Abstract
Background
Osteosarcoma is a malignant bone cancer of which the survival rate is still low. One reason for this low survival rate is drug resistance. In the past, it has been shown that microRNAs may play critical roles in osteosarcoma development and drug resistance. The mechanisms by which osteosarcoma cells acquire this resistance have, however, remained largely unknown. Here, we aimed at assessing the role of microRNA-488 in the acquisition of drug resistance by osteosarcoma cells.
Methods
Quantitative RT-PCR was used to measure the expression of microRNA-488 in primary osteosarcoma samples and in osteosarcoma-derived cells, whereas microRNA-488 mimics and inhibitors were used to modify its expression in these cells. Luciferase reporter, Western blotting, cell viability, apoptosis and ChIP assays were used to assess the various effects of modified microRNA-488 expression in osteosarcoma-derived cells.
Results
We found that microRNA-488 is over-expressed in primary osteosarcoma tissues and osteosarcoma-derived cells and that hypoxia can induce microRNA-488 expression via binding to the hypoxia response element (HRE) in its promoter. We also found that exogenous over-expression of microRNA-488 promotes the proliferation, reduces the apoptosis and decreases the sensitivity to chemotherapy (doxorubicin) of osteosarcoma cells via direct targeting of the tumor suppressor Bim, which is a mediator of apoptosis. In contrast, we found that transfection of a microRNA-488 inhibitor resulted in an increase in both apoptosis and drug sensitivity, and a decrease in proliferation.
Conclusions
Our data suggest that miRNA-488 may serve as a predictor of response to chemotherapy and as a therapeutic target in human osteosarcomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Osteosarcoma is a malignant bone cancer primarily found in children and adolescents, which is characterized by an aggressive clinical course [1]. Despite multiple options that have been reported for the treatment of osteosarcoma, the 5-year overall survival rate has remained ~70 % [2]. The occurrence of metastases, recurrences and chemo-resistance are the main causes of treatment failure [3]. Therefore, a comprehensive understanding of the mechanisms underlying treatment failure is urgently needed.
microRNAs (miRNAs) are small 19–25 nucleotide-long noncoding RNAs that participate in gene-regulatory networks governing various biological processes, including cellular proliferation, differentiation, metastasis and apoptosis [4]. They post-transcriptionally regulate gene expression by binding to the 3′ untranslated regions (3’UTR) of downstream target messenger RNAs [5, 6]. Growing evidence has indicated that deregulated miRNAs may be involved in cancer development and may be indicative for a clinical outcome of human cancer patients [7–11]. However, our understanding of the mechanisms underlying miRNA deregulation and their biological role(s) in cancer initiation and progression, especially osteosarcoma, is still limited.
Previous reports have suggested that microRNAs may play critical roles in the drug resistance of tumor cells via the suppression of apoptosis. Hypoxia-induced miR-424 has e.g. been found to decrease the sensitivity of cancer cells to chemotherapy and to inhibit apoptosis via targeting PDCD4 [12], whereas microRNA-587 has been found to antagonize 5-FU-induced apoptosis and to confer drug resistance to colorectal cancer cells by regulating PPP2R1B expression [13]. miR-197 has been found to be able to act at different levels within the p53 pathway to counteract the induction of apoptosis, thus allowing cells to proliferate in an uncontrolled manner [14]. miR-143 expression has been reported to be decreased in tumor tissues and knockdown of miR-143 has been found to promote cell proliferation and to hamper cell apoptosis by directly targeting PKC [15]. Over-expression of miR-101 has led to a decrease in EZH2 expression and a concomitant decrease in the proliferative and invasive abilities of non-small cell lung cancer (NSCLC) cells, resulting in a sensitization of NSCLC cells to paclitaxel [16].
Previously, microRNA-488 has been reported to be associated with panic disorder and to regulate several anxiety candidate genes and related pathways, as also with the pathogenesis of osteoarthritis by regulating the zinc transporter SLC39A8/ZIP [17, 18]. More recently, aberrant expression of microRNA-488 has been observed during cancer development. For example, microRNA-488 has been found to be over-expressed and to inhibit androgen receptor (AR) expression in prostate cancer cells [19]. As yet, the role of microRNA-488 in osteosarcoma is insufficiently understood.
Here, we show that microRNA-488 is over-expressed in primary human osteosarcoma tissues and osteosarcoma-derived cell lines, and that hypoxia can induce microRNA-488 expression at the transcriptional level. In addition, we show that exogenous over-expression of microRNA-488 promotes the proliferation, reduces the apoptosis and decreases the sensitivity to chemotherapy of osteosarcoma cells via directly targeting the tumor suppressor Bim, a mediator of apoptosis.
2 Materials and methods
2.1 Cell culture, human tissues and reagents
The MG-63, Saos2 and G293 osteosarcoma-derived cells lines and the hFOB1.19 normal human osteoblastic cell line were purchased from the American Type Culture Collection (ATCC), whereas the HEK293T cells used were maintained in our lab. All cells were cultured in RPMI-1640 medium supplemented with 10 % heat inactivated fetal calf serum (FCS) in a 5 % CO2 atmosphere. Primary antibodies directed against Bim (catalog number: 2933), PARP (catalog number: 9532), caspase 3 (catalog number: 9662), HIF1-α (catalog number: 14,179) and actin (catalog number: 4970) were purchased from Cell Signaling Technology (Boston, MA, USA). Paired primary osteosarcoma tissues and normal adjacent tissues were obtained from patients who had undergone surgical removal. They were histologically verified as high grade osteosarcoma with either stage IIA or IIB (Enneking system).
2.2 RNA extraction and quantitative RT- PCR
Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer’s protocol. microRNA expression levels were measured using a TaqMan MicroRNA Assay (Applied Biosystems) according to the manufacturer’s instructions. Briefly, cDNAs were synthesized from 200 ng total RNA in a 15 μl reaction mixture. Next, these cDNAs were amplified by quantitative PCR using TaqMan microRNA Assays Human Panel sequence-specific primers. RNU48 was used as an internal control. The primers used for microRNA-488 and RNU48 were: microRNA-488-F: 5′-gcggcgcccagauaaug-3′; microRNA-488-R: 5′-gtgcagggtccgaggt-3′; RNU48-F: 5′-taatgatgaccccaggtaactc-3′; RNU48-R: 5′-gagcgctgcggtgatg-3′.
2.3 Transfection assays
The microRNA-488 mimics, microRNA-488 inhibitor and scramble control were purchased from GenePharma (Shanghai, China). The indicated cells were cultured till 60 % confluency and then transfected with the microRNA-488 mimics, microRNA-488 inhibitor and scramble control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After 24 or 48 h the cells were collected for further experiments.
2.4 Vector construction
For the construction of a reporter vector containing the Bim 3’UTR region, the 3’UTR of the human Bim mRNA (GenBank accession number: NM_001204106.1) containing the putative microRNA-488 binding sequence was amplified by PCR and cloned into a pmirGLO-control vector using the XhoI and SacI sites. Bim 3’UTR mutagenesis, inducing mutations in the complementary sequence in the seed region of microRNA-488, was performed using a QuickChange Kit (Stratagene).
For the construction of a hypoxia response element (HRE) containing reporter vector, a HRE-containing fragment from the microRNA-488 promoter was amplified by PCR using the following primers: F: 5′-tctaacagcaacaataggga-3′; R: 5′- caaattgtttgaaaggctat-3′ and, subsequently, cloned into the promoter of the luciferase reporter gene. pGL3-mutHRE, which harbors a mutation in the HRE, was generated by site-specific mutagenesis using the following primers: F: 5′-tggcacacagtggctgcgtggaaggacagc-3′; R: 5′-cgtggacagagctgtccttcGTGCGagcca-3′.
2.5 Luciferase reporter assay
HEK293T cells were seeded at a density of 5000 cells/well in a 96-well plate. The next day, luciferase vectors (pmirGLO-Bim-3’UTR-WT or pmirGLO-Bim-3’UTR-MUT; see above) and microRNA-301b mimics or scramble controls were transfected into the HEK293T cells using Lipofectamine 2000. After 48 h, the cells were harvested and analyzed using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). HEK293T cells were also transfected with pGL3-mutHRE and pGL3-wtHRE (see above) and cultured under hypoxic conditions (1 % O2) to assess the functionality of the putative HRE in the microRNA-488 promoter. The cells were collected 48 h after transfection and, again, analyzed using a Dual-Luciferase Reporter Assay System (Promega). A plasmid constitutively expressing renilla luciferase was used as an internal control to correct for differences in both transfection and harvesting efficiencies. The transfections were performed in duplicate, and at least three independent experiments were performed.
2.6 Western blot analyses
Cells were collected, washed twice in ice-cold phosphate buffered saline (PBS) and spun down. The resulting cell pellets were dissolved in lysis buffer containing a protease inhibitor mixture for 15 min on ice. The cell lysates were centrifuged at 12,000 g for 10 min and the supernatants were recovered, followed by boiling for 10 min. Equal amounts of total protein were separated by 10 % SDS-PAGE and transferred to polyvinylidene fluoride microporous membranes (PVDF, Bio-Rad). The membranes were blocked with 5 % non-fat milk for 1 h at 37 °C and subsequently incubated with an appropriate primary antibody. After being washed, the membranes were incubated with a secondary antibody. The proteins were detected by chemiluminescence.
2.7 Cell viability assay
Cell viability was measured as reported before [20]. Briefly, cells were seeded at a density of 5000 cells/well into 96-well plates and transfected with microRNA-301b mimics or a microRNA-301b inhibitor using Lipofectamine 2000. 1, 2, 3, 4 and 5 days post-transfection the cells were labeled using a Cell Counting Kit-8 (CCK8; DOJINDO, Tabaru, Japan) for 2 h, after which the absorbance was measured at 450 nm.
2.8 Apoptosis assay
Osteosarcoma-derived cells were transiently transfected with microRNA-488 mimics or a microRNA-488 inhibitor and a scramble control and, subsequently, incubated for 24 h. At the end of the incubation period, the cells were harvested and stained with 25 ng/ml Annexin V and 10 mg/ml propidium iodide (PI) via incubation for 15 min at 25 °C in the dark. Subsequently, apoptosis was measured using routine flow cytometry (BD Biosciences, San Jose, CA, USA).
2.9 ChIP assay
Chromatin immunoprecipitation (ChIP) was performed as reported before [21]. Briefly, cells were cross-linked with 1 % formaldehyde for 30 min at 37 °C and subsequently quenched in 0.125 M glycine. Immunoprecipitated DNA from sonicated cell lysates was quantified by real-time PCR using a SYBR Green system (ThermoFisher Scientific, NY, USA).
2.10 Statistical analyses
Data are presented as mean ± S.D. Statistical analyses were performed using the Graphpad Prism software package (Graphpad Software Inc., La Jolla, CA, USA). Student’s t-test was used to determine statistical significance. P < 0.05 was considered statistically significant in all analyses.
3 Results
3.1 microRNA-488 is over-expressed in human osteosarcomas
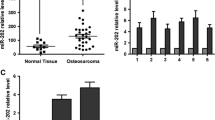
A miRNA expression profile was downloaded from TCGA to assess the expression of microRNA-488 in human osteosarcomas (Gene Expression Omnibus accession # 39,040). We found that the level of microRNA-488 expression was higher in osteosarcoma tissues than in normal tissues (Fig. 1A). To confirm this high expression level in human osteosarcomas, we next set out to perform quantitative RT-PCR (qRT-PCR) on primary human tissues. By doing so, we found that the level of microRNA-488 was indeed significantly higher in the five primary osteosarcoma tissues tested compared to its adjacent normal tissues (Fig. 1B). In addition, we assessed the microRNA-488 expression level in three human osteosarcoma-derived cell lines and a normal human osteoblastic cell line using qRT-PCR. Again, the microRNA-488 level was found to be significantly higher in the MG-63, G-293 and Saos2 cells than the hFOB1.19 cells (Fig. 1C). Taken together, we conclude that microRNA-488 is over-expressed in human osteosarcomas.
microRNA-488 is over-expressed in osteosarcoma. (a) TCGA data analysis showing that microRNA-488 is over-expressed in osteosarcoma tissues compared to normal tissues. (b) qRT-PCR analysis showing that microRNA-488 is significantly over-expressed in primary human osteosarcoma tissues compared to adjacent normal tissues. (c) qRT-PCR analysis showing that microRNA-488 is significantly over-expressed in the osteosarcoma-derived cell lines MG-63, G-292 and Saos2 compared to the osteoblastic cell line hFOB 1.19. ** p < 0.05
3.2 Hypoxia induces the expression of microRNA-488 in human osteosarcoma cells
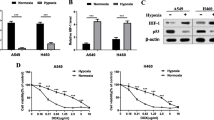
As shown previously, hypoxia can induce microRNA expression in human cancer cells. So far, however, it has not been established whether hypoxia can induce microRNA-488 expression in human osteosarcoma cells. To test this option, MG-63 and G-292 cells were cultured for 12 h under hypoxic conditions (see materials and methods). After this, mature microRNA-488 expression was measured by qRT-PCR, and we found that its expression levels were significantly increased in these two cell lines under hypoxic conditions (Fig. 2A, B). H2O2 is a well-known reagent that can induce the accumulation of HIF1-α, which functions as a hypoxia inducible transcription factor. To examine whether HIF1-α can up-regulate the expression of microRNA-488 in human osteosarcoma cells, we treated MG-63 and G-292 cells with DMOG, a prolyl hydroxylase domain (PHD) enzyme inhibitor, which can increase the stability of HIF1-α. We indeed found that DMOG enhances the expression level of microRNA-488 in these cells compared to untreated control cells (Fig. 2A, B). Concomitantly, we found that the HIF1-α protein level was up-regulated in MG-63 and G-292 cells treated with hypoxia or DMOG using Western blotting (Fig. 2C, D). In contrast, we found that under hypoxic conditions, siRNA-mediated knockdown of HIF1-α led to a decrease in the expression of microRNA-488 in MG-63 cells (Fig. 2E, F). Together, these data suggest that HIF1-α is involved in the regulation of microRNA-488 expression in human osteosarcoma cells.
Hypoxia induces the expression of microRNA-488 in osteosarcoma cells. qRT-PCR analyses showing that hypoxia and DMOG treatment significantly increase the expression level of mature microRNA-488 in (a) MG-63 cells and (b) G-292 cells. Western blot analyses showing that the HIF1-α protein level is significantly increased in (c) MG-63 cells and (d) G-292 cells under hypoxic conditions and after DMOG treatment. qRT-PCR analyses showing that the microRNA-488 level is decreased in (e) MG-63 cells and (f) MG-63 cells treated with shRNA-HIF1-α under hypoxic conditions. ** p < 0.05
3.3 HIF1-α a induces microRNA-488 expression at the transcriptional level
Since HIF1-α is a well-known hypoxia inducible transcription factor, we hypothesized that HIF1-α may directly be involved in the up-regulation of the expression of microRNA-488. To test this hypothesis, we first set out to assess the expression of pri-microRNA-488 in cells treated with hypoxia or DMOG. Using qRT-PCR we found that both DMOG and hypoxia can significantly up-regulate the expression of pri-microRNA-488 (Fig. 3A). To further explore the question how HIF1-α regulates the expression of pri-microRNA-488, a 3 kb region upstream of the pri-microRNA-488 start site was analyzed. By doing so, a conserved HIF1-α binding sequence GCGTG (hypoxia response element, HRE) was encountered at ~500 bp (Fig. 3B). Next, chromatin immunoprecipitation (ChIP) was performed to determine whether HIF1-α indeed binds to the pri-microRNA-488 promoter. We found that HIF1-α was significantly enriched at the HRE element (Fig. 3C), indicating that HIF1-α may indeed regulate microRNA-488 expression at the transcriptional level via binding to its promoter. To assess the functionality of the putative HRE in the microRNA-488 promoter, a 500 bp fragment containing the HRE element was cloned into the pGL3 promoter. Next, HEK293T cells transfected with the control reporter, the wild-type microRNA-488 promoter reporter, the mutant microRNA-488 promoter reporter or the control pTK-Renilla vector, were cultured under hypoxic or normal conditions for 12 h. By doing so, we found that the wild-type microRNA-488 promoter readily induced luciferase activity in the hypoxic cells, whereas the promoter with the mutant HRE, lacking the HIF1-α binding sequence, readily suppressed the hypoxia-induced luciferase activity in these cells (Fig. 3D). Together, these data suggest that HIF1-α can bind to the HRE within the microRNA-488 promoter and, by doing so, directly regulate its transcription.
HIF1-α induces microRNA-488 expression at the transcriptional level. (a) qRT-PCR analysis showing that hypoxia significantly increases the expression level of pri-microRNA-488 in MG-63 and G-292 cells. (b) Putative HRE (hypoxia response element, HRE1) in the pri-microRNA-488 promoter. (c) ChIP analysis showing that HIF1-α is significantly enriched in HRE1; NC represents a negative control without HRE sequence. (d) Luciferase reporter assay showing that wild-type microRNA-488 HRE1 significantly increases luciferase activity under hypoxic conditions and that mutant HRE1 inhibits the increased luciferase activity; mut1-HRE1 represents a mutation of 5′-gcgtg-3′ to 5′-aaatg-3′. ** p < 0.05
3.4 microRNA-488 enhances cell proliferation, reduces cell apoptosis and induces drug resistance
The observed increased expression of microRNA-488 in primary human osteosarcoma tissues and cell lines inspired us to explore the question whether microRNA-488 may act as an oncomiR. We found that in MG-63 cells, transfection of microRNA-488 inhibitors or mimics significantly reduced or restored the intracellular microRNA-488 expression levels, respectively (Fig. 4A). In addition, we assessed the effect of microRNA-488 expression on MG-63 cell proliferation using a CCK8 assay. We found that microRNA-488 over-expression increased cell proliferation and that, in contrast, microRNA-488 expression knockdown reduced cell proliferation (Fig. 4B). To next examine the effect of microRNA-488 expression on apoptosis, we analyzed MG-63 cells transfected with microRNA-488 inhibitors, microRNA-488 mimics or a scramble control by Western blotting to assess the expression of cleaved PARP and cleaved caspase 3, both of which are well-known markers of apoptosis. We found that microRNA-488 over-expression decreased the expression of cleaved PARP and cleaved caspase 3 (Fig. 4C), suggesting that this over-expression results in suppression of apoptosis. In contrast, we found that microRNA-488 knockdown resulted in a reduction in apoptosis (Fig. 4C). In addition, the survival rate of MG-63 cells treated with 10 nM doxorubicin (Dox) was determined using a CCK8 assay. The results obtained suggest that microRNA-488 over-expression improves the survival rate of MG-63 cells (Fig. 4D), indicating that microRNA-488 over-expressing cells exhibit a decreased sensitivity to drug treatment. To further explore the role of microRNA-488 on apoptosis in osteosarcoma cells, Dox-induced apoptosis was analyzed by Western blotting. The data obtained showed that Dox treatment resulted in increases in the cleaved PARP and cleaved caspase 3 levels (Fig. 4E), and that the application of microRNA-488 mimics resulted in suppressed cleaved caspase 3 and cleaved PARP levels, indicating that microRNA-488 can inhibit Dox-induced apoptosis. Taken together, these observations indicate that microRNA-488 can enhance the proliferation, reduce the apoptosis and increase the resistance of osteosarcoma cells to drug treatment.
microRNA-488 increases cell proliferation, reduces apoptosis and enhances drug resistance in osteosarcoma cells. (a) qRT-PCR microRNA-488 expression analysis of MG-63 cells transfected with microRNA-488 mimics or inhibitor showing a significant increase and a significant decrease, respectively. (b) CCK8 analysis showing that the proliferation rate of microRNA-488 (mimics) over-expressing MG-63 cells is increased compared to that of control cells. Conversely, the proliferation rate is enhanced in inhibitor-based microRNA-488 knockdown MG-63 cells. (c) Western blot analysis showing that microRNA-488 over-expression decreases cleaved PARP and cleaved caspase 3 levels, whereas microRNA-488 knockdown increases cleaved PARP and cleaved caspase 3 levels. (d) CCK8 analysis showing that over-expression of microRNA-488 increases the resistance to doxorubicin of MG-63 cells. (e) Western blot analysis showing that microRNA-488 over-expression suppresses the expression of apoptosis-related proteins of MG-63 cells induced by doxorubicin (Dox). ** p < 0.05
3.5 5 microRNA-488 reduces apoptosis via directly suppressing Bim expression
Based on the above observations we concluded that microRNA-488 is involved in osteosarcoma cell proliferation and apoptosis. It is conceivable that this biological function results from the (de)regulation of apoptosis-related factors by microRNA-488. To address this question, we used Targetscan and miRanda to predict potential microRNA-488 targets related to apoptosis. This led to the identification of a candidate target, Bim (Bcl-2-interacting mediator of cell death), which has a predicted binding sequence in its 3’UTR for microRNA-488 (Fig. 5A). To substantiate the notion that Bim may serve as a direct target of microRNA-488, we tested the Bim protein level in cells transiently transfected with microRNA-488 mimics. The results obtained showed that exogenous microRNA-488 over-expression markedly down-regulated the protein expression level of Bim in osteosarcoma cells (Fig. 5B). Additional real-time PCR results showed that the Bim mRNA level was dramatically decreased in cells transfected with microRNA-488 mimics compared to that in untransfected control cells (Fig. 5C). These observations suggest that microRNA-488 can regulate Bim expression both at the mRNA level and the protein level. To further explore whether Bim serves as a direct target of microRNA-488, we cloned the wild-type 3’UTR and a mutant 3’UTR of Bim into a dual-luciferase vector and performed a luciferase reporter assay. As expected, microRNA-488 markedly suppressed the luciferase activity in the wild-type 3’UTR expressing cells, but not in the cells expressing the mutant 3’UTR of Bim (Fig. 5D). These results indicate that microRNA-488 directly regulates Bim expression by binding to its 3’UTR. To assess whether Bim is required for the role of microRNA-488 in apoptosis induction, MG-63 cells transfected with microRNA-488 mimics were subjected to exogenous Bim over-expression, after which the percentage of apoptotic cells was determined. Through Western blotting it was found that Bim over-expression significantly rescued the Bim protein expression level in cells transfected with microRNA-488 mimics (Fig. 5E). As expected, we found that the apoptotic rate was higher in cells over-expressing microRNA-488 mimics and Bim than in cells over-expressing microRNA-488 alone (Fig. 5F). From this, we conclude that the biological role of microRNA-488 in apoptosis may be mediated by Bim.
microRNA-488 down-regulates the expression level of Bim by directly binding to its 3’UTR. (a) Sequences in the 3’UTR of Bim predicted to bind to microRNA-488; wild-type and mutant sequences are depicted. (b) Western blot analysis showing that microRNA-488 over-expression leads to a suppression of the Bim protein level in G-292 and MG-63 cells. (c) qRT-PCR analysis showing that microRNA-488 over-expression (OE) leads to inhibition of the Bim mRNA level in G-292 and MG-63 cells. (d) Luciferase reporter activity analysis showing that microRNA-488 suppresses the luciferase activity of the wild-type UTR, but not the mutant UTR. (e) Western blot analysis showing that exogenous Bim over-expression rescues the Bim protein level in MG-63 cells transfected with microRNA-488 mimics. (f) CCK8 analysis showing that restored Bim expression significantly increases the apoptosis rate (%).** p < 0.05
4 Discussion
Osteosarcoma is a devastating and common type of tumor that usually develops in children and young adults [22]. Although several treatment options are available, the 5-year survival rate of osteosarcoma is still low due to, among others, the occurrence of drug resistance [1]. Therapy with anticancer drugs, including methotrexate, doxorubicin and cisplatin, is usually the first option for osteosarcoma patients [3]. However, many patients are not very responsive to chemotherapy and, as a consequence, they have a poor prognosis. Thus, it has become imperative to explore the mechanisms underlying osteosarcoma development and chemotherapy resistance.
Different mechanisms, including apoptosis, autophagy and dysfunctional membrane transport have been reported to explain chemotherapy resistance [23]. Apoptosis plays an important role in the development and maintenance of multi-cellular organisms through the removal of damaged, aged or autoimmune cells [23]. In contrast to normal cells, cancer cells have the ability to evade apoptosis and to enhance cell survival under drug treatment pressure [23]. Recently, it has been reported that deregulated apoptosis-related miRNAs may be involved in cancer development [24]. Some miRNAs may function as tumor suppressors, which are down-regulated, whereas others may serve as oncogenes (oncomiRs), which are up-regulated in cancer tissues and cancer-derived cell lines [11, 24]. Therefore, a potential therapeutic strategy would be to target oncomiRs or restore the expression of tumor suppressor miRNAs in the treatment of osteosarcomas.
Recently, aberrant microRNA-488 expression has been reported to be involved in cancer development. Our current experimental data, along with our analysis of a publicly available dataset, revealed that microRNA-488 is over-expressed in primary osteosarcoma tissues compared to normal tissues, as well as in osteosarcoma-derived cell lines. We also found that hypoxia can induce microRNA-488 expression in osteosarcoma cells. Hypoxia is a common micro-environmental factor that is manifest in various cancers, and growing evidence indicates that hypoxia may induce the expression of several miRNAs. To additionally determine whether the microRNA-488 promoter can bind HIF1-α, we performed ChIP experiments and found that HIF1-α can indeed associate with the microRNA-488 promoter. A luciferase assay further confirmed that HIF1-α can bind to a conserved HRE sequence within the pri-microRNA-488 promoter and, thus, can regulate microRNA-488 expression at the transcriptional level. To the best of our knowledge, this is the first report showing that microRNA-488 is up-regulated in osteosarcoma and can be induced by hypoxia.
We also found that exogenous microRNA-488 expression increased the proliferation of osteosarcoma cells, reduced their apoptosis and decreased their sensitivity to drug (doxorubicin) treatment by targeting Bim. Expression knockdown of microRNA-488 resulted in the opposite effect. Restoring the expression of Bim in MG-63 cells treated with microRNA-488 mimics led to an increase in apoptosis. These observations suggest that Bim is required for microRNA-488 in regulating the apoptosis and drug resistance in osteosarcoma cells.
Taken together, we found that microRNA-488 is over-expressed in osteosarcomas and can be induced by hypoxia at the transcriptional level. We also found that microRNA-488 may act as a regulator of apoptosis via targeting Bim. Our data suggest that microRNA-488 may serve as a predictor of response to doxorubicin and may serve as a therapeutic target in human osteosarcomas.
References
D. S. Geller, R. Gorlick, Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 8, 705–718 (2010)
W. S. Ferguson, A. M. Goorin, Current treatment of osteosarcoma. Cancer Investig. 19, 292–315 (2001)
A. Luetke, P. A. Meyers, I. Lewis, H. Juergens, Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat. Rev. 40, 523–532 (2014)
Z. Wei, L. Cui, Z. Mei, M. Liu, D. Zhang, miR-181a mediates metabolic shift in colon cancer cells via the PTEN/AKT pathway. FEBS Lett. 588, 1773–1779 (2014)
R. Mitra, J. Sun, Z. Zhao, microRNA regulation in cancer: One arm or two arms? Int. J. Cancer 137, 1516-1518 (2015)
E. Prodromaki, A. Korpetinou, E. Giannopoulou, E. Vlotinou, M. Chatziathanasiadou, N. I. Papachristou, C. D. Scopa, H. Papadaki, H. P. Kalofonos, D. J. Papachristou, Expression of the microRNA regulators Drosha, dicer and Ago2 in non-small cell lung carcinomas. Cell. Oncol. 38, 307–317 (2015)
Y. S. Lee, A. Dutta, MicroRNAs in cancer. Annu. Rev. Pathol. 4, 199–227 (2009)
C. Salazar, R. Nagadia, P. Pandit, J. Cooper-White, N. Baneriee, N. Dimitrova, W. B. Coman, C. Punyadeera, A novel saliva-based microRNA biomarker panel to detect head and neck cancers. Cell. Oncol. 37, 331–338 (2014)
L. Rask, E. Balslev, R. Sokilde, E. Hogdall, H. Flyger, J. Eriksen, T. Litman, Differential expression of miR-139, miR-486 and miR-21 in breast cancer patients sub-classified according to lymph node status. Cell. Oncol. 37, 215–227 (2014)
C. Yu, M. Wang, Z. Li, J. Xiao, F. Peng, X. Guo, Y. Deng, J. Jiang, C. Sun, MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cell. Oncol. 38, 173–181. (2015)
I. Fkih M'hamed, M. Privat, F. Ponelle, F. Penault-Llorca, A. Kenani, Y. J. Bignon, Identification of miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative breast cancer biomarkers. Cell. Oncol. 38, 433–442 (2015)
D. Zhang, Z. Shi, M. Li, J. Mi, Hypoxia-induced miR-424 decreases tumor sensitivity to chemotherapy by inhibiting apoptosis. Cell. Death Dis. 5, e1301 (2014)
Y. Zhang, G. Talmon, J. Wang, MicroRNA-587 antagonizes 5-FU-induced apoptosis and confers drug resistance by regulating PPP2R1B expression in colorectal cancer. Cell. Death. Dis. 6, e1845 (2015)
M. E. Fiori, C. Barbini, T. L. Haas, N. Marroncelli, M. Patrizii, M. Biffoni, R. De Maria, Antitumor effect of miR-197 targeting in p53 wild-type lung cancer. Cell. Death Differ. 21, 774–782 (2014)
K. Jain, A. Basu, The multifunctional protein kinase C-epsilon in cancer development and progression. Cancers (Basel) 6, 860–878 (2014)
J. G. Zhang, J. F. Guo, D. L. Liu, Q. Liu, J. J. Wang, MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J. Thorac. Oncol. 6, 671–678 (2011)
J. Song, D. Kim, C.H. Lee, M.S. Lee, C.H. Chun, E.J. Jin, MicroRNA-488 regulates zinc transporter SLC39A8/ZIP8 during pathogenesis of osteoarthritis. J. Biomed. Sci. 20, 20–31 (2013)
M. Muinos-Gimeno, Y. Espinosa-Parrilla, M. Guidi, B. Kagerbauer, T. Sipila, E. Maron, K. Pettai, L. Kananen, R. Navines, R. Martin-Santos, M. Gratacos, A. Metspalu, I. Hovatta, X. Estivilla, Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol. Psychiatry 69, 526–533 (2011)
K. Sikand, J.E. Slaibi, R. Singh, S.D. Singh, G.C. Shukla, miR 488* inhibits androgen receptor expression in prostate carcinoma cells. Int. J. Cancer 129, 810–819 (2011)
D. Zhang, Y. Wang, Z. Shi, J. Liu, P. Sun, X. Hou, J. Zhang, S. Zhao, B. P. Zhou, J. Mi, Metabolic reprogramming of cancer-associated fibroblasts by IDH3alpha downregulation. Cell. Rep. 10, 1335–1348 (2015)
Q. Li, D. Zhang, Y. Wang, P. Sun, X. Hou, J. Larner, W. Xiong, J. Mi, MiR-21/Smad 7 signaling determines TGF-beta1-induced CAF formation. Sci. Rep. 3, 2038 (2013)
A. J. Chou, R. Gorlick, Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev. Anticancer Ther. 6, 1075–1085 (2006)
S. W. Lowe, A. W. Lin, Apoptosis in cancer. Carcinogenesis 21, 485–495 (2000)
Y. Wang, C. G. Lee, MicroRNA and cancer--focus on apoptosis. J. Cell. Mol. Med. 13, 12–23 (2009)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Funding
This study was supported by grants from the National Science Foundation of China (81,372,869).
Ethical standard
This article does not contain any studies with animals performed by any of the authors. All patients provided informed consent prior to inclusion in this study.
Rights and permissions
About this article
Cite this article
Zhou, C., Tan, W., Lv, H. et al. Hypoxia-inducible microRNA-488 regulates apoptosis by targeting Bim in osteosarcoma. Cell Oncol. 39, 463–471 (2016). https://doi.org/10.1007/s13402-016-0288-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-016-0288-2