Abstract
Purpose
Several prognostic models have been developed to assess the efficacy and safety of sorafenib for metastatic renal cell carcinoma (mRCC), but few studies have validated its use in Chinese patients. The objective of this single center, single arm retrospective study was to examine the efficacy and safety of sorafenib and its related prognostic clinico-pathologic factors in Chinese mRCC patients.
Methods
One hundred thirty four mRCC patients were enrolled. All patients received 400 mg of sorafenib orally twice daily. The dose was subsequently adjusted in the event of treatment-induced toxicity. Tumor response, progression-free survival (PFS), overall survival (OS) and adverse events (AEs) were determined.
Results
The median PFS and OS were 10 months (1–36 months) and 22 months (2–37 months), respectively. Complete, partial, and stable disease were observed in two (1.49 %), 24 (17.91 %), and 99 (73.88 %) patients, respectively. Hand/foot skin reactions, diarrhea and fatigue were the most commonly observed AEs following sorafenib treatment. Among the AEs, only 13 grades 3 and 4 were observed. Multivariate analysis revealed that independent predictive factors for PFS included Eastern Cooperative Oncology Group (ECOG) status, Memorial Sloan-Kettering Cancer Center (MSKCC) risk status, and bone metastasis (all p < 0.05). Factors associated with OS included MSKCC risk values, bone metastasis and sorafenib-induced hypertension (all p < 0.05).
Conclusion
The introduction of sorafenib therapy for mRCC in Chinese patients may lead to a favorable disease control with acceptable tolerability. In addition, the parameters predicting favorable outcomes, including ECOG status, MSKCC risk status and bone metastasis, may have prognostic value in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Renal cell carcinoma (RCC) accounts for the majority of renal malignancies, of which 75 % are clear cell carcinomas [1, 2]. For patients with early-stage disease, surgery can be curative [3]. Nearly 30 % of the patients have metastatic RCC (mRCC) at diagnosis [4], and another 25–30 % of the patients subsequently develop metastatic disease following resection with curative intent [5]. The median survival of patients with mRCC is approximately 8 months [6], whereas the overall 5-year survival rate of patients with RCC is less than 10 % [7].
In addition to the high propensity of RCC to metastasize, RCC is also highly resistant to conventional chemotherapy, including cytokine therapy (i.e., interferon and interleukin-2), as well as radiotherapy [6]. Thus, targeted systemic therapies for mRCC, including sunitinib and pazopanib, are now the gold standard for treatment. In addition, phase II and III trials have shown the efficacy of sorafenib (BAY 43–9006), a multi-kinase inhibitor of c-RAF, b-RAF, VEGFR-2 and PDGFR [8], as both a first- and second-line treatment option for patients with mRCC [9–13]. However, most studies assessing the safety and efficacy of sorafenib have been conducted in North America and Europe [13], whereas few studies have assessed the efficacy of sorafenib in Chinese mRCC patients [14].
Given that the expression of small molecule targets can vary in different ethnic groups, resulting in different disease characteristics [15], it is important to assess the safety and efficacy of treatments in different populations, especially given the fact that Zhang et al. [14] reported a higher proportion of hand and foot skin reactions in their study population compared to studies assessing sorafenib in Caucasian populations [10, 11]. Thus, the objective of this single center, single arm retrospective study was to examine the efficacy and safety of sorafenib, as well as its associated prognostic clinico-pathologic factors, in Chinese mRCC patients receiving first-line sorafenib therapy. In addition to overall survival (OS) and progression-free survival (PFS), the occurrence of adverse events (AEs) was also examined.
2 Materials and methods
2.1 Patients
From June 2007 to December 2013, all cases of RCC treated at the Peking Union Medical College Hospital were included for analysis if they met the following inclusion criteria: (1) > 18 years of age, (2) diagnosed with pathologically proven RCC, (3) advanced RCC with foci that could be evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) [16], (4) treated with sorafenib alone without other anti-tumor therapy, and (5) sufficient organ function, which was defined by routine blood tests (i.e., absolute neutrophil count (NAC) ≥ 1500 cells/mm3; platelet count ≥ 75,000 cells/mm3; hemoglobin (Hgb) ≥ 9.0 g/dl), and by analyzing liver and kidney functions (serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5-fold above the upper limit of normal (ULN); total bilirubin ≤ 1.5 ULN; serum creatinine (Scr) ≤ 1.5 ULN). Patients were excluded from the study if they (1) received anti-angiogenic or other anti-tumor therapy (i.e., radiotherapy and chemotherapy), (2) had gastrointestinal dysfunction, including the inability to receive oral medication and requirement for intravenous nutrition, and (3) were pregnant or breastfeeding. This study was approved by the institutional review board of the Peking Union Medical College Hospital, and informed consent was obtained from each patient prior to enrollment in the study.
2.2 Sorafenib treatment protocol
All patients received an initial dose of 400 mg of sorafenib orally twice daily. The dose was subsequently adjusted in the event of toxicity. Patients with grade I and II toxicity continued to receive 400 mg sorafenib orally twice daily. For those with grade III toxicity, the dose of sorafenib was reduced to 400 mg sorafenib daily at 8:00 am and 200 mg sorafenib daily at 16:00 pm. In patients with grade IV toxicity, therapy was initially discontinued and then resumed with 200 mg of sorafenib orally twice daily when the side effects resolved.
2.3 Follow-up
All patients were routinely followed in outpatient care clinics until their death or the end of our analysis at June 30, 2014. No patients were lost at follow-up. The responses were evaluated following RECIST guidelines [16].
2.4 Statistical analyses
Patients’ demographics, baseline characteristics and AEs were summarized as n (%) for categorical variables and median (range: min. to max.) for survival. A Kaplan-Meier survival curve was used to analyze PFS and OS. Moreover, a Cox-regression model was applied to identify the factors associated with PFS and OS. Results were presented as hazard ratios (HRs) with corresponding 95 % confidence intervals (95 % CIs). All statistical assessments were two-tailed, and p-values < 0.05 were considered significant. All statistical analyses were performed using the SAS 9.0 statistical software package (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Patient outcome characteristics after sorafenib treatment
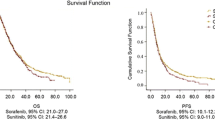
A total of 134 patients (94 males and 40 females) with mRCC that received sorafenib were included for evaluation. The patients’ demographics and baseline characteristics are listed in Table 1. The median age of the patients was 59.8 years (range: 22–82 years). 53 of the patients (39.6 %) died during the study period (Table 1). The median PFS and OS were 10 months (range: 1–36 months) and 22 months (range: 2–37 months), respectively (Fig. 1a and b). As shown in Table 2, 26 (19.40 %) patients exhibited a response to sorafenib treatment. Specifically, two patients (1.49 %) achieved a complete response and 24 patients (17.91 %) achieved a partial response. In addition, 99 patients (73.88 %) showed stable disease and 9 patients (6.72 %) showed disease progression (Table 2).
3.2 Occurrence of adverse events (AEs) after sorafenib treatment
As shown in Table 3, all mRCC patients experienced hand/foot skin reactions after sorafenib treatment. Diarrhea and fatigue were the second and third most commonly observed AEs, occurring in 76.87 % and 30.60 % of the patients, respectively. Among the AEs, 11 patients (8.21 %) showed grades 3 and 4 hand/foot skin reactions. However, only two patients (1.49 %) showed grades 3 and 4 diarrhea, and no patient showed grade 3 fatigue (Table 3). Furthermore, no patient terminated the treatment prematurely due to toxicity.
3.3 Factors associated with survival in mRCC patients treated with sorafenib
Next, we analyzed the patients’ demographics and baseline characteristics associated with PFS and OS by multiple Cox regression analysis. As shown in Fig. 2a, PFS was associated with an Eastern Cooperative Oncology Group (ECOG) [17] performance status of 1 (vs. 0), Memorial Sloan-Kettering Cancer Center (MSKCC) [18] risk groups of 3 (vs. 1), and bone metastasis (vs. no bone metastasis) (all p < 0.05). However, no association was observed between PFS and MSKCC values of 2 (vs. 1). Similarly, OS was associated with MSKCC values of 3 (vs. 1), bone metastasis (vs. no bone metastasis), and sorafenib-induced hypertension (vs. no hypertension) (all p < 0.05; Fig. 2b). Among the 31 patients with non-clear-cell histology, 13 patients (41.9 %) had deceased by the end of the study and 24 patients (77.4 %) showed disease progression. The median OS and PFS times were 20 months (range: 2–70 months) and 9 months (range: 2–36 months), respectively (Fig. S1).
Factors associated with survival in mRCC patients treated with sorafenib. Cox multiple regression analysis of patients’ demographics and baseline characteristics associated with (a) progression-free survival (PFS) and (b) overall survival (OS). HR, hazard ratio; 95 %CI, 95 % confidence interval of HR. * p < 0.05, significantly associated with PFS or OS
4 Discussion
Despite advances in the development of multiple targeted systemic therapies for mRCC, its prognosis remains poor. Nevertheless, the median OS of mRCC patients has increased from 10 months in the era of cytokines to about 30 months in the more recent era of targeted therapies [19]. Although studies have shown the efficacy and safety of sorafenib as both first- and second-line treatment options for mRCC [9–13], only few have specifically analyzed its effects in Chinese patients [14]. In this single center, single arm retrospective study of 134 Chinese mRCC patients, the median PFS and OS were 10 and 22 months, respectively. In addition, sorafenib induced a response in 19.40 % of the patients with stable disease, which was observed in 73.88 % of the cases, and only a few grade 3 or 4 toxicities were noted. Thus, as reported by Zhang et al. [14], sorafenib was found to be both efficacious and well-tolerated by Chinese mRCC patients.
The results of the present study indicate that the median PFS was 10 months. In addition, complete, partial, and stable disease were observed in two (1.49 %), 24 (17.91 %), and 99 (73.88 %) of the patients, respectively. The two patients with a complete response, a 59-year-old male and a 69-year-old female, had clear cell carcinomas, ECOG statuses of 1 and 2 and moderate MSKCC classifications. The outcomes observed in the present study are better than those in previous studies that assessed the efficacy of sorafenib in Caucasian patients [10, 11, 20, 21]. Specifically, among patients previously treated with immunotherapy, those treated with sorafenib had an improved median PFS of 5.5 months compared to 2.8 months in the placebo group [10]. In addition, in patients receiving sorafenib as first-line treatment, the median PFS was 5.7 months as compared to 5.6 months in patients treated with interferon [11, 21]. Our results were similar to a Phase II Japanese study that reported a PFS of 32 weeks with sorafenib [22], but lower than the 60 week median PFS reported in Chinese patients by Zhang et al. [14]. This latter discrepancy may be due to differences in previous treatments received by the patients, since 39 (39.8 %) patients in the study by Zhang et al. received sorafenib as second-line treatment [14]. In addition, 16 (16.3 %) patients in the study by Zhang et al. received concurrent cytokine therapy [14]. Further studies with larger numbers of patients are needed to examine the efficacy as well as tolerability of administering cytokine therapy along with sorafenib as first-line treatment for mRCC. In the ROSORC trial [23] that included 128 Italian patients with mRCC, no difference in PFS was observed between patients receiving sorafenib alone versus those administered sorafenib plus interleukin-2. However, response to combination therapy may differ by ethnicity and, therefore, these observations warrant further analysis.
In mRCC patients treated with sunitinib, hypertension has been found to be associated with clinical outcome [24]. Its relation with ECOG status has previously been associated with disease control following sorafenib treatment in Chinese patients [14], and the latter authors suggested that ECOG status may be reflective of a better tolerance to treatment. Until now, the most frequently used predictive model for patients with mRCC was the MSKCC model developed by Motzer [18], which categorizes patients into favorable-, intermediate-, and poor-risk groups, according to the number of adverse factors, time from diagnosis to start of systemic therapy of less than 1 year, elevated serum LDH level, high corrected serum calcium level, anemia and low performance status. In 2009, Heng et al. [25] confirmed the prognostic factors for OS in patients receiving VEGF-targeted agents and internally validated a model that relies on 4 of the 5 Motzer criteria (i.e., high serum corrected calcium level, low hemoglobin level, low Karnofsky performance score and time from diagnosis to therapy of less than 1 year) in addition to absolute neutrophil and platelet counts greater than ULN and, subsequently, validated these factors in a population-based study in 2013 [26]. In the present study, multivariate analysis revealed that the independent predictive factors for PFS included ECOG status, MSKCC risk status and bone metastasis. Factors associated with OS included MSKCC risk values, bone metastasis and sorafenib-induced hypertension. The association of the MSKCC risk classification with both PFS and OS is similar to that observed in mRCC patients treated with sunitinib and cytokine as first-line therapy [27]. Further studies will be aimed at examining the International mRCC Database Consortium (IMDC) criteria and their association with survival in Chinese mRCC patients treated with sorafenib.
The results of the current study revealed that hand/foot skin reactions, diarrhea and fatigue were the most commonly observed AEs following sorafenib treatment. The treatment was, however, generally well-tolerated as only 13 grade 3 and 4 AEs were observed. These observations are consistent with those reported by Zhang et al. [14] in patients that received sorafenib as first- or second-line treatment, but are in contrast to some studies in which up to 9 % of the patients had to discontinue the treatment due to drug-related toxicity [7, 28]. Of note, all of the patients in the present study experienced low-grade hand/foot skin reactions in response to sorafenib treatment. In a small Japanese study of mRCC patients, this reaction was found to serve as an independent predictive factor for a better clinical outcome as determined by MSKCC risk classification, tumor response, and PFS [29]. In a national registry-based study carried out in the Czech Republic hand/foot skin reaction was, however, not found to be associated with PFS or OS [30], suggesting that this reaction may be an independent predictive factor only in patients with specific ethnicities.
The present study has the following limitations that warrant further discussion. Firstly, this was a single arm, retrospective study of a relatively small cohort of patients from a single center in China, which limits the generalizability of the results and may account for the low toxicity observed. Furthermore, the impact of co-administration of low-dose interferon was not ascertained in the present study. In addition, our study did not address the prognostic value of molecular markers for mRCC. Therefore, a multi-center prospective investigation of conventional clinico-pathological parameters in addition to biomarkers in a large number of mRCC patients receiving sorafenib as first-line therapy is required to confirm the results of the present study.
5 Conclusion
In conclusion, this retrospective study confirms that introduction of sorafenib as first-line therapy in Chinese mRCC patients can lead to favorable disease control with acceptable tolerability. In addition, we identified the prognostic parameters for predicting outcomes in Chinese mRCC patients receiving sorafenib as first-line therapy, which may be useful for defining prognosis in clinical practice.
References
B. Ljungberg, N.C. Cowan, D.C. Hanbury, M. Hora, M.A. Kuczyk, A.S. Merseburger, J.J. Patard, P.F. Mulders, I.C. Sinescu, European Association of Urology Guideline Group, EAU guidelines on renal cell carcinoma: the 2010 update. Eur. Urol. 58, 398–406 (2010)
C.L. Martel, P.N. Lara, Renal cell carcinoma: current status and future directions. Crit. Rev. Oncol. Hematol. 45, 177–190 (2003)
J. Bellmunt, M. Fishman, T. Eisen, D. Quinn, Expert opinion on the use of first-line sorafenib in selected metastatic renal cell carcinoma patients. Expert. Rev. Anticancer. Ther. 10, 825–835 (2010)
H.T. Cohen, F.J. McGovern, Renal-cell carcinoma. N. Engl. J. Med. 353, 2477–2490 (2005)
F. Rasmussen, Metastatic renal cell cancer. Cancer Imaging 13, 374–380 (2013)
B.I. Rini, Stabilization of disease in patients with metastatic renal cell carcinoma using sorafenib. Nat. Clin. Pract. Oncol. 3, 602–603 (2006)
R.J. Motzer, N.H. Bander, D.M. Nanus, Renal-cell carcinoma. N. Engl. J. Med. 335, 865–875 (1996)
K.T. Flaherty, Sorafenib in renal cell carcinoma. Clin. Cancer Res. 13, 747s–752s (2007)
M.J. Ratain, T. Eisen, W.M. Stadler, K.T. Flaherty, S.B. Kaye, G.L. Rosner, M. Gore, A.A. Desai, A. Patnaik, H.Q. Xiong, E. Rowinsky, J.L. Abbruzzese, C. Xia, R. Simantov, B. Schwartz, P.J. O’Dwyer, Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 24, 2505–2512 (2006)
B. Escudier, T. Eisen, W.M. Stadler, C. Szczylik, S. Oudard, M. Siebels, S. Negrier, C. Chevreau, E. Solska, A.A. Desai, F. Rolland, T. Demkow, T.E. Hutson, M. Gore, S. Freeman, B. Schwartz, M. Shan, R. Simantov, R.M. Bukowski, TARGET Study Group, Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007)
B. Escudier, C. Szczylik, T.E. Hutson, T. Demkow, M. Staehler, F. Rolland, S. Negrier, N. Laferriere, U.J. Scheuring, D. Cella, S. Shah, R.M. Bukowski, Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27, 1280–1289 (2009)
C. Guevremont, C. Jeldres, P. Perrotte, P.I. Karakiewicz, Sorafenib in the management of metastatic renal cell carcinoma. Curr. Oncol. 16, S27–32 (2009)
G. Procopio, E. Verzoni, I. Testa, N. Nicolai, R. Salvioni, F. Debraud, Experience with sorafenib in the treatment of advanced renal cell carcinoma. Ther. Adv. Urol. 4, 303–313 (2012)
H. Zhang, B. Dong, J.J. Lu, X. Yao, S. Zhang, B. Dai, Y. Shen, Y. Zhu, D. Ye, Y. Huang, Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer 9, 249 (2009)
H.S. Stafford, S.L. Saltzstein, S. Shimasaki, C. Sanders, T.M. Downs, G.R. Sadler, Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival. J. Urol. 179, 1704–1708 (2008)
E.A. Eisenhauer, P. Therasse, J. Bogaerts, L.H. Schwartz, D. Sargent, R. Ford, J. Dancey, S. Arbuck, S. Gwyther, M. Mooney, L. Rubinstein, L. Shankar, L. Dodd, R. Kaplan, D. Lacombe, J. Verweij, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009)
M.M. Oken, R.H. Creech, D.C. Tormey, J. Horton, T.E. Davis, E.T. McFadden, P.P. Carbone, Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 5, 649–655 (1982)
R.J. Motzer, M. Mazumdar, J. Bacik, W. Berg, A. Amsterdam, J. Ferrara, Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 17, 2530–2540 (1999)
R.J. Motzer, T.E. Hutson, L. McCann, K. Deen, T.K. Choueiri, Overall survival in renal-cell carcinoma with pazopanib versus sunitinib(J). N. Engl. J. Med. 370, 1769–1770 (2014)
A. Mancuso, E.D. Di Paola, A. Leone, A. Catalano, F. Calabrò, L. Cerbone, A. Zivi, C. Messina, S. Alonso, L. Vigna, R. Caristo, C.N. Sternberg, Phase II escalation study of sorafenib in patients with metastatic renal cell carcinoma who have been previously treated with anti-angiogenic treatment. BJU Int. 109, 200–206 (2012)
B. Escudier, Sorafenib for the management of advanced renal cell carcinoma. Expert. Rev. Anticancer. Ther. 11, 825–836 (2011)
H. Akaza, T. Tsukamoto, M. Murai, K. Nakajima, S. Naito, Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn. J. Clin. Oncol. 37, 755–762 (2007)
G. Procopio, E. Verzoni, S. Bracarda, S. Ricci, C. Sacco, L. Ridolfi, C. Porta, R. Miceli, N. Zilembo, E. Bajetta, Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. Br. J. Cancer 104, 1256–1261 (2011)
B.I. Rini, D.P. Cohen, D.R. Lu, I. Chen, S. Hariharan, M.E. Gore, R.A. Figlin, M.S. Baum, R.J. Motzer, Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl. Cancer Inst. 103, 763–773 (2011)
D.Y. Heng, W. Xie, M.M. Regan, M.A. Warren, A.R. Golshayan, C. Sahi, B.J. Eigl, J.D. Ruether, T. Cheng, S. North, P. Venner, J.J. Knox, K.N. Chi, C. Kollmannsberger, D.F. McDermott, W.K. Oh, M.B. Atkins, R.M. Bukowski, B.I. Rini, T.K. Choueiri, Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J. Clin. Oncol. 27, 5794–5799 (2009)
D.Y. Heng, W. Xie, M.M. Regan, L.C. Harshman, G.A. Bjarnason, U.N. Vaishampayan, M. Mackenzie, L. Wood, F. Donskov, M.H. Tan, S.Y. Rha, N. Agarwal, C. Kollmannsberger, B.I. Rini, T.K. Choueiri, External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 14, 141–148 (2013)
S. Patil, R.A. Figlin, T.E. Hutson, M.D. Michaelson, S. Négrier, S.T. Kim, X. Huang, R.J. Motzer, Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann. Oncol. 22, 295–300 (2011)
B.I. Rini, S.C. Campbell, B. Escudier, Renal cell carcinoma. Lancet 373, 1119–1132 (2009)
K. Nakano, K. Komatsu, T. Kubo, S. Natsui, A. Nukui, S. Kurokawa, M. Kobayashi, T. Morita, Hand-foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn. J. Clin. Oncol. 43, 1023–1029 (2013)
A. Poprach, T. Pavlik, B. Melichar, I. Puzanov, L. Dusek, Z. Bortlicek, R. Vyzula, J. Abrahamova, T. Buchler, Czech Renal Cancer Cooperative Group, skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann. Oncol. 23(3137–3143) (2012)
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
mRCC patient survival in those with non-clear cell histology after sorafenib treatment. Kaplan-Meier curves of (A) progression-free survival (PFS) and (B) overall survival (OS) for 31 mRCC patients with non-clear-cell histology after sorafenib treatment. (GIF 20 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, Y., Cai, Y. et al. Efficacy of sorafenib correlates with Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification and bone metastasis in Chinese patients with metastatic renal cell carcinoma. Cell Oncol. 39, 15–21 (2016). https://doi.org/10.1007/s13402-015-0245-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-015-0245-5