddad F, Holbrook I, Whiting PH. Urinary enzyme measurements as early indicators of renal insult in type 2 diabetes. Br J Biomed Sci. 2007;64(4):153–6.
Vestra MD, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031–5.
Ding H, He Y, Lia K, Yang J, Li X, Lu R, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Cin Immu. 2007;123:227–34.
Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–97.
Floege J, Feehally J. IgA nephropathy: recent developments. J Am Soc Nephrol. 2000;11:2395–403.
Soler MJ, Mir M, Rodriguez E, OrWla A, Munne A, Vazquez S, et al. Recurrence of IgA nephropathy and Henoch-Schoenlein purpura after kidney transplantation: risk factors and graft survival. Transplant Proc. 2005;37:3705–9.
Acknowledgments
The authors are grateful to the Muljibhai Patel Urological Hospital, Nadiad, Gujarat, India, for their support to provide blood and urine samples of patients used for the present study. The authors thank the University Grant Commission, New Delhi, for providing research fellowship to pursue this work.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, D.N., Kalia, K. Efficacy of urinary N-acetyl-β-D-glucosaminidase to evaluate early renal tubular damage as a consequence of type 2 diabetes mellitus: a cross-sectional study. Int J Diabetes Dev Ctries 35 (Suppl 3), 449–457 (2015). https://doi.org/10.1007/s13410-015-0404-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-015-0404-2