Abstract
Circulating Retinol-Binding Protein 4 (RBP4) has recently been identified as a marker of insulin resistance. We tested this hypothesis in patients with coronary artery disease (CAD), patients with type 2 diabetes mellitus (T2DM), and in non-diabetic control subjects. We studied plasma RBP4 levels and RBP4-to-transthyrethin (TTR) ratio, estimating the excess circulating RBP4 in proportion to TTR, in 45 individuals divided into three groups (15 CAD, 15 T2DM, and 15 controls). Plasma RBP4 levels were significantly lower in patients with T2DM than in non-diabetic control subjects (P = 0.05). The RBP4/TTR ratio was not statistically different between the groups. There was no difference in plasma RBP4 levels and RBP4/TTR ratio between non-diabetic CAD patients and control subjects or those with and without metabolic syndrome. No significant associations were found between RBP4 and RBP4/TTR ratio, as dependent parameters, with markers of the metabolic syndrome and lipid metabolism. RBP4 does not seem to be a valuable marker for identification of the metabolic syndrome or insulin resistance in patients with T2DM or CAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose tissues function as an endocrine organ, secreting several bioactive molecules collectively known as adipokines [1]. Retinol-binding protein 4 (RBP4) is a newly discovered adipokine with potential implications in the development of obesity-associated diseases, including insulin resistance, type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD) [2]. RBP4 is mainly synthesized in the liver and secreted bound to retinol, along with transthyretin (TTR), a homotetrameric thyroid hormone transport protein, forming a 1:1 molar complex in the human plasma [3].
Serum RBP4 has been recently reported to be associated with insulin resistance in subjects with obesity, Impaired Glucose Tolerance (IGT), or T2DM and lean non-diabetic individuals with a strong family history of diabetes [4]. However, whilst the role of RBP4 in human glucose metabolism is considered controversial [5-8], its close association with proatherogenic lipid profile makes it a potential novel marker for identifying those at increased risk of cardiovascular events and premature death [8, 9]. The purpose of the present study was to investigate circulating RBP4 concentrations in patients with CAD, patients with T2DM, and non-diabetic control subjects, correlating RBP4 levels with components of the metabolic syndrome and circulating lipoproteins.
Patients and methods
Participants
A total of 45 subjects were included in this study. All participants underwent elective coronary angiography for the evaluation of stable CAD based on clinical indication. Three cohorts were studied: (1) 15 patients with angiographically confirmed CAD without T2DM; (2) 15 patients with T2DM without CAD by coronary angiography; and (3) 15 non-diabetic control subjects without CAD by coronary angiography. CAD was diagnosed angiographically and defined as ≥50 % diameter stenosis in at least one of the coronary arteries or their branches. T2DM was defined according to the criteria of the American Diabetes Association [10]. Previously diagnosed DM, receiving oral hypoglycemic drugs and/or insulin, and fasting plasma glucose levels of ≥126 mg/dL were considered exclusion criteria for the CAD and control groups. Patients with active infections, autoimmune diseases, malignancies, and recent myocardial infarction were excluded. Written informed consent was obtained from all participants. This study was approved by the Ethics Committee of Tehran University of Medical Sciences and Health Services.
Data collection included demographics, cardiovascular risk factors including diabetes, hypertension, hypercholesterolemia, family history of CAD, smoking, medication in use, measures of weight, height, waist circumference at its narrowest point, and Body Mass Index (BMI) calculated as weight in kilograms divided by height square in meters. Metabolic syndrome was diagnosed according to National Cholesterol Education Program Adult Treatment Panel III criteria [11]. Participants were also divided in two groups according to BMI (BMI > 30 or ≤30 kg/m2).
Biochemical assays
Fasting blood samples were collected for the measurement of plasma glucose, total cholesterol, Low-Density Lipoprotein (LDL) cholesterol, High-Density Lipoprotein (HDL) cholesterol, triglyceride, and C-Reactive Protein (CRP). All samples were frozen at −80°C until assayed. Laboratory measurements were performed using automated enzymatic commercial kits (Pars Azmoon, Tehran, Iran).
RBP4 and TTR assays
Plasma RBP4 and TTR levels were determined in duplicate by sandwich Enzyme-Linked Immunosorbent Assay (ELISA).
Statistical analyses
The statistical analyses were performed using SPSS software version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). RBP4 and TTR form a 1:1 molar complex in plasma [3]. In order to estimate the excess RBP4 in proportion to TTR, the molar concentrations of RBP4 were divided to the molar concentrations of TTR. Continuous variables were expressed as mean±standard deviation (SD). Comparison between two cohorts of patients was performed by unpaired Student’s t-test or Mann–Whitney U test. Three-way analysis of variance (ANOVA) was used to compare RBP4, TTR, and RBP4/TTR ratio according to age and sex among the groups. Categorical variables were expressed as percentages and compared by Pearson’s Chi-square test and Fisher’s exact test. In univariate analysis models, the associations of plasma RBP4 levels and RBP4/TTR ratio, as dependent parameters, were investigated with markers of the metabolic syndrome and lipid metabolism, as independent variables. Age and sex were also included in all models. Results were reported as Regression Coefficients (RC) with 95 % Confidence interval (CI). A p value of less than 0.05 was considered to be statistically significant.
Results
The baseline characteristics of participants are summarized in Table 1. Compared with control subjects, non-diabetic CAD patients were older and had a lower mean HDL cholesterol level. The prevalence of hypertension was higher in non-diabetic CAD patients than in non-diabetic controls. Abdominal obesity, hypercholesterolemia, and hypertension were more prevalent in patients with T2DM than in non-diabetic controls. The metabolic syndrome was diagnosed in approximately half of all participants (N = 23). Subjects diagnosed with metabolic syndrome had a higher proportion of diabetes, hypertension, hypertriglyceridemia, abdominal obesity, and angiographically confirmed CAD. CRP was significantly higher in subjects with than in those without metabolic syndrome (4.34 ± 4.66 vs. 1.64 ± 1.58 mg/dL, p = 0.014).
Plasma RBP4 and TTR
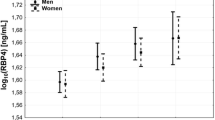
Univariate linear regression analysis showed a significant correlation between plasma RBP4 levels as the dependent parameter and plasma TTR levels (P < 0.0001, r2: 0.291, RC: 0.539, CI: 0.292–0.718) (Fig. 1). Similar results were obtained after age and sex adjustment.
Plasma RBP4
Plasma RBP4 levels were lower in individuals with T2DM compared with non-diabetic control subjects (1.09 ± 0.23 vs.1.24 ± 0.15 μmol/L, p = 0.05). There was no difference between non-diabetic CAD patients and control subjects (P = 0.418) or those with and without metabolic syndrome (P = 0.054). There was no difference in plasma RBP4 according to obesity (P = 0.261) or sex (P = 0.969).
Plasma TTR
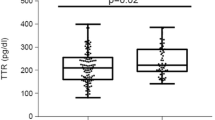
Plasma TTR levels were not significantly different between patients with T2DM and non-diabetic control subjects (P = 0.90) or those with and without metabolic syndrome (P = 0.537). Plasma TTR levels were significantly higher in non-diabetic CAD patients compared with control subjects (0.56 ± 0.15 vs. 0.45 ± 0.11 μmol/L, P = 0.027). There was no difference in plasma TTR according to obesity (P = 0.063) or sex (P = 0.709).
RBP4/TTR ratio
RBP4/TTR ratio was not statistically different between T2DM patients and controls (P = 0.182), non-diabetic CAD patients and controls (P = 0.085), or those with and without metabolic syndrome (P = 0.433)
Associations of RBP4 and RBP4/TTR ratio with markers of the metabolic syndrome and lipid metabolism
In patients with T2DM, univariate regression analyses revealed no significant associations between RBP4 and RBP4/TTR ratio as dependent parameters with markers of the metabolic syndrome and lipid metabolism, including waist circumference, BMI, history of hypertension, triglyceride, total cholesterol, LDL, and HDL (Table 2). There were also no associations found in non-diabetic CAD patients (Table 3).
Discussion
RBP4 was recently reported to be expressed by adipose tissue and associated with insulin resistance and components of the metabolic syndrome in patients with T2DM, IGT, or obesity [4, 5]. No associations between RBP4 and markers of insulin sensitivity have also been reported in patients with T2DM [8] or non-diabetic, insulin-resistant individuals [12, 13]. These findings are in accordance with recent published data showing that circulating RBP4 concentrations were similar in lean, overweight, and obese postmenopausal women and that there was no relationship between the HOMA index of insulin resistance and circulating RBP4 concentrations [14]. Moreover lower RBP4 levels have also been reported in patients with T2DM [15] or IGT [16]. Erikstrup et al. showed that plasma RBP4 and retinol levels were lower, whereas RBP4/retinol ratio was elevated in patients with T2DM compared to controls [15]. It has been postulated that, since the RBP4/retinol ratio estimates the excess of circulating RBP4 in proportion to retinol, free rather than total RBP4 concentration is related to insulin resistance [15]. RBP4 and TTR are also thought to exist as a 1:1 molar complex [3]. Akbay et al. reported comparable levels of RBP4 in type 2 diabetic patients and controls, whereas, the molar ratio of RBP4 to TTR was found to be higher in diabetic patients than that of the control group [17]. We found lower plasma RBP4 levels in patients with T2DM compared to non-diabetic control subjects; however, RBP4/TTR ratio was not significantly different between the groups. Plasma retinol level was not determined in the present study, nevertheless, there might be differences in the ratio between the groups.
It has been demonstrated recently that plasma RBP4 increases with decreasing renal function in type 2 diabetes [17-19]. Since we did not assess renal function in our cohort of patients, it should be considered as a potential confounding factor which might account for the above discrepancies.
These discrepancies among human studies have been attributed to differences in study populations (age, sex composition, levels of fasting insulin, use of glucose or lipid lowering agents, adjusting for cardiovascular risk factors, components of the metabolic syndrome and kidney function), divergent measures of insulin sensitivity (clamp studies vs. HOMA-IR), as well as methodological differences in measuring serum RBP4 levels (ELISA vs. Western blotting) [4, 8, 14, 15]. Therefore, the lack of associations of RBP4 with glucose metabolism and insulin sensitivity needs to be further investigated in a large, well-defined cohort of patients with more sophisticated measures of insulin sensitivity.
Increased RBP4 levels were significantly associated with prior cerebrovascular disease, but not with prior myocardial infarction, in large community-based samples of elderly individuals [9]. It has been postulated that circulating RBP4, as a marker of metabolic complications, might serve as a useful marker in identifying individuals at increased risk for CVD [9]. Additionally, the risk of incident CAD was associated with increasing quartiles of RBP4 concentrations in a nested case–control study; however, RBP4 mostly correlated with triglyceride levels and failed to provide added value beyond traditional cardiovascular risk factors [20]. The presence of clinical atherosclerosis, including coronary, cerebrovascular or peripheral vascular disease, but not sub-clinical atherosclerosis, was associated with higher plasma RBP4 concentrations [18]. On the contrary, serum RBP4 levels have been reported to be significantly lower in patients with CAD than in non-diabetic control subjects [8]. Mean intima media thickness has been reported to correlate with RBP4, retinol, RBP/TTR ratio, and retinol/RBP4 ratio [21]. However, we found no difference in plasma RBP4 levels and RBP4/TTR ratio between non-diabetic CAD patients and controls.
Based on current data, the contributing roles of RBP4 in insulin resistance and metabolic syndrome has been debated, whilst its associations with proatherogenic lipid profile, including increased triglyceride, LDL cholesterol, and very low-density lipoprotein cholesterol and decreased HDL cholesterol levels, as well as key enzymes of lipoprotein metabolism may imply a plausible contribution to the pathogenesis of CVD [2, 4, 5, 8, 9, 15, 22, 23]. However, we failed to demonstrate such an association which may be partly due to recruitment of individuals who were on lipid lowering medications and lack of adjustment for potential confounders. This statement needs to be clarified in experimental and prospective studies.
References
Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39.
von Eynatten M, Humpert PM. Retinol-binding protein-4 in experimental and clinical metabolic disease. Expert Rev Mol Diagn. 2008;8:289–99.
Raghu P, Sivakumar B. Interactions amongst plasma retinol-binding protein, transthyretin and their ligands: implications in vitamin A homeostasis and transthyretin amyloidosis. Biochim Biophys Acta. 2004;1703:1–9.
Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63.
Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–61.
Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, Franco OH, Wang J, Li H, Liu Y, Lin X. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92:4827–34.
Broch M, Vendrell J, Ricart W, Richart C, Fernandez-Real JM. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. 2007;30:1802–6.
von Eynatten M, Lepper PM, Liu D, Lang K, Baumann M, Nawroth PP, Bierhaus A, Dugi KA, Heemann U, Allolio B, Humpert PM. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930–7.
Ingelsson E, Sundstrom J, Melhus H, Michaelsson K, Berne C, Vasan RS, Riserus U, Blomhoff R, Lind L, Arnlov J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009;206:239–44.
Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer 3rd HJ, Rashidi AA, McGehee Jr RE, Fried SK, Kern PA. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–7.
Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587–94.
Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–10.
Erikstrup C, Mortensen OH, Nielsen AR, Fischer CP, Plomgaard P, Petersen AM, Krogh-Madsen R, Lindegaard B, Erhardt JG, Ullum H, Benn CS, Pedersen BK. RBP-to-retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes Metab. 2009;11:204–12.
Sasaki H, Iwasaki T, Kato S, Tada N. High retinol/retinol-binding protein ratio in noninsulin-dependent diabetes mellitus. Am J Med Sci. 1995;310:177–82.
Akbay E, Muslu N, Nayir E, Ozhan O, Kiykim A. Serum retinol binding protein 4 level is related with renal functions in Type 2 diabetes. J Endocrinol Invest. 2010;33:725–9.
Cabre A, Lazaro I, Girona J, Manzanares J, Marimon F, Plana N, Heras M, Masana L. Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med. 2007;262:496–503.
Henze A, Frey SK, Raila J, Tepel M, Scholze A, Pfeiffer AF, Weickert MO, Spranger J, Schweigert FJ. Evidence that kidney function but not type 2 diabetes determines retinol-binding protein 4 serum levels. Diabetes. 2008;57:3323–6.
Mallat Z, Simon T, Benessiano J, Clement K, Taleb S, Wareham NJ, Luben R, Khaw KT, Tedgui A, Boekholdt SM. Retinol-binding protein 4 and prediction of incident coronary events in healthy men and women. J Clin Endocrinol Metab. 2009;94:255–60.
Bobbert T, Raila J, Schwarz F, Mai K, Henze A, Pfeiffer AF, Schweigert FJ, Spranger J. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis. 2010;213:549–51.
Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schon MR, Stumvoll M, Bluher M, Kahn BB. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87.
Lee JW, Im JA, Lee HR, Shim JY, Youn BS, Lee DC. Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity (Silver Spring). 2007;15:2225–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmoudi, M.J., Mahmoudi, M., Siassi, F. et al. Circulating retinol-binding protein 4 concentrations in patients with coronary artery disease and patients with type 2 diabetes mellitus. Int J Diabetes Dev Ctries 32, 105–110 (2012). https://doi.org/10.1007/s13410-012-0077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-012-0077-z