Abstract
Background
Patients with esophageal cancer are confronted with high mortality rates. Whether it is esophageal squamous cell carcinoma (ESCC) or esophageal adenocarcinoma (EAC), patients usually present at advanced stages, with treatment options traditionally involving chemotherapy in metastatic settings. With the comprehensive genomic characterization of esophageal cancers, targeted therapies are gaining interest and agents such as ramucirumab, trastuzumab and pembrolizumab are already being used for the treatment of EAC.
Conclusions
Pembrolizumab has recently been FDA-approved for PD-L1 positive, locally advanced or metastatic ESCC. Despite comprehensive molecular characterization, however, available targed therapies for ESCC are still lagging behind. Herein, we discuss current trends towards more targeted therapies in esophageal cancers, taking into consideration unique features of ESCCs and EACs. Patients progressing on standard therapies should be subjected to genomic profiling and considered for clinical trials aimed at testing targeted therapies. Future targeted therapies may include CDK4/6 inhibitors, PARP inhibitors and inhibitors targeting the NRF2 and Wnt signaling pathways. Ultimately, optimized biomarker assays and next generation sequencing platforms may allow for the identification of subcategories of ESCC and EAC patients that will benefit from selective targeted therapies and/or combinations thereof.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The esophageal cancer incidence has been estimated to be ~500,000 cases worldwide in 2018. Most patients present in advanced stages and, therefore, the overall survival rate remains dismal: 20% at five years [1,2,3]. It is a heterogeneous disease that can broadly be categorized into esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). To date, treatment options have been largely similar for the different types of esophageal cancer [1]. More recent cellular and molecular data suggest, however, that these types of cancer represent different entities [2]. ESCCs closely resemble head and neck cancers, whereas EACs mimic gastric cancers [2,3,4]. Herein, we review the latest findings on the molecular pathways driving esophageal cancer and highlight recent advances in targeted therapies. We also present a summary of clinical outcomes with respect to currently available targeted therapies. Finally, we focus on ongoing research in the field and discuss prospective options for translation into clinical use.
2 The esophagus
2.1 Anatomy and histology
Anatomically, the esophagus comprises three distinct sections of clinical relevance: cervical, thoracic and abdominal [1, 5, 6]. The cervical portion extends from the pharyngo-esophageal junction to the suprasternal notch. Esophageal cancers that arise here are mostly of squamous histology. The thoracic segment extends from the suprasternal notch to the diaphragmatic hiatus. The abdominal portion extends from the diaphragmatic hiatus to the esophago-gastric junction. Histologically, adenocarcinomas typically arise in this latter segment. For gastroesophageal adenocarcinoma, the Siewert-Stein classification uses anatomical location to help guide surgical intervention and treatment [7]. Siewert Type 1 cancers constitute adenocarcinoma of the distal esophagus and are treated as esophageal adenocarcinoma [7]. Siewert Type 2 cancers constitute adenocarcinoma of the cardia and Siewert Type III cancers constitute subcardial adenocarcinoma, both of which are treated in a similar fashion to gastric cancers [7].
Histologically, the esophageal wall consists of innermost mucosa, submucosa, muscularis propria and outermost adventitia. The mucosa is further subdivided into stratified squamous epithelium, lamina propria and muscularis mucosa. The muscularis propria consists of purely skeletal muscle (upper third of the esophagus), purely smooth muscle (lower third) and a transition mix of skeletal and smooth muscle in between. The adventitia includes loose connective tissue and connects the esophagus with its surrounding tissue [1, 5, 6].
2.2 Esophageal cancers
The majority of esophageal cancers are ESCC and EAC. Additionally, there exist small cell/neuroendocrine and rare basaloid esophageal cancers. ESCC occurs in the upper and mid-esophagus and originates from squamous epithelial lining of the esophagus [1, 5]. It is associated with smoking, alcohol consumption, diet (hot beverages, red meat and nutritional deficiencies), caustic injury and poor oral hygiene. ESCC has a relatively higher incidence among Eastern Asian and Eastern and Southern African populations [1, 5]. The mechanism of carcinogenesis involves direct mechanical injury secondary to exposure of the esophageal mucosa to carcinogens [1, 5]. This leads to epithelial changes from normal epithelium to basal cell hyperplasia, low-grade and high grade intra-epithelial neoplasia and, finally, invasive carcinoma [1, 5]. EAC occurs in the lower third of the esophagus and is thought to initiate around glandular cells near the stomach [1, 8]. It is associated with male gender, gastroesophageal reflux disease (GERD), obesity, reduced H. pylori infection, and has an increased incidence among European, North American and Australian populations [6, 8]. In this case, the mechanism involves a chronic process of GERD causing metaplastic changes in the esophageal epithelium from normal squamous cell epithelium to columnar epithelium, known as Barrett’s esophagus, to low grade dysplasia, high grade dysplasia and ultimately invasive adenocarcinoma [6]. According to a population-based national registry, the Surveillance Epidemiology and End Results (SEER) database for outcomes on ESCC versus EAC, there are no differences in overall survival between ESCC and EAC [9]. Chinese ESCC patients, however, tend to have a better overall survival compared to Caucasian ESCC patients [9].
Thus far, treatment options have been largely similar for both ESCC and EAC, and generally depend on the stage of esophageal cancer (Tables 1 and 2) [10,11,12,13]. Endoscopic resection for mucosal lesions versus esophagectomy for submucosal lesions is the main approach for early stage esophageal cancer [10,11,12]. For locally advanced cancers, based on the patient’s performance status, neoadjuvant chemo-radiation followed by esophagectomy versus definitive chemo-radiation is offered in the case of ESCC [14,15,16,17]. For locally advanced EAC, neoadjuvant chemotherapy or chemo-radiation is offered with surgery, or surgery alone in low risk patients with well-differentiated tumors that are less than 2 cm in size. In the case of unresectable tumors, definitive chemo-radiation has been found to be associated with a better overall survival compared to radiation therapy alone [10,11,12, 18]. Management of metastatic disease includes palliative systemic therapy, radiation therapy or supportive care [10,11,12]. Endoscopic therapy of metastatic disease is offered for palliative purposes and includes balloon dilation to relieve dysphagia, resection of advanced lesions and placement of endoscopic stents [10,11,12]. Esophagectomy is associated with high morbidity and mortality rates [19]. Chemotherapy options include platinum-based regimens with 5-fluorouracil or taxanes and sometimes anthracyclines, all of which exhibit considerable cytotoxic effects. To mitigate these limited therapeutic options, there is an increasing interest in the molecular characterization of esophageal cancers allowing the design of personalized targeted therapies that can improve overall patient survival and quality of life.
3 Molecular characterization of esophageal cancers
A number of studies has used molecular analyses to characterize esophageal cancers, to identify driver mutations and to uncover therapeutic targets (Tables 3 and 4). Here we review several seminal studies that highlight the molecular characteristics of ESCCs and EACs, respectively.
3.1 ESCC
A number of studies from China and Japan has provided comprehensive analyses of driver mutations in ESCCs as listed in Table 3 [3, 4, 20,21,22,23,24,25,26,27]. One of the earlier studies considered 158 Chinese patients with ESCC and identified 8 significantly mutated genes and highlighted alterations in key pathways including cell cycle, TP53, NOTCH, Wnt and RTK/PI3K pathways [25]. ZNF750 is a known tumour suppressor gene, with mutations leading to impaired differentiation causing ESCC [28]. Genetic alterations in CDKN2A, PIK3CA, TGFBR2 and, less commonly in ERBB, EGFR and BRCA have been noted and may provide opportunities to improve clinical outcomes through targeted therapies.
The Cancer Genome Atlas (TCGA) integrated genomic analyses of 164 esophageal cancers from eastern and western hemispheres, including ESCCs and EACs [2]. Similar to previous studies, common mutations were found in the TP53 gene, which occur in the majority of dysplastic lesions and malignant ESCC lesions [2]. In addition, mutations were found in NOTCH1, NFE2L2, ZNF750, PIK3CA and CDKN2A. Notable amplifications of SOX2, TERT, FGFR1, MDM2, NKX2–1 and CCND1, and deletions of RB1, VGLL4 and the negative regulator of the Hippo pathway and autophagy factor ATG7 were highlighted. Genomic alterations affecting the histone-modifying factors KDM6A, KMT2D and KMT2C were also found to be more common in ESCCs. The TCGA also integrated clustering of somatic copy number alterations, DNA methylation, mRNA and microRNA expression levels in ESCCs to further classify these cancers into three subtypes [2]. Type I ESCCs tend to be more common among Vietnamese, exhibit alterations in the NRF2 pathway, are associated with a poor prognosis and are resistant to chemo-radiotherapy [2]. This subtype has a higher frequency of SOX2 and/or TP63 amplifications and resembles lung SCC and head and neck SCC [2]. Type II ESCCs tend to be more common among Eastern Europeans and South Americans and are associated with higher mutations rates in the NOTCH1 and ZNF750 genes, more frequent inactivating alterations of KDM6A and KDM2D, CDK6 amplifications and inactivation of PTEN or PIK3R1 [2]. This type of ESCC also exhibits a greater leukocyte infiltration. Finally, type III ESCCs, which represent the lowest number of cases, occur more commonly among North Americans and exhibit alterations predicted to activate the PI3K pathway [2].
3.2 EAC
Based on TCGA data, gastric cancers are classified into four subtypes including EBV-positive cancers, genomic stable cancers, microsatellite unstable cancers and cancers with chromosomal instability (CIN) [2]. EACs tend to more closely resemble gastric cancers with CIN, characterized by structural DNA variations and copy number changes. These cancers exhibit oncogene amplifications, including ERBB2, KRAS, EGFR, IGF1R and VEGFA. With respect to cell cycle dysregulation, EACs exhibit mutations in CDKN2A and amplifications of CCNE1. Regarding epigenetic modifications, alterations affecting the SWI/SNF-encoding genes ARID1A, SMARCA4 and PBRM1 have been found to be more common in EAC [2].
Using a cohort of 551 genomically characterized EACs with matched RNA sequencing data, a recent study identified 77 EAC driver genes, 21 noncoding driver elements, and highlighted potential therapeutic targets [29]. Of note, GATA4 amplification and SMAD4 mutation or homozygous deletion were found to be associated with significantly poorer prognoses. Novel EAC drivers included B2M, which encodes a core component of the MHC class I complex and is a marker of acquired resistance to immunotherapy and ABCB1, which encodes a channel pump protein that is associated with drug resistance [29]. Chromatin-modifying genes belonging to the SWI/SNF complex were also found to be selectively mutated. In terms of sub-classification of EACs, mutation analysis of 129 EACs identified 3 dominant subtypes with potential personalized therapeutic targets. The DNA damage response (DDR)-impaired subtype was found to be genomically unstable and to be deficient in the homologous recombination repair (HR) pathway, suggesting a potential role for PARP inhibitors in addition to DNA-damaging agents. The C > A/T dominant mutation subtype exhibited age as a risk factor and a higher frequency of ERBB2/MET co-amplifications that might benefit from combined receptor tyrosine kinase (RTK) inhibitors. The mutagenic subtype showed higher mutation rates as well as higher levels of immune-mediated signaling with neo-antigen presentation, which could benefit from PD1 blockade therapy [29].
4 Targeted therapies
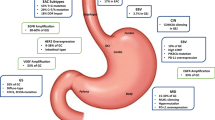
Despite recent advances in identifying genomic drivers of esophageal cancer, only a handful of targeted therapies are clinically available. Trastuzumab, ramucirumab and pembrolizumab are three agents that have been approved by the US FDA for the treatment of advanced and/or metastatic gastroesophageal cancers [30,31,32,33,34]. Below, we review completed and ongoing clinical trials of targeted therapies and suggest potential options for future targets (Fig. 1).
Schematic representation of targeted pathways in esophageal cancer. Current FDA-approved therapies include trastuzumab for advanced HER2-positive esophageal/GEJ adenocarcinomas, ramucirumab for advanced esophageal/GEJ adenocarcinomas and pembrolizumab for advanced esophageal cancers, whether ESCC or EAC, expressing PD-L1. Potential targets include CDk4/6 inhibitors, such as palbociclib in esophageal cancers that over-express cell cycle genes, PARP inhibitors for esophageal cancers with HR deficiency and/or BRCA 1 mutations, LGK974 for esophageal cancers with an activated Wnt signaling pathway, and a possibility for NRF2 targeting in ESCC
4.1 Anti-angiogenesis agents
EACs tend to over-express pro-angiogenesis factors, such as vascular endothelial growth factor (VEGF), which is secreted by both tumor and stromal cells to form blood vessels allowing tumor progression [35]. Targeting angiogenesis is used as a second line treatment in advanced EACs. Various clinical trials have been conducted with bevacizumab as a VEGF inhibitor, but without clear clinical benefit [35, 36]. As yet, ramucirumab, an anti-VEGFR2 monoclonal antibody, is the only anti-angiogenesis agent that is approved as a single agent or in combination with paclitaxel to treat patients with advanced esophageal and gastro-esophageal junction (GEJ) adenocarcinomas who have progressed on fluoropyrimidine/platinum chemotherapy [32, 34]. In a ramucirumab monotherapy trial for previously treated advanced gastric or GEJ adenocarcinomas (REGARD), a total of 355 patients was randomized to ramucirumab versus placebo [32]. The results showed that patients who received ramucirumab had a higher median progression-free survival (PFS) (2.1 vs 1.3 months) and a higher overall survival (OS) (5.2 vs 3.8 months, p = 0.047) compared to those who received conservative treatment [32]. In a phase III ramucirumab plus paclitaxel versus placebo plus paclitaxel trial in patients with previously treated advanced gastric or GEJ adenocarcinomas (RAINBOW), a total of 665 patients who progressed on fluoropyrimidine/platinum-containing chemotherapy was randomized to receive ramucirumab combined with weekly paclitaxel versus placebo plus weekly paclitaxel. Patients who received the treatment combination showed an improved OS (9.6 vs 7.4 months) compared to those who received paclitaxel alone [34]. The side effect profile of ramucirumab includes hypertension, thromboembolic events, rash, diarrhea and myelosuppression [31, 34].
4.2 Anti-proliferation agents
As previously noted, EACs may exhibit activating mutations in proliferation-related pathways, including those in the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 2 (HER2) [30, 35, 37, 38]. Targeting EGFR has been investigated in various clinical trials without clinical benefit [39]. In contrast, targeting HER2 has become standard of care in HER2-positive (HER2+) metastatic EACs. Tumors are considered to be HER2-positive when they exhibit a 3+ HER2 expression score by immunohistochemistry (IHC) or when they exhibit HER2 amplification as detected by FISH [30].
Trastuzumab is a monoclonal antibody that selectively targets the extracellular domain of HER2 and causes antibody- mediated toxicity in HER2 over-expressing cells [30]. In the ToGa trial, assessing the addition trastuzumab to chemotherapy for HER2-positive advanced gastric or GEJ cancers, 3665 patients were screened for HER2 by IHC/FISH and 22% were scored HER2-positive; 584 patients were randomly assigned to receive trastuzumab plus chemotherapy versus chemotherapy alone (cisplatin/fluoropyrimidine). Patients meeting the current standards for HER2-positive disease that received trastuzumab showed a higher median OS (mOS) (16.0 vs 11.8 months) [30]. The side effect profile of trastuzumab includes cardio-toxicity, which is largely reversible, nausea, vomiting, diarrhea and musculoskeletal pain [30].
Pertuzumab is a monoclonal antibody that inhibits dimerization of HER2 with other HER family members [36]. It can result in cell-mediated toxicity as well as inhibition of downstream MAP kinase and PI3K signaling, leading to growth arrest and apoptosis [36]. Unlike breast cancer [40], adding pertuzumab to trastuzumab did not significantly improve the OS in EACs [37]. In the phase III JACOB trial, 780 patients with metastatic gastric and GEJ cancers were randomized to receive trastuzumab/chemotherapy ± pertuzumab. After a median follow-up of approximately 2 years, the addition of pertuzumab provided a non-significant improvement in mOS (17.5 months vs 14.2 months) [37]. Currently, a trial is ongoing assessing the addition of trastuzumab, with or without pertuzumab, to chemotherapy versus chemotherapy alone in the neoadjuvant treatment of gastric/GEJ adenocarcinomas [41].
Lapatinib, a small molecule inhibitor of both EGFR type 1 and HER2, was not found to improve the OS when combined with chemotherapy as a first line treatment in HER2-positive esophageal cancers. This was noted in the phase III TRIO-013/LoGiC trial, where 545 previously untreated patients with HER2-positive gastric, esophageal and GEJ adenocarcinomas received capecitabine/oxalipatin with or without lapatinib. No statistically significant differences were observed in the mOS and/or PFS between the two treatment arms [42].
Trastuzumab emtansine (T-DM1), an antibody-drug conjugate of trastuzumab and the cytotoxic agent emtansine (DM1), a microtubule inhibitor, did not yield any clinical benefit in patients with advanced gastroesophageal cancers who were previously treated with chemotherapy plus HER2-targeted therapy. T-DM1 was compared to chemotherapy (docetaxel or paclitaxel) as a second line treatment for HER2-positive advanced gastric and GEJ cancers in the phase 2/3 GATSBY trial with no effect on mOS [38].
4.3 Immunotherapy
Tumor cells evade the immune system by modulating inhibitory signals on T cell receptors resulting in immune tolerance [43, 44]. In particular, tumor cells express programmed death ligand 1 (PD-L1), which binds programmed death (PD) receptors on T cells and inhibit their function. They can modulate CTLA4, which is known to inhibit T cell function. The overall aim of immunotherapy in cancer management is to release the inhibition on T cells such that they can attack the tumor cells. The risk is activation of the immune system elsewhere in the body, which can cause immune-mediated adverse effects including, but not limited to, colitis, transaminitis, pneumonitis, thyroiditis and dermatitis [43].
As indicated above, molecular characterization of esophageal cancers has led to the identification of subsets of ESCCs and EACs that could benefit from immunotherapy [26]. Checkpoint inhibitors include monoclonal antibodies directed against PD1/PD-L1/CTLA4, which are used in various solid tumors such as melanoma, renal cell carcinoma and non-small cell lung carcinoma [43]. Key clinical trials have shown significantly improved OS rates when using immunotherapy compared to conventional chemotherapies.
In the phase II KEYNOTE-059 trial, patients with previously treated advanced gastric and GEJ cancers who had progressed on at least two lines of therapy received pembrolizumab [31]. The objective response rate (ORR) was 11.6% with a median response duration of 8.4 months. Interestingly, the ORR was 15.5% for patients with a tumor PD-L1 expression ≥ 1% and 6.4% for patients with a tumor PD-L1 expression < 1% [31]. PD-L1 expression status was determined by IHC using a PD-L1 IHC 22C3 pharmDx assay. PD-L1 positive tumors exhibited combined positive scores (CPS) ≥ 1 calculated as the number of PD-L1-positive tumor cells, lymphocytes and macrophages divided by the total number of tumor cells [31]. Based on these results, pembrolizumab is currently approved by the FDA as a third-line treatment modality for advanced gastric and GEJ adenocarcinomas with a PD-L1 expression score ≥ 1% [45].
In the phase II KEYNOTE-180 study, 121 previously treated esophageal cancer patients with ESCC, EAC or GEJ who progressed after two or more lines of therapy received the single agent pembrolizumab. The overall response rate was 10% with a mOS of 5.8 months [33]. The ORR was 14.3% among patients with ESCC, 5.2% among patients with EAC,13.8% among patients with PD-L1 positive tumors and 6.3% among patients with PD-L1 negative tumors [33]. In this study, PD-L1 positive tumors had CPS scores ≥ 10. The data suggest that immunotherapy may be most beneficial in PD-L1 positive ESCCs. As a result of this study and the KEYNOTE-181 trial [46], pembrolizumab has recently been FDA approved as a second-line option for recurrent, locally advanced or metastatic PD-L1 positive ESCCs with CPS scores ≥ 10% [47].
In the phase III KEYNOTE-061 trial, 395 patients requiring second-line treatment for gastric or GEJ adenocarcinomas with a PD-L1 CPS score of ≥ 1% received either pembrolizumab or paclitaxel. The mOS was not found to be significantly longer with pembrolizumab (9.1 months) versus paclitaxel (8.3 months), HR = 0.82, 95% CI 0.66–1.02 [48]]. This study suggested that a PD-L1 CPS score of ≥ 1% may not be enough to identify cancers that will preferentially benefit from immunotherapy. Considerations of higher scores or other biomarkers are warranted.
In the phase III ATTRACTION-2 trial, which was conducted in Asia, patients with advanced gastric or GEJ cancers who were refractory to at least two previous lines of therapy received nivolumab versus placebo [49]. Nivolumab was found to improve the mOS (5.3 months) compared to placebo (4.1 months) and the ORR of 11% was found to be independent of PD-L1 positivity [49]. In the ATTRACTION-4 trial, the same research group evaluated combinations of nivolumab with different chemotherapy regimens, including S-1 plus oxaliplatin (SOX) or capecitabine plus oxaliplatin (CAPOX) as first-line therapies for unresectable advanced HER2-negative gastric and GEJ cancers. The study included 40 patients and the results were promising, showing that the combination of nivolumab with SOX resulted in an ORR of 57.1% and a PFS of 9.7 months, while the combination of nivolumab with CAPOX resulted in an ORR of 76.5% and a PFS of 10.6 months. A phase III clinical trial is currently underway [50]. In the JAVELIN Gastric 300 trial avelumab did not improve the OS compared to third-line chemotherapy or best supportive care (BSC) [51]. Ipilumumab did not improve the PFS compared to BSC in a phase II study of 143 patients with pretreated gastric or GEJ adenocarcinomas [52]. In the CheckMate-032 phase I/II trial 160 patients with advanced or metastatic chemotherapy-refractory gastric, esophageal and GEJ cancers received nivolumab or nivolumab/ipilimumab in different dosing schedules [53]. The ORRs ranged from 8 to 24% with a median duration of response > 7 months [53]. A phase III trial is underway.
In the phase III KEYNOTE-062 randomized clinical trial 763 patients with advanced gastric or GEJ adenocarcinomas were randomly assigned to receive first line pembrolizumab, pembrolizumab plus chemotherapy or chemotherapy alone [54]. All patients had PD-L1 CPS scores ≥ 1, and 37% had a score ≥ 10 [54]. This was the first study showing that pembrolizumab monotherapy was non-inferior to chemotherapy as a first line treatment. The greatest benefit was seen in patients with CPS scores ≥ 10 where the 2-year OS was 39% for pembrolizumab vs 22% for standard chemotherapy, and the mOS was 17.4 months vs 10.8 months [54]. Moving forward, pembrolizumab monotherapy will likely be offered as a first line treatment option in advanced gastric and GEJ cancers that express PD-L1 with CPS scores ≥ 10. Ongoing phase III clinical trials include CheckMate-577, which is assessing stage II/III esophageal/GEJ cancer status post chemo-radiation therapy, followed by surgery randomized to receive nivolumab or placebo in the adjuvant setting [55]. CheckMate-649 is assessing the efficacy of nivolumab plus ipilimumab or nivolumab with chemotherapy compared to chemotherapy in previously untreated advanced gastric/GEJ cancers [56].
Of note, there are various assays to test for PD-L1 expression depending on which epitope was designed for the antibody used in IHC. There are different antibodies for different checkpoint inhibitors, as well as different cut-offs for PD-L1 positive versus negative scores in different cancers [57]. The variance in PD-L1 testing creates confusion and affects patient outcome as it dictates who is included/excluded from immunotherapy. There is a pressing need for a uniform assay [58].
Tumors with a high microsatellite instability (MSI) and mismatch repair (MMR) deficiency exhibit high mutational burdens and generate neo-antigens that can be used as response predictors to immunotherapy [59]. Identifying EACs and ESCCs with MMR deficiency provides opportunities for immunotherapy. MSI-high tumors can be identified via immunohistochemistry for the mismatch repair proteins MLH1, MSH2, MSH6 and PMS2 [60] or via PCR-based techniques to assess the mononucleotide repeat markers NR1, NR24, BAT25, BAT26 and MONO27 [30]. Future tests will be aimed at combining next generation sequencing, MSI testing and tumor mutational burden to identify cancers eligible for immunotherapy [60]. Targeted immunotherapy refinement in esophageal cancer will likely include uniform assays to accurately measure PD-L1 expression levels as well as assays to select for MSI-high tumors.
5 Emerging and future therapies
5.1 CDK4/6 inhibitors
The CyclinD/CDK4/6 pathway is known to play a central role in the regulation of cell cycle progression and to be activated in various cancers [61]. CDK4/6 inhibitors prevent cell cycle progression from the G1 to the S phase and are currently indicated as first line treatment options for metastatic ER-positive breast cancers [61]. Since both ESCCs and EACs exhibit alterations that activate cell cycle regulatory genes, CDK4/6 inhibitors may serve as attractive agents to be explored in clinical esophageal cancer trials. EACs that over-express cyclin E are more likely to be CDK4/6 resistant, as they are able to bypass CDK4/6 and activate downstream signals in the cell cycle pathway [2]. Early preclinical studies have shown that a CDK4/6 inhibitor, PD-0332991, can inhibit ESCC cell growth, induce ESCC cell apoptosis and suppress ESCC cell migration and invasion [62]. Additional in vivo xenograft experiments have shown that PD-0332991 can potently inhibit ESCC tumor growth and lung metastasis [62]. Currently, CDK4/6 inhibitors are being tested in SCC biomarker-driven trials, including patients with ESCC [63, 64].
5.2 Targeting the NRF2 pathway
Genomic data suggest that NRF2 is hyperactive in human ESCC [65] and that mutations in the NRF2 gene range from 7.3% to 20%, depending on the studies reported [3, 20, 24, 25, 66]. Also KEAP1, the inhibitor of NRF2, has been found to exhibit inactivating mutations in ESCC albeit at a lower frequency [20, 66]. Keap1 knockout mice show constitutive Nrf2 activation and exhibit esophageal hyper-proliferation and hyperkeratosis [67]. It has been found that ESCCs with a high NRF2 protein expression are more resistant to chemo-radiotherapy and exhibit a poorer survival compared to ESCCs with a low expression [68,69,70]. This is likely secondary to antioxidant effects of NRF2 signaling that reduce the accumulation of reactive oxygen species (ROS) and allow tumors cells to survive in highly oxidative environments [71,72,73]. In preclinical studies it has been found that NRF2 inhibition may lead to a repressed migration and invasion of ESCC cells in hypoxic microenvironments [74]. Furthermore, it has been found that inhibition of the NRF2 downstream target NQO1 may enhance the antitumor effects of curcumin in ESCC patient-derived xenograft tumors [74].
5.3 Poly (ADP-ribose) polymerase (PARP) inhibitors
Both EACs and ESCCs encompass subtypes with alterations in DNA damage response (DDR) genes and, as such, they may benefit from PARP inhibitors [2, 26, 29]. In EACs, a DDR impaired subgroup comprising 22% of the cancers has been found to be enriched for a BRCA mutation signature and a deficient HR pathway [29]. A BRCA mutation signature was also identified in 7 out of 39 ESCCs [75]. Among the DNA damage response pathways, both EACs and ESCCs exhibit mutations in the TP53 pathway. EACs also exhibit mutations in ARID1A (8%), which is recruited to double strand breaks to process it to single strand breaks. PARP inhibitors are FDA approved for platinum-sensitive relapsed BRCA1/2-associated high-grade ovarian, fallopian tube and primary peritoneal cancers [76], and have shown efficacy in BRCA-associated breast and pancreatic cancers [77,78,79]. Using the concept of synthetic lethality, PARP inhibitors impair the repair of single strand breaks in cells that are already deficient in the repair of double strand breaks by HR. The resulting genomic instability leads to apoptosis [76]. In a preclinical study using the ESCC-derived cell lines KYSE70 and KYSE140, olaparib, a PARP inhibitor, has been found to enhance the cytotoxicity of chemotherapeutic agents [80]. Ultimately, identifying ESCCs and EACs exhibiting DDR deficiency through molecular profiling or immunohistochemistry will allow the selection of cancers that may be sensitive to PARP inhibitors. This will be an important step forward in facilitating personalized medicine in these specific cancer cases. Currently, there are trials ongoing testing PARP inhibitors in combination with other therapies for the treatment of patients with metastatic or locally recurrent esophageal cancers [81, 82].
5.4 Targeting Wnt signaling
Wnt signaling has been found to be activated in the majority ESCCs through up-regulation of Wnt activators and down-regulation of Wnt inhibitors [25, 66, 83, 84]. Wnt signaling controls stemness and is thought to drive carcinogenesis by promoting the growth of pluripotent stem cells [83]. In vitro studies have shown that WNT10a over-expression can promote the migration and invasion and enhance the proliferation of transformed esophageal cells, and increase the proportion of cells with a stem cell-like phenotype [83]. Other studies have shown that Wnt inhibitory factor-1 (WIF1), a secreted antagonist of the Wnt pathway, may be inactivated through epigenetic mechanisms. Over-expression of WIF1 in ESCC-derived cells was found to result in a significant inhibition of tumor cell colony formation [84]. A phase 1 clinical trial is currently underway using LGK974, a drug that targets Porcupine, a Wnt-specific acyltransferase that is important for the processing of Wnt ligand secretion in various cancers, including ESCC [85]. Although to a lesser extent, activation of the Wnt pathway has been found to occur in ~19% of EAC cases [86] through a different mechanism: β-catenin mutation preventing the degradation of APC, which in turn reduces Wnt destruction [87,88,89]. A putative role of Wnt signaling in the esophageal cancer precursor Barrett’s esophagus is emerging [87,88,89], but further research is needed.
6 Conclusions and perspectives
Trastuzumab is used in combination with chemotherapy as first line treatment in metastatic EAC. Ramucirumab is available as monotherapy or in combination with chemotherapy in the second line setting in locally advanced or metastatic EAC. Pembrolizumab is currently available in the second line setting for locally advanced, metastatic or recurrent PDL-1 positive ESCCs and EACs with CPS ≥ 10 or MSI-high/deficient MMR cancers and as a third line agent in EACs with CPS ≥ 1. Patients with GEJ cancers progressing on standard chemotherapy, either 5FU + platinum ± anthracycline or taxane + ramucirumab, should be subjected to PD-L1 testing (if not already done) and next generation sequencing (NGS), ideally on biopsies of progressive lesions, to assess for targeted therapy and clinical trial inclusion eligibility. With recent data showing that pembrolizumab is non-inferior to chemotherapy as fist line agent in advanced gastric and GEJ cancers expressing PD-L1, immunotherapy is an attractive option. Moving forward, identifying gene expression/mutation signatures or biochemical markers (other than PD-L1 staining) including MSI and MMR deficiency will help to identify ESCC and EAC subtypes that will benefit most from immunotherapy.
Targeted therapies in ESCCs are limited and immunotherapy is an option only in the second line setting. Molecular classification of esophageal cancers has facilitated the design of new potential therapeutic options, which are precise and targeted. These emerging alternatives are awaiting clinical trials: CDK4/6 inhibitors, PARP inhibitors and Wnt signaling modulators. Targeting NRF2 in ESCCs is a possibility that remains to be fully explored. Ultimately, personalized medicine in esophageal cancer will likely rely on NGS analysis of ESCCs and EACs to pinpoint subtypes that will most likely benefit from targeted therapies. The costs of targeted therapies in conjunction with drug access variability in various countries still pose challenges. Moreover, incorporating diagnostic tests, such as genomic sequencing to identify gene signatures, assays to test for PD-L1 staining, MSI and MMR deficiency, go along with healthcare costs that will need to be balanced with any improvement in survival outcome and/or quality of life, particularly for patients with advanced esophageal cancer.
References
J. Lagergren, E. Smyth, D. Cunningham, P. Lagergren, Oesophageal cancer. Lancet 390, 2383–2396 (2017)
Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency; Brigham and Women’s Hospital; Broad Institute; Brown University; Case Western Reserve University; Dana-Farber Cancer Institute; Duke University; Greater Poland Cancer Centre; Harvard Medical School; Institute for Systems Biology; KU Leuven; Mayo Clinic; Memorial Sloan Kettering Cancer Center; National Cancer Institute; Nationwide Children’s Hospital; Stanford University; University of Alabama; University of Michigan; University of North Carolina; University of Pittsburgh; University of Rochester; University of Southern California; University of Texas MD Anderson Cancer Center; University of Washington; Van Andel Research Institute; Vanderbilt University; Washington University; Genome Sequencing Center: Broad Institute; Washington University in St. Louis; Genome Characterization Centers: BC Cancer Agency; Broad Institute; Harvard Medical School; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University; University of North Carolina; University of Southern California Epigenome Center; University of Texas MD Anderson Cancer Center; Van Andel Research Institute; Genome Data Analysis Centers: Broad Institute; Brown University:; Harvard Medical School; Institute for Systems Biology; Memorial Sloan Kettering Cancer Center; University of California Santa Cruz; University of Texas MD Anderson Cancer Center; Biospecimen Core Resource: International Genomics Consortium; Research Institute at Nationwide Children’s Hospital; Tissue Source Sites: Analytic Biologic Services; Asan Medical Center; Asterand Bioscience; Barretos Cancer Hospital; BioreclamationIVT; Botkin Municipal Clinic; Chonnam National University Medical School; Christiana Care Health System; Cureline; Duke University; Emory University; Erasmus University; Indiana University School of Medicine; Institute of Oncology of Moldova; International Genomics Consortium; Invidumed; Israelitisches Krankenhaus Hamburg; Keimyung University School of Medicine; Memorial Sloan Kettering Cancer Center; National Cancer Center Goyang; Ontario Tumour Bank; Peter MacCallum Cancer Centre; Pusan National University Medical School; Ribeirão Preto Medical School; St. Joseph’s Hospital &Medical Center; St. Petersburg Academic University; Tayside Tissue Bank; University of Dundee; University of Kansas Medical Center; University of Michigan; University of North Carolina at Chapel Hill; University of Pittsburgh School of Medicine; University of Texas MD Anderson Cancer Center; Disease Working Group: Duke University; Memorial Sloan Kettering Cancer Center; National Cancer Institute; University of Texas MD Anderson Cancer Center; Yonsei University College of Medicine; Data Coordination Center: CSRA Inc.; Project Team: National Institutes of Health, Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017)
Y.B. Gao, Z.L. Chen, J.G. Li, X.D. Hu, X.J. Shi, Z.M. Sun, F. Zhang, Z.R. Zhao, Z.T. Li, Z.Y. Liu, Y.D. Zhao, J. Sun, C.C. Zhou, R. Yao, S.Y. Wang, P. Wang, N. Sun, B.H. Zhang, J.S. Dong, Y. Yu, M. Luo, X.L. Feng, S.S. Shi, F. Zhou, F.W. Tan, B. Qiu, N. Li, K. Shao, L.J. Zhang, Q. Xue, S.G. Gao, J. He, Genetic landscape of esophageal squamous cell carcinoma. Nat Genet 46, 1097–1102 (2014)
D.C. Lin, J.J. Hao, Y. Nagata, L. Xu, L. Shang, X. Meng, Y. Sato, Y. Okuno, A.M. Varela, L.W. Ding, M. Garg, L.Z. Liu, H. Yang, D. Yin, Z.Z. Shi, Y.Y. Jiang, W.Y. Gu, T. Gong, Y. Zhang, X. Xu, O. Kalid, S. Shacham, S. Ogawa, M.R. Wang, H.P. Koeffler, Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet 46, 467–473 (2014)
C.C. Abnet, M. Arnold, W.Q. Wei, Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154, 360–373 (2018)
E.C. Smyth, J. Lagergren, R.C. Fitzgerald, F. Lordick, M.A. Shah, P. Lagergren, D. Cunningham, Oesophageal cancer. Nat Rev Dis Primers 3, 17048 (2017)
J. Rüdiger Siewert, M. Feith, M. Werner, H.J. Stein, Adenocarcinoma of the esophagogastric junction: Results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 232, 353–361 (2000)
F.L. Huang, S.J. Yu, Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 41, 210–215 (2018)
M.Q. Lin, Y.P. Li, S.G. Wu, J.Y. Sun, H.X. Lin, S.Y. Zhang, Z.Y. He, Differences in esophageal cancer characteristics and survival between Chinese and Caucasian patients in the SEER database. Onco Targets Ther 9, 6435–6444 (2016)
Y. Kitagawa, T. Uno, T. Oyama, K. Kato, H. Kato, H. Kawakubo, O. Kawamura, M. Kusano, H. Kuwano, H. Takeuchi, Y. Toh, Y. Doki, Y. Naomoto, K. Nemoto, E. Booka, H. Matsubara, T. Miyazaki, M. Muto, A. Yanagisawa, M. Yoshida, Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 2. Esophagus 16, 25–43 (2019)
F. Lordick, C. Mariette, K. Haustermans, R. Obermannová, D. Arnold, E.G. Committee, Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27(suppl 5), v50–v57 (2016)
J.A. Ajani, T.A. D’Amico, D.J. Bentrem, J. Chao, C. Corvera, P. Das, C.S. Denlinger, P.C. Enzinger, P. Fanta, F. Farjah, H. Gerdes, M. Gibson, R.E. Glasgow, J.A. Hayman, S. Hochwald, W.L. Hofstetter, D.H. Ilson, D. Jaroszewski, K.L. Johung, R.N. Keswani, L.R. Kleinberg, S. Leong, Q.P. Ly, K.A. Matkowskyj, M. McNamara, M.F. Mulcahy, R.K. Paluri, H. Park, K.A. Perry, J. Pimiento, G.A. Poultsides, R. Roses, V.E. Strong, G. Wiesner, C.G. Willett, C.D. Wright, N.R. McMillian, L.A. Pluchino, Esophageal and esophagogastric junction cancers. J Natl Compr Cancer Netw 17(7), 855–883 (2019)
T.W. Rice, D.T. Patil, E.H. Blackstone, 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann Cardiothorac Surg 6, 119–130 (2017)
T.N. Walsh, N. Noonan, D. Hollywood, A. Kelly, N. Keeling, T.P. Hennessy, A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 335, 462–467 (1996)
V. Gebski, B. Burmeister, B.M. Smithers, K. Foo, J. Zalcberg, J. Simes, Australasian Gastro-Intestinal Trials Group, Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol 8, 226–234 (2007)
J. Shapiro, J.J.B. van Lanschot, M.C.C.M. Hulshof, P. van Hagen, M.I. van Berge Henegouwen, B.P.L. Wijnhoven, H.W.M. van Laarhoven, G.A.P. Nieuwenhuijzen, G.A.P. Hospers, J.J. Bonenkamp, M.A. Cuesta, R.J.B. Blaisse, O.R.C. Busch, F.J.W. Ten Kate, G.M. Creemers, C.J.A. Punt, J.T.M. Plukker, H.M.W. Verheul, E.J.S. Bilgen, H. van Dekken, M.J.C. van der Sangen, T. Rozema, K. Biermann, J.C. Beukema, A.H.M. Piet, C.M. van Rij, J.G. Reinders, H.W. Tilanus, E.W. Steyerberg, A. van der Gaast, CROSS Study Group, Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol 16, 1090–1098 (2015)
J. Tepper, M.J. Krasna, D. Niedzwiecki, D. Hollis, C.E. Reed, R. Goldberg, K. Kiel, C. Willett, D. Sugarbaker, R. Mayer, Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26, 1086–1092 (2008)
J.S. Cooper, M.D. Guo, A. Herskovic, J.S. Macdonald, J.A. Martenson, M. Al-Sarraf, R. Byhardt, A.H. Russell, J.J. Beitler, S. Spencer, S.O. Asbell, M.V. Graham, L.L. Leichman, Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 281, 1623–1627 (1999)
S. Paul, N. Altorki, Outcomes in the management of esophageal cancer. J Surg Oncol 110, 599–610 (2014)
J. Chang, W. Tan, Z. Ling, R. Xi, M. Shao, M. Chen, Y. Luo, Y. Zhao, Y. Liu, X. Huang, Y. Xia, J. Hu, J.S. Parker, D. Marron, Q. Cui, L. Peng, J. Chu, H. Li, Z. Du, Y. Han, Z. Liu, Q. Zhan, Y. Li, W. Mao, C. Wu, D. Lin, Genomic analysis of oesophageal squamous-cell carcinoma identifies alcohol drinking-related mutation signature and genomic alterations. Nat Commun 8, 15290 (2017)
C. Cheng, Y. Zhou, H. Li, T. Xiong, S. Li, Y. Bi, P. Kong, F. Wang, H. Cui, Y. Li, X. Fang, T. Yan, J. Wang, B. Yang, L. Zhang, Z. Jia, B. Song, X. Hu, J. Yang, H. Qiu, G. Zhang, J. Liu, E. Xu, R. Shi, Y. Zhang, H. Liu, C. He, Z. Zhao, Y. Qian, R. Rong, Z. Han, W. Luo, S. Peng, X. Yang, X. Li, L. Li, H. Fang, X. Liu, L. Ma, Y. Chen, S. Guo, X. Chen, Y. Xi, G. Li, J. Liang, J. Guo, J. Jia, Q. Li, X. Cheng, Q. Zhan, Y. Cui, Whole-genome sequencing reveals diverse models of structural variations in esophageal squamous cell carcinoma. Am J Hum Genet 98, 256–274 (2016)
J. Deng, H. Chen, D. Zhou, J. Zhang, Y. Chen, Q. Liu, D. Ai, H. Zhu, L. Chu, W. Ren, X. Zhang, Y. Xia, M. Sun, H. Zhang, J. Li, X. Peng, L. Li, L. Han, H. Lin, X. Cai, J. Xiang, S. Chen, Y. Sun, Y. Zhang, S. Zhang, Y. Zhao, Y. Liu, H. Liang, K. Zhao, Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nat Commun 8, 1533 (2017)
J. Deng, X. Weng, J. Ye, D. Zhou, Y. Liu, K. Zhao, Identification of the germline mutation profile in esophageal squamous cell carcinoma by whole exome sequencing. Front Genet 10, 47 (2019)
H.D. Qin, X.Y. Liao, Y.B. Chen, S.Y. Huang, W.Q. Xue, F.F. Li, X.S. Ge, D.Q. Liu, Q. Cai, J. Long, X.Z. Li, Y.Z. Hu, S.D. Zhang, L.J. Zhang, B. Lehrman, A.F. Scott, D. Lin, Y.X. Zeng, Y.Y. Shugart, W.H. Jia, Genomic characterization of esophageal squamous cell carcinoma reveals critical genes underlying tumorigenesis and poor prognosis. Am J Hum Genet 98, 709–727 (2016)
Y. Song, L. Li, Y. Ou, Z. Gao, E. Li, X. Li, W. Zhang, J. Wang, L. Xu, Y. Zhou, X. Ma, L. Liu, Z. Zhao, X. Huang, J. Fan, L. Dong, G. Chen, L. Ma, J. Yang, L. Chen, M. He, M. Li, X. Zhuang, K. Huang, K. Qiu, G. Yin, G. Guo, Q. Feng, P. Chen, Z. Wu, J. Wu, J. Zhao, L. Luo, M. Fu, B. Xu, B. Chen, Y. Li, T. Tong, M. Wang, Z. Liu, D. Lin, X. Zhang, H. Yang, Q. Zhan, Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509, 91–95 (2014)
T. Xiong, M. Wang, J. Zhao, Q. Liu, C. Yang, W. Luo, X. Li, H. Yang, K. Kristiansen, B. Roy, Y. Zhou, An esophageal squamous cell carcinoma classification system that reveals potential targets for therapy. Oncotarget 8, 49851–49860 (2017)
L. Zhang, Y. Zhou, C. Cheng, H. Cui, L. Cheng, P. Kong, J. Wang, Y. Li, W. Chen, B. Song, F. Wang, Z. Jia, L. Li, B. Yang, J. Liu, R. Shi, Y. Bi, Y. Zhang, Z. Zhao, X. Hu, J. Yang, H. Li, Z. Gao, G. Chen, X. Huang, X. Yang, S. Wan, C. Chen, B. Li, Y. Tan, L. Chen, M. He, S. Xie, X. Li, X. Zhuang, M. Wang, Z. Xia, L. Luo, J. Ma, B. Dong, J. Zhao, Y. Song, Y. Ou, E. Li, L. Xu, Y. Xi, G. Li, E. Xu, J. Liang, J. Guo, X. Chen, Q. Li, L. Liu, X. Zhang, H. Yang, D. Lin, X. Cheng, Y. Guo, Q. Zhan, Y. Cui, Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet 96, 597–611 (2015)
M. Hazawa, D.C. Lin, H. Handral, L. Xu, Y. Chen, Y.Y. Jiang, A. Mayakonda, L.W. Ding, X. Meng, A. Sharma, S. Samuel, M.M. Movahednia, R.W. Wong, H. Yang, C. Tong, H.P. Koeffler, ZNF750 is a lineage-specific tumour suppressor in squamous cell carcinoma. Oncogene 36, 2243–2254 (2017)
M. Secrier, X. Li, N. de Silva, M.D. Eldridge, G. Contino, J. Bornschein, S. MacRae, N. Grehan, M. O'Donovan, A. Miremadi, T.P. Yang, L. Bower, H. Chettouh, J. Crawte, N. Galeano-Dalmau, A. Grabowska, J. Saunders, T. Underwood, N. Waddell, A.P. Barbour, B. Nutzinger, A. Achilleos, P.A. Edwards, A.G. Lynch, S. Tavaré, R.C. Fitzgerald, Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium, Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet 48, 1131–1141 (2016)
Y.J. Bang, E. Van Cutsem, A. Feyereislova, H.C. Chung, L. Shen, A. Sawaki, F. Lordick, A. Ohtsu, Y. Omuro, T. Satoh, G. Aprile, E. Kulikov, J. Hill, M. Lehle, J. Rüschoff, Y.K. Kang, T.T. Investigators, Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010)
C.S. Fuchs, T. Doi, R.W. Jang, K. Muro, T. Satoh, M. Machado, W. Sun, S.I. Jalal, M.A. Shah, J.P. Metges, M. Garrido, T. Golan, M. Mandala, Z.A. Wainberg, D.V. Catenacci, A. Ohtsu, K. Shitara, R. Geva, J. Bleeker, A.H. Ko, G. Ku, P. Philip, P.C. Enzinger, Y.J. Bang, D. Levitan, J. Wang, M. Rosales, R.P. Dalal, H.H. Yoon, Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 Trial. JAMA Oncol 4, e180013 (2018)
C.S. Fuchs, J. Tomasek, C.J. Yong, F. Dumitru, R. Passalacqua, C. Goswami, H. Safran, L.V.D. Santos, G. Aprile, D.R. Ferry, B. Melichar, M. Tehfe, E. Topuzov, J.R. Zalcberg, I. Chau, W. Campbell, C. Sivanandan, J. Pikiel, M. Koshiji, Y. Hsu, A.M. Liepa, L. Gao, J.D. Schwartz, J. Tabernero, R.T. Investigators, Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383, 31–39 (2014)
M.A. Shah, T. Kojima, D. Hochhauser, P. Enzinger, J. Raimbourg, A. Hollebecque, F. Lordick, S.B. Kim, M. Tajika, H.T. Kim, A.C. Lockhart, H.T. Arkenau, F. El-Hajbi, M. Gupta, P. Pfeiffer, Q. Liu, J. Lunceford, S.P. Kang, P. Bhagia, K. Kato, Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol 5, 546-550 (2019)
H. Wilke, K. Muro, E. Van Cutsem, S.C. Oh, G. Bodoky, Y. Shimada, S. Hironaka, N. Sugimoto, O. Lipatov, T.Y. Kim, D. Cunningham, P. Rougier, Y. Komatsu, J. Ajani, M. Emig, R. Carlesi, D. Ferry, K. Chandrawansa, J.D. Schwartz, A. Ohtsu, RAINBOW Study Group, Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol 15, 1224–1235 (2014)
S. Kasper, M. Schuler, Targeted therapies in gastroesophageal cancer. Eur J Cancer 50, 1247–1258 (2014)
P. Samson, A.C. Lockhart, Biologic therapy in esophageal and gastric malignancies: Current therapies and future directions. J Gastrointest Oncol 8, 418–429 (2017)
J. Tabernero, P.M. Hoff, L. Shen, A. Ohtsu, M.A. Shah, K. Cheng, C. Song, H. Wu, J. Eng-Wong, K. Kim, Y.K. Kang, Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 19, 1372–1384 (2018)
P.C. Thuss-Patience, M.A. Shah, A. Ohtsu, E. Van Cutsem, J.A. Ajani, H. Castro, W. Mansoor, H.C. Chung, G. Bodoky, K. Shitara, G.D.L. Phillips, T. van der Horst, M.L. Harle-Yge, B.L. Althaus, Y.K. Kang, Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 18, 640–653 (2017)
T. Waddell, I. Chau, D. Cunningham, D. Gonzalez, A.F. Okines, A. Frances, C. Okines, A. Wotherspoon, C. Saffery, G. Middleton, J. Wadsley, D. Ferry, W. Mansoor, T. Crosby, F. Coxon, D. Smith, J. Waters, T. Iveson, S. Falk, S. Slater, C. Peckitt, Y. Barbachano, Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol 14, 481–489 (2013)
N. Mavaddat, D. Barrowdale, I.L. Andrulis, S.M. Domchek, D. Eccles, H. Nevanlinna, S.J. Ramus, A. Spurdle, M. Robson, M. Sherman, A.M. Mulligan, F.J. Couch, C. Engel, L. McGuffog, S. Healey, O.M. Sinilnikova, M.C. Southey, M.B. Terry, D. Goldgar, F. O'Malley, E.M. John, R. Janavicius, L. Tihomirova, T.V. Hansen, F.C. Nielsen, A. Osorio, A. Stavropoulou, J. Benítez, S. Manoukian, B. Peissel, M. Barile, S. Volorio, B. Pasini, R. Dolcetti, A.L. Putignano, L. Ottini, P. Radice, U. Hamann, M.U. Rashid, F.B. Hogervorst, M. Kriege, R.B. van der Luijt, S. Peock, D. Frost, D.G. Evans, C. Brewer, L. Walker, M.T. Rogers, L.E. Side, C. Houghton, J. Weaver, A.K. Godwin, R.K. Schmutzler, B. Wappenschmidt, A. Meindl, K. Kast, N. Arnold, D. Niederacher, C. Sutter, H. Deissler, D. Gadzicki, S. Preisler-Adams, R. Varon-Mateeva, I. Schönbuchner, H. Gevensleben, D. Stoppa-Lyonnet, M. Belotti, L. Barjhoux, C. Isaacs, B.N. Peshkin, T. Caldes, M. de la Hoya, C. Cañadas, T. Heikkinen, P. Heikkilä, K. Aittomäki, I. Blanco, C. Lazaro, J. Brunet, B.A. Agnarsson, A. Arason, R.B. Barkardottir, M. Dumont, J. Simard, M. Montagna, S. Agata, E. D'Andrea, M. Yan, S. Fox, T.R. Rebbeck, W. Rubinstein, N. Tung, J.E. Garber, X. Wang, Z. Fredericksen, V.S. Pankratz, N.M. Lindor, C. Szabo, K. Offit, R. Sakr, M.M. Gaudet, C.F. Singer, M.K. Tea, C. Rappaport, P.L. Mai, M.H. Greene, A. Sokolenko, E. Imyanitov, A.E. Toland, L. Senter, K. Sweet, M. Thomassen, A.M. Gerdes, T. Kruse, M. Caligo, P. Aretini, J. Rantala, A. von Wachenfeld, K. Henriksson, L. Steele, S.L. Neuhausen, R. Nussbaum, M. Beattie, K. Odunsi, L. Sucheston, S.A. Gayther, K. Nathanson, J. Gross, C. Walsh, B. Karlan, G. Chenevix-Trench, D.F. Easton, A.C. Antoniou, HEBON, EMBRACE, GEMO Study Collaborators, kConFab Investigators, SWE-BRCA Collaborators and Consortium of Investigators of Modifiers of BRCA1/2, Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 21, 134–147 (2012)
A.D. Wagner, H.I. Grabsch, M. Mauer, S. Marreaud, C. Caballero, P. Thuss-Patience, L. Mueller, A. Elme, M.H. Moehler, U. Martens, Y.K. Kang, S.Y. Rha, A. Cats, M. Tokunaga, F. Lordick, EORTC-1203-GITCG – The “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: A randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer 19, 494 (2019)
J.R. Hecht, Y.J. Bang, S.K. Qin, H.C. Chung, J.M. Xu, J.O. Park, K. Jeziorski, Y. Shparyk, P.M. Hoff, A. Sobrero, P. Salman, J. Li, S.A. Protsenko, Z.A. Wainberg, M. Buyse, K. Afenjar, V. Houé, A. Garcia, T. Kaneko, Y. Huang, S. Khan-Wasti, S. Santillana, M.F. Press, D. Slamon, Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC – A randomized phase III trial. J Clin Oncol 34, 443–451 (2016)
R.J. Kelly, The emerging role of immunotherapy for esophageal cancer. Curr Opin Gastroenterol, 35(4), 337–343 (2019). https://doi.org/10.1097/MOG.0000000000000542
A. Ghahremanloo, A. Soltani, S.M.S. Modaresi, S.I. Hashemy, Recent advances in the clinical development of immune checkpoint blockade therapy. Cell Oncol 42, 609–626 (2019)
L. Fashoyin-Aje, M. Donoghue, H. Chen, K. He, J. Veeraraghavan, K.B. Goldberg, P. Keegan, A.E. McKee, R. Pazdur, FDA approval summary: Pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist 24, 103–109 (2019)
T.A. Post, KEYNOTE-181: Pembrolizumab vs chemotherapy in second-line treatment of advanced esophageal cancer. 2019; Available from: https://www.ascopost.com/issues/april-10-2019-supplement-conference-highlights-gugi-2019/keynote-181-in-esophageal-cancer/. Accessed 19 October 2019
FDA. FDA approves pembrolizumab for advanced esophageal squamous cell cancer. July 31; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-esophageal-squamous-cell-cancer. Accessed 19 October 2019
K. Shitara, M. Özgüroğlu, Y.J. Bang, M. Di Bartolomeo, M. Mandalà, M.H. Ryu, L. Fornaro, T. Olesiński, C. Caglevic, H.C. Chung, K. Muro, E. Goekkurt, W. Mansoor, R.S. McDermott, E. Shacham-Shmueli, X. Chen, C. Mayo, S.P. Kang, A. Ohtsu, C.S. Fuchs, KEYNOTE-061 Investigators, Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133 (2018)
Y.K. Kang, N. Boku, T. Satoh, M.H. Ryu, Y. Chao, K. Kato, H.C. Chung, J.S. Chen, K. Muro, W.K. Kang, K.H. Yeh, T. Yoshikawa, S.C. Oh, L.Y. Bai, T. Tamura, K.W. Lee, Y. Hamamoto, J.G. Kim, K. Chin, D.Y. Oh, K. Minashi, J.Y. Cho, M. Tsuda, L.T. Chen, Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 2461–2471 (2017)
N. Boku, M.H. Ryu, K. Kato, H.C. Chung, K. Minashi, K.W. Lee, H. Cho, W.K. Kang, Y. Komatsu, M. Tsuda, K. Yamaguchi, H. Hara, S. Fumita, M. Azuma, L.T. Chen, Y.K. Kang, Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: Interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 30, 250–258 (2019)
Y.J. Bang, E.Y. Ruiz, E. Van Cutsem, K.W. Lee, L. Wyrwicz, M. Schenker, M. Alsina, M.H. Ryu, H.C. Chung, L. Evesque, S.E. Al-Batran, S.H. Park, M. Lichinitser, N. Boku, M.H. Moehler, J. Hong, H. Xiong, R. Hallwachs, I. Conti, J. Taieb, Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann Oncol 29, 2052–2060 (2018)
Y.J. Bang, J.Y. Cho, Y.H. Kim, J.W. Kim, M. Di Bartolomeo, J.A. Ajani, K. Yamaguchi, A. Balogh, T. Sanchez, M. Moehler, Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res 23, 5671–5678 (2017)
Y.Y. Janjigian, J. Bendell, E. Calvo, J.W. Kim, P.A. Ascierto, P. Sharma, P.A. Ott, K. Peltola, D. Jaeger, J. Evans, F. de Braud, I. Chau, C.T. Harbison, C. Dorange, M. Tschaika, D.T. Le, CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 36, 2836–2844 (2018)
T.A. Post, 2019 ASCO: KEYNOTE-062: Pembrolizumab with or without chemotherapy vs chemotherapy in advanced gastric or GEJ adenocarcinoma; Available from: https://www.ascopost.com/News/60099. Accessed 7 July 2019
Health, N.I.o. An investigational immuno-therapy study of nivolumab or placebo in patients with resected esophageal or gastroesophageal junction cancer (CheckMate 577); Available from: https://clinicaltrials.gov/ct2/show/NCT02743494. Accessed 24 July 2019
Health, N.I.o. Efficacy study of nivolumab plus ipilimumab or nivolumab plus chemotherapy against chemotherapy in stomach cancer or stomach/esophagus junction cancer (CheckMate649); Available from: https://clinicaltrials.gov/ct2/show/NCT02872116. Accessed 29 July 2019
D.N. Ionescu, M.R. Downes, A. Christofides, M.S. Tsao, Harmonization of PD-L1 testing in oncology: A Canadian pathology perspective. Curr Oncol 25, e209–e216 (2018)
H. Polioudaki, A. Chantziou, K. Kalyvianaki, P. Malamos, G. Notas, D. Mavroudis, M. Kampa, E. Castanas, P.A. Theodoropoulos, Nuclear localization of PD-L1: Artifact or reality? Cell Oncol 42, 237–242 (2019)
M. Yi, S. Qin, W. Zhao, S. Yu, Q. Chu, K. Wu, The role of neoantigen in immune checkpoint blockade therapy. Exp Hematol Oncol 7, 28 (2018)
C. Luchini, F. Bibeau, M.J.L. Ligtenberg, N. Singh, A. Nottegar, T. Bosse, R. Miller, N. Riaz, J.Y. Douillard, F. Andre, A. Scarpa, ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann Oncol, doi: 10.1093/annonc/mdz116 (2019)
F. Schettini, I. De Santo, C.G. Rea, P. De Placido, L. Formisano, M. Giuliano, G. Arpino, M. De Laurentiis, F. Puglisi, S. De Placido, L. Del Mastro, CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol 8, 608 (2018)
L. Chen, J. Pan, Dual cyclin-dependent kinase 4/6 inhibition by PD-0332991 induces apoptosis and senescence in oesophageal squamous cell carcinoma cells. Br J Pharmacol 174, 2427–2443 (2017)
Health, N.I.o. Korean Cancer Study Group: Translational bIomarker Driven UMbrella Project for Head and Neck (TRIUMPH), Esophageal Squamous Cell Carcinoma- Part 1 (HNSCC); Available from: https://clinicaltrials.gov/ct2/show/NCT03292250. Accessed 11 October 2017
Health, N.I.o. Phase Ib study of TNO155 in combination with spartalizumab or ribociclib in selected malignancies; Available from: https://clinicaltrials.gov/ct2/show/NCT04000529. Accessed 16 July 2019
Q. Zhang, J.E. Burdette, J.P. Wang, Integrative network analysis of TCGA data for ovarian cancer. BMC Syst Biol 8, 1338 (2014)
G. Sawada, A. Niida, R. Uchi, H. Hirata, T. Shimamura, Y. Suzuki, Y. Shiraishi, K. Chiba, S. Imoto, Y. Takahashi, T. Iwaya, T. Sudo, T. Hayashi, H. Takai, Y. Kawasaki, T. Matsukawa, H. Eguchi, K. Sugimachi, F. Tanaka, H. Suzuki, K. Yamamoto, H. Ishii, M. Shimizu, H. Yamazaki, M. Yamazaki, Y. Tachimori, Y. Kajiyama, S. Natsugoe, H. Fujita, K. Mafune, Y. Tanaka, D.P. Kelsell, C.A. Scott, S. Tsuji, S. Yachida, T. Shibata, S. Sugano, Y. Doki, T. Akiyama, H. Aburatani, S. Ogawa, S. Miyano, M. Mori, K. Mimori, Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology 150, 1171–1182 (2016)
N. Wakabayashi, K. Itoh, J. Wakabayashi, H. Motohashi, S. Noda, S. Takahashi, S. Imakado, T. Kotsuji, F. Otsuka, D.R. Roop, T. Harada, J.D. Engel, M. Yamamoto, Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35, 238–245 (2003)
Y. Kawasaki, H. Okumura, Y. Uchikado, Y. Kita, K. Sasaki, T. Owaki, S. Ishigami, S. Natsugoe, Nrf2 is useful for predicting the effect of chemoradiation therapy on esophageal squamous cell carcinoma. Ann Surg Oncol 21, 2347–2352 (2014)
T. Shibata, A. Kokubu, S. Saito, M. Narisawa-Saito, H. Sasaki, K. Aoyagi, Y. Yoshimatsu, Y. Tachimori, R. Kushima, T. Kiyono, M. Yamamoto, NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia 13, 864–873 (2011)
J. Zhang, Q. Jiao, L. Kong, J. Yu, A. Fang, M. Li, Nrf2 and Keap1 abnormalities in esophageal squamous cell carcinoma and association with the effect of chemoradiotherapy. Thorac Cancer 9, 726–735 (2018)
C. Gorrini, P.S. Baniasadi, I.S. Harris, J. Silvester, S. Inoue, B. Snow, P.A. Joshi, A. Wakeham, S.D. Molyneux, B. Martin, P. Bouwman, D.W. Cescon, A.J. Elia, Z. Winterton-Perks, J. Cruickshank, D. Brenner, A. Tseng, M. Musgrave, H.K. Berman, R. Khokha, J. Jonkers, T.W. Mak, M.L. Gauthier, BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J Exp Med 210, 1529–1544 (2013)
S. Ma, C. Paiboonrungruan, T. Yan, K.P. Williams, M.B. Major, X.L. Chen, Targeted therapy of esophageal squamous cell carcinoma: The NRF2 signaling pathway as target. Ann N Y Acad Sci 1434, 164–172 (2018)
M. Seervi, S. Sumi, A. Chandrasekharan, A.K. Sharma, T.R. SanthoshKumar, Molecular profiling of anastatic cancer cells: Potential role of the nuclear export pathway. Cell Oncol 42, 645–661 (2019)
A. Mizumoto, S. Ohashi, M. Kamada, T. Saito, Y. Nakai, K. Baba, K. Hirohashi, Y. Mitani, O. Kikuchi, J. Matsubara, A. Yamada, T. Takahashi, H. Lee, Y. Okuno, M. Kanai, M. Muto, Combination treatment with highly bioavailable curcumin and NQO1 inhibitor exhibits potent antitumor effects on esophageal squamous cell carcinoma. J Gastroenterol 54, 687-698 (2019)
T. Yan, H. Cui, Y. Zhou, B. Yang, P. Kong, Y. Zhang, Y. Liu, B. Wang, Y. Cheng, J. Li, S. Guo, E. Xu, H. Liu, C. Cheng, L. Zhang, L. Chen, X. Zhuang, Y. Qian, J. Yang, Y. Ma, H. Li, F. Wang, J. Liu, X. Liu, D. Su, Y. Wang, R. Sun, Y. Li, X. Cheng, Z. Liu, Q. Zhan, Y. Cui, Multi-region sequencing unveils novel actionable targets and spatial heterogeneity in esophageal squamous cell carcinoma. Nat Commun 10, 1670 (2019)
A. Ashworth, C.J. Lord, Synthetic lethal therapies for cancer: What’s next after PARP inhibitors? Nat Rev Clin Oncol 15, 564–576 (2018)
J.K. Litton, H.S. Rugo, J. Ettl, S.A. Hurvitz, A. Gonçalves, K.H. Lee, L. Fehrenbacher, R. Yerushalmi, L.A. Mina, M. Martin, H. Roché, Y.H. Im, R.G.W. Quek, D. Markova, I.C. Tudor, A.L. Hannah, W. Eiermann, J.L. Blum, Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 379, 753–763 (2018)
M. Robson, C. Goessl, S. Domchek, Olaparib for metastatic germline BRCA-mutated breast cancer. N Engl J Med 377, 1792–1793 (2017)
T.A. Post, 2019 ASCO: POLO: Maintenance olaparib in germline BRCA-mutated pancreatic cancer; Available from: https://www.ascopost.com/News/60105. Accessed 16 July 2019
K. Miyamoto, T. Minegaki, M. Tanahashi, A. Yamamoto, Y. Moriyama, A. Wada, A. Matsumoto, K. Ota, M. Tanaka, U. Masuda, M. Tsujimoto, K. Nishiguchi, Synergistic effects of olaparib and DNA-damaging agents in oesophageal squamous cell carcinoma cell lines. Anticancer Res 39, 1813–1820 (2019)
Health, N.I.o. Olaparib and ramucirumab in treating patients with metastatic or locally recurrent gastric or gastroesophageal junction cancer that cannot be removed by surgery; Available from: https://clinicaltrials.gov/ct2/show/NCT03008278. Accessed 7 July 2019
Health, N.I.o. Veliparib, paclitaxel, and carboplatin in treating patients with solid tumors that are metastatic or cannot be removed by surgery and liver or kidney dysfunction; Available from: https://clinicaltrials.gov/ct2/show/NCT01366144. Accessed 4 June 2019
A. Long, V. Giroux, K.A. Whelan, K.E. Hamilton, M.P. Tétreault, K. Tanaka, J.S. Lee, A.J. Klein-Szanto, H. Nakagawa, A.K. Rustgi, WNT10A promotes an invasive and self-renewing phenotype in esophageal squamous cell carcinoma. Carcinogenesis. 36, 598–606 (2015)
S.H. Yang, S.L. Li, Z.M. Dong, Q.C. Kan, Epigenetic inactivation of Wnt inhibitory factor-1 in human esophageal squamous cell carcinoma. Oncol Res 20, 123–130 (2012)
Health, N.I.o. A study of LGK974 in patients with malignancies dependent on Wnt ligands; Available from: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2011-03693&r=1. Accessed 7 July 2019
A.M. Frankell, S. Jammula, X. Li, G. Contino, S. Killcoyne, S. Abbas, J. Perner, L. Bower, G. Devonshire, E. Ococks, N. Grehan, J. Mok, M. O'Donovan, S. MacRae, M.D. Eldridge, S. Tavaré, R.C. Fitzgerald, Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium, The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat Genet 51, 506–516 (2019)
G. Clément, D.M. Jablons, J. Benhattar, Targeting the Wnt signaling pathway to treat Barrett's esophagus. Expert Opin Ther Targets 11, 375–389 (2007)
O. Lyros, P. Rafiee, L. Nie, R. Medda, N. Jovanovic, M.F. Otterson, B. Behmaram, I. Gockel, A. Mackinnon, R. Shaker, Wnt/β-catenin signaling activation beyond robust nuclear β-catenin accumulation in nondysplastic Barrett’s esophagus: Regulation via Dickkopf-1. Neoplasia 17, 598–611 (2015)
L.H. Moyes, H. McEwan, S. Radulescu, J. Pawlikowski, C.G. Lamm, C. Nixon, O.J. Sansom, J.J. Going, G.M. Fullarton, P.D. Adams, Activation of Wnt signalling promotes development of dysplasia in Barrett’s oesophagus. J Pathol 228, 99–112 (2012)
Y. Kitagawa, T. Uno, T. Oyama, K. Kato, H. Kato, H. Kawakubo, O. Kawamura, M. Kusano, H. Kuwano, H. Takeuchi, Y. Toh, Y. Doki, Y. Naomoto, K. Nemoto, E. Booka, H. Matsubara, T. Miyazaki, M. Muto, A. Yanagisawa, M. Yoshida, Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: Part 1. Esophagus. 16, 1–24 (2019)
A.M. Dulak, P. Stojanov, S. Peng, M.S. Lawrence, C. Fox, C. Stewart, S. Bandla, Y. Imamura, S.E. Schumacher, E. Shefler, A. McKenna, S.L. Carter, K. Cibulskis, A. Sivachenko, G. Saksena, D. Voet, A.H. Ramos, D. Auclair, K. Thompson, C. Sougnez, R.C. Onofrio, C. Guiducci, R. Beroukhim, Z. Zhou, L. Lin, J. Lin, R. Reddy, A. Chang, R. Landrenau, A. Pennathur, S. Ogino, J.D. Luketich, T.R. Golub, S.B. Gabriel, E.S. Lander, D.G. Beer, T.E. Godfrey, G. Getz, A.J. Bass, Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 45, 478–486 (2013)
J.Y. Dai, X. Wang, M.F. Buas, C. Zhang, J. Ma, B. Wei, Y. Li, B. Zhao, T.S. Hyun, X. Chen, K.R. Loeb, R. Odze, L. Yao, X. Sun, S. Self, T.L. Vaughan, Y. Guo, Whole-genome sequencing of esophageal adenocarcinoma in Chinese patients reveals distinct mutational signatures and genomic alterations. Commun Biol 1, 174 (2018)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics
No cell lines or human tissues were used for this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatehi Hassanabad, A., Chehade, R., Breadner, D. et al. Esophageal carcinoma: Towards targeted therapies. Cell Oncol. 43, 195–209 (2020). https://doi.org/10.1007/s13402-019-00488-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-019-00488-2