Abstract
Purpose
MicroRNAs (miRNAs) may act as oncogenes or tumor suppressor genes and, as such, they may play a role in cancer development. We investigated miR-429 expression levels in a cohort of esophageal carcinomas (EC) to assess its impact on EC cell growth, apoptosis and invasion.
Methods
qRT-PCR assays were used to quantify miR-429 expression levels in 32 paired EC samples and adjacent non-neoplastic tissues. Assays for cell growth, apoptosis, caspase activity and trans-well invasion were used to evaluate the effects of miR-429 expression on EC cells. Luciferase reporter and Western blotting assays were used to test whether the Bcl-2 and specificity protein 1 (SP1) mRNAs serve as major targets of miR-429.
Results
The expression levels of miR-429 in EC tissues were found to be lower than those in adjacent non-neoplastic tissues (P < 0.05). This relatively low expression was found to be significantly associated with the occurrence of lymph node metastases (P < 0.05). Apoptosis and migration rates were found to be significantly higher in two EC-derived cell lines (EC9706 and KYSE30) transfected with a miR-429 agomir (P < 0.05). Subsequent Western blotting and luciferase reporter assays showed that miR-429 can bind to putative binding sites within the Bcl-2 and SP1 mRNA 3′ untranslated regions (UTRs) to reduce their expression.
Conclusions
In primary EC tissues miR-429 is expressed at low levels. Up-regulation of miR-429 inhibits invasion and promotes apoptosis in EC cells by targeting Bcl-2 and SP1. Our findings suggest that Bcl-2 and SP1 may serve as major targets of miR-429. This study paves the way for a better understanding of the mechanism underlying EC pathogenesis and the development of novel, targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
MicroRNAs (miRNAs), typically 18–25 nucleotides long, constitute a class of small non-coding single-stranded RNAs (ssRNAs) that are highly conserved and endogenously expressed across many species. These miRNAs regulate gene expression by targeting complementary sequences that are primarily located within the 3′ untranslated regions (UTRs) of mRNAs [1–6]. Although the full extent of miRNA biological functions have yet to be elucidated, they have been suggested to act as intrinsic regulators of many cellular processes, including cell invasion, differentiation, proliferation and apoptosis [7–12]. Aberrant expression of miRNAs has been linked to the development and progression of several cancers and they have been shown to have prognostic significance for certain types, such as lung and esophageal cancer, neuroblastoma and lymphocytic leukemia [13–16].
Esophageal cancer (EC) is a major cause of cancer-related death worldwide and the fourth most lethal malignant tumor in China. In EC, altered expression of miRNAs has previously been observed [17], indicating that deregulation of miRNAs may play a role in the development of EC. We have previously conducted a miRNA chip-based expression analysis of primary EC tissues and found that the expression of miR-429 in these tissues was lower than that in adjacent paired non-tumor tissues. Previous reports have also shown that miR-429 levels are down-regulated in gastric and breast cancers [18, 19]. Bcl-2 is a well-known inhibitor of apoptosis and plays a key role in anti-apoptotic mechanisms in tumors [20, 21]. Recent studies have shown that the Bcl-2 protein is overexpressed in approximately one-third of esophageal squamous cell carcinomas (ESCCs), as well as in a significant portion of preneoplastic lesions of esophageal cancer, and that Bcl-2 overexpression is associated with a poor tumor differentiation, but not with patients’ survival [22, 23]. SP1 is a member of the SP/KLF (Krüppel-like factor) family of transcription factors [24] and is thought to act as a metastasis promoter [25–29]. SP1 overexpression in tumor tissues has been associated with aggressive disease, poor prognosis and is inversely correlated with survival [30].
As yet, the effects of miR-429 on apoptosis and metastasis of EC cells are unclear. In the present study, we investigated miR-429 expression levels in tumor and normal tissues from 32 EC patients, and assessed concomitant alterations in EC cell apoptosis and metastasis.
2 Materials and methods
2.1 Patient sample collection
EC specimens (n = 32) were obtained from eligible patients between June 2011 and May 2012, from the First Affiliated Hospital of Zhengzhou University (listed in Table 1). None of the patients had received preoperative adjuvant therapy. Postoperative pathological staging was determined for each individual according to the seventh edition of the UICC/AJCC TNM staging system for EC. This study was approved by the ethics committee of Zhengzhou University, and informed consent was obtained from each patient.
2.2 RNA extraction and quantitative real-time PCR
Total RNA was isolated from EC tissue samples and adjacent non-tumor tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. To verify mature miRNA expression levels, quantitative real-time PCR (qRT-PCR) was performed using a High-Specificity miR-429 qRT-PCR Detection Kit (Stratagene Corp, La Jolla, CA) in conjunction with an ABI 7500 thermal cycler, according to the manufacturer’s recommendations. We used U6 small nuclear RNA (U6 snRNA) as an endogenous control for normalization. The qRT-PCR results were expressed relative to miR-429 expression levels at the threshold cycle (Ct) and were converted to fold changes (2−ΔΔCt).
2.3 Cell culture
Human EC cell lines (EC9706 and KYSE30) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Both cell lines were maintained in RPMI 1640 medium supplemented with 10 % fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, USA) and incubated at 37 °C/5 % CO2.
2.4 miRNA transfection
The miR-429 agomir (GMR-miR™ microRNA-429 agomir) used in this study was synthesized by Shanghai GenePharma Co. Ltd. Prior to transfection, EC cell lines EC9706 and KYSE30 were plated at a density of 1.5 × 105 cells/well in 6-well plates. Once cells reached ~50 % confluence, transient transfections were conducted using Lipofectamine™2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Transfection efficiencies were evaluated in every experiment by qRT-PCR at 24 h post-transfection. Cells from each cell line were subdivided into three groups: a non-transfected blank group (Blank), a scrambled miR-429 transfected negative control group (NC) and a miR-429 agomir transfected group (miR-429).
2.5 Cell growth assay
Cells were seeded into a 96-well plate at a density of 1 × 104 cells/well, with five replicate wells per group. The absorbance value for each well was determined using cell counting kit-8 (CCK-8; Dojindo, Japan). The optical density (OD) was measured daily over four consecutive days at a wavelength of 490 nm (OD490) to estimate viable cell numbers.
2.6 Apoptosis assay
Cells from each group were harvested at 48 h post-transfection by trypsinization. Cells were resuspended at a density of 1 × 106 cells/mL in 1 × binding buffer. After double staining with FITC-Annexin V and propidium iodide (PI) using the FITC Annexin V Apoptosis Detection Kit I (BestBio, Shanghai, China), cells were analyzed using a FACScan® flow cytometer (BD Biosciences) equipped with Cell Quest software (BD Biosciences).
2.7 Caspase activity assay
Cells from each treatment group were harvested at 48 h post-transfection and, subsequently, caspase activity was measured using a caspase activity assay kit (Beyotime, Haimen, China). Cellular extracts and substrates (Ac-DEVD-pNA) were kept in 96-well plates for 2 h at 37 °C. Absorbance values were measured using a microplate reader at 405 nm (Infinite M200, Tecan, Switzerland).
2.8 Trans-well assay
The concentration of cells in each group (see under Section 2.4) was adjusted to 2 × 105 cells/mL at 48 h post-transfection. The upper chamber of a 24-well Transwell Permeable Support (Costar, USA) with 8 μm pores was loaded with 200 μL of cell suspension and the lower chamber was supplied with 500 μL of medium containing 10 % FBS, followed by an incubation at 37 °C/5 % CO2 for 48 h. Five wells were used for each group. After the incubation, the medium was removed from the upper chamber and cells in the upper chamber were scraped off with a cotton swab. Cells that had migrated to the other side of the membrane were fixed with methanol, stained with hematoxylin, mounted and dried at 80 °C for 30 min. The number of cells invading the matrigel was counted in three randomly selected fields using an inverted microscope (200× magnification).
2.9 Dual-luciferase assay
The human Bcl-2 3′ UTR (bases 2733–2875) and SP1 3′ UTR (bases 2410–2581) fragments containing putative binding sites for miR-429 were amplified by PCR from human genomic DNA. The mutant Bcl-2 and SP1 3′ UTRs were obtained by overlap extension PCR. The fragments were cloned into a pGL3 reporter vector (Promega), downstream of the luciferase gene, to generate the recombinant vectors pGL3-Bcl-2-wt, pGL3-Bcl-2-mut, pGL3-SP1-wt and pGL3-SP1-mut. For the luciferase reporter assay, EC9706 and KYSE30 cells were transiently co-transfected with miRNA (miR-429 agomir or scrambled-miR-429 negative control) and reporter vectors (wild-type reporter vectors or mutant-type reporter vectors), using LipofectamineTM2000. Luciferase activities were measured using a Dual-Luciferase assay kit (Promega) according to the manufacturer’s instructions at 48 h post-transfection.
2.10 Western blotting
Total proteins of cultured cells were extracted using RIPA buffer containing phenylmethanesulfonylfluoride (PMSF). A BCA protein assay kit (Beyotime, Haimen, China) was used to determine the protein concentrations. Proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes. After blocking, the membranes were incubated overnight at 4 °C with diluted (1:300) primary antibodies (polyclonal rabbit anti-Bcl-2 or polyclonal rabbit anti-SP1; Santa Cruz). Following extensive washing, the membranes were incubated with diluted (1:3000) horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz). Signals were determined using a chemiluminescence detection kit (Amersham Pharmacia Biotech, Piscataway, NJ). An antibody against β-actin (Santa Cruz Biotechnology) served as endogenous reference.
2.11 Statistical analysis
Statistical testing was conducted with the assistance of SPSS 17.0 software. All data are expressed as means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to analyze data. Results were considered significant when P-values were <0.05.
3 Results
3.1 Down-regulation of miR-429 and up-regulation of Bcl-2 and SP1 in EC
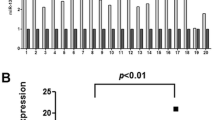
Using adjacent non-tumor tissues as reference, miR-429 expression in EC tissues was found to be significantly reduced (P < 0.05; Fig. 1a; Table 2). We also found that miR-429 expression levels in EC tissues were associated with the occurrence of lymph node metastases, differentiation status and TNM stage (P < 0.05; Fig. 1b, h; Table 2). In contrast, no significant differences were observed between miR-429 expression and either gender, age or tumor location (P >0.05; Table 2). Compared to adjacent non-cancerous tissues, the expression levels of Bcl-2 and SP1 in EC tissues were found to be higher (P < 0.05; Fig. 1c, d, and f; Table 2). Both Bcl-2 and SP1 expression levels in EC tissues were found to be associated with the occurrence of lymph node metastases, differentiation status and TNM stage (P < 0.05; Fig. 1e, g, h; Table 2), and again not by gender, age or tumor location (P > 0.05; Table 2). The expression levels of miR-429, SP1 and Bcl-2 exhibited significant differences in different TNM stages (P < 0.05; Fig. 1h; Table 2).
Expression levels of miR-429, Bcl-2 and SP1 in EC tissues and adjacent non-cancerous tissues. a The relative expression levels of miR-429 in paired EC tissues and adjacent non-tumor tissues were determined by qPCR. A statistically significant difference in expression levels (P < 0.05) was observed. b qPCR results showing that miR-429 expression in the metastasis-positive group was notably higher than in the metastasis-free group (P < 0.05). c Western blot results showing that Bcl-2 and SP1 expression levels in EC tissues were higher than in adjacent non-cancerous tissues. d, f Compared with adjacent non-cancerous tissues, Bcl-2 and SP1 expression levels in EC tissues were significantly higher (P < 0.05). e, g Among EC tissues, expression levels of Bcl-2 and SP1 in cases with lymph nodes positive for metastases were lower than those in cases with lymph nodes negative for metastases (P < 0.05). h The expression levels of miR-429, SP1 and Bcl-2 were significantly different in different TNM stages (P < 0.05). T tumor tissue (n = 32); N adjacent non-tumor tissue (n = 32). * P < 0.05 compared to the control group
3.2 Up-regulation of miR-429 inhibits proliferation in EC9706 and KYSE30 cells
The levels of miR-429, Bcl-2 and SP-1 after transfection of the miR-429 agomir in EC9706 and KYSE30 cells are shown in Fig. 2. Expression levels of miR-429 in the agomir transfected group (miR-429) were found to be significantly higher (P < 0.05) (Fig. 2a) than in the non-transfected blank group (Blank) and the scrambled miR-429 transfected negative control group (NC). Concomitantly, the Bcl-2 and SP-1 expression levels were found to be significantly lower in the miR-429 group compared to the Blank and NC groups (Fig. 2b). The corresponding cell growth curves are presented in Fig. 3. No significant differences were noted in OD450 values between the Blank and NC groups (P > 0.05). However, compared to the Blank and NC groups, the OD490 values for the miR-429 group on days 2, 3 and 4 were significantly decreased (P < 0.05) in both EC9706 (Fig. 3a) and KYSE30 (Fig. 3b) cells.
Expression levels of miR-429, Bcl-2 and SP-1 after transfection in EC9706 and KYSE30 cells were determined using qPCR and Western blot assays. a Expression levels of miR-429 in the three treatment groups: miR-429, cells transfected with miR-429 agomir; NC, cells transfected with scrambled miR-429 negative control; Blank, untransfected cells. *Indicates statistical significance (P < 0.05). b Western blot results showing that Bcl-2 and SP-1 expression levels in the miR-429 group were lower than in the other treatment groups: miR-429, cells transfected with miR-429 agomir; NC, cells transfected with scrambled miR-429 negative control; Blank, untransfected cells. * P < 0.05 compared to the control group
CCK8 assays analyzing the biological effects of exogenous miR-429 agomir expression in EC9706 and KYSE30 cells. a A statistically significant decrease in EC9706 cell proliferation in the miR-429 group was observed compared to the NC group (2 day, P = 0.0191; 3 day, P = 0.0032; 4 day, P = 0.0046). miR-429, cells transfected with miR-429 agomir; NC, cells transfected with scrambled miR-429 negative control; Blank, non-transfected cells. b A statistically significant decrease in KYSE30 cell proliferation in the miR-429 group was seen compared with the NC group (2 day, P = 0.0211; 3 day, = 0.0076; 4 day, P = 0.0059). No significant differences were seen between the Blank and NC groups. (P < 0.05). miR-429, cells transfected with miR-429 agomir; NC, cells transfected with scrambled miR-429 negative control; Blank, non-transfected cells. * P < 0.05 compared to the control group
3.3 Up-regulation of miR-429 induces apoptosis of EC9706 and KYSE30 cells
Our flow cytometry results indicated that the apoptosis levels of cells transfected with the miR-429 agomir were significantly enhanced compared to those of cells in the Blank and NC groups (P < 0.01; Fig. 4a). Similarly, transfection of EC cells with the miR-429 agomir was foud to significantly increase caspase-3/7 activity compared to cells in the Blank and NC groups (P < 0.01; Fig. 4b). From these results we conclude that up-regulation of miR-429 induces apoptosis of EC9706 and KYSE30 cells.
miR-429 agomir induces apoptosis in EC9706 and KYSE30 cells. Blank, non-transfected cells; NC, cells transfected with scrambled miR-429 negative control; miR-429, cells transfected with miR-429 agomir. a Statistically significant increases in the number of apoptotic cells were observed in miR-429 agomir-transfected EC9706 and KYSE30 cells compared to cells in the NC and Blank groups (P < 0.01). b Results from the caspase activity assay showing that transfection with the miR-429 agomir led to a significant increase in caspase-3/7 activity compared to the NC and Blank groups (P < 0.01). * P < 0.05 compared to the control group
3.4 Up-regulation of miR-429 restricts cell invasion in EC9706 and KYSE30 cells
Using a trans-well assay, we found that the mean numbers of cells penetrating the membrane in the Blank and NC groups were not significantly different (P > 0.05; Fig. 5). However, compared to the Blank and NC groups, the mean number of cells penetrating the trans-well membrane was significantly lower in the miR-429 group (P < 0.05). Based on these results we conclude that exogenous over-expression of miR-429 decreases the invasive ability of both EC9706 and KYSE30 cells.
Invasive ability of EC cells after transfection were assessed using trans-well assays. a The number of invasive miR-429-transfected EC9706 cells was significantly lower than in the Blank and NC groups (P < 0.05). Blank, non-transfected cells; NC, cells transfected with scrambled miR-429 negative control; miR-429, cells transfected with miR-429 agomir. b The numbers of miR-429-transfected KYSE30 cells passing through the trans-well membrane were significantly lower compared to those from the Blank and NC groups (P < 0.05). * P < 0.05 compared to the control group
3.5 BCL-2 and SP1 are direct targets of miR-429
Bioinformatic analyses using TargetScan and miRanda predicted that the 3′ UTRs of Bcl-2 and SP1 contain binding sites for miR-429 (Fig. 6a). Subsequent Western blot analysis indeed showed that Bcl-2 and SP1 expression were down-regulated in EC9706 and KYSE30 cells following transfection with the miR-429 agomir (Fig. 6b). To verify whether Bcl-2 and SP1 are direct targets of miR-429, we used a Dual-Luciferase reporter system containing either wild-type or mutant 3′ UTRs of Bcl-2 and SP1, respectively. Co-transfection with miR-429 significantly suppressed the luciferase activity of the reporter containing the wild-type 3′ UTR (Fig. 6c). These results indicate that miR-429 negatively regulates Bcl-2 and SP1 expression by directly binding to putative binding sites in the 3′ UTR.
Bcl-2 and SP1 were identified as targets of miR-429 in EC9706 and KYSE30 cells. a The putative miR-429 binding sequences for the Bcl-2 and SP1 3′ UTRs. b Western blot analysis of Bcl-2 and SP1 expression in transfected cells. β-actin was used as a reference. Blank, non-transfected cells; NC, cells transfected with scrambled miR-429 negative control; miR-429, cells transfected with miR-429 agomir. c Luciferase activity determined 48 h after transfection. Control, scrambled-miRNA; miR-429, miR-429 agomir; wt-Bcl-2, wild-type pGL3-Bcl-2; mut-Bcl-2, mutant pGL3-Bcl-2; wt-SP1, wild-type pGL3-SP1; mut-SP1, mutant pGL3-SP1. * P < 0.05 compared to the control group
3.6 Expression of Bcl-2 restores the pro-apoptotic function of miR-429
Our apoptosis assay indicated that exogenous expression of miR-429 increased cell apoptosis induced by serum starvation (Fig. 7a). Subsequently, we exogenously expressed recombinant Bcl-2 lacking the 3′ UTR sequence (pcDNA3.1-Bcl-2) in EC9706 and KYSE30 cells. Subsequent Western blotting showed that co-transfection of pcDNA3.1-Bcl-2 and miR-429 abrogated the effects of miR-429 on Bcl-2 expression (Fig. 7b). Cells transfected with pcDNA3.1-Bcl-2 alone showed significantly decreased levels of apoptosis (Fig. 7c). When we, however, co-transfected cells with pcDNA3.1-Bcl-2 and miR-429, the expression of Bcl-2 lacking the 3′ UTR sequence was found to override the pro-apoptotic functions of miR-429.
Expression of Bcl-2 abrogates miR-429 pro-apoptotic functions. a Cells were transfected with miR-429, serum starved and, next, the apoptosis rate was determined. Expression of miR-429 significantly increased cell apoptosis. b Western blot analysis of EC cells transfected with pcDNA3.1-Bcl-2 lacking the 3′ UTR sequence, and miR-429. β-actin was used as a reference. c Apoptosis assays conducted on cells transfected with pcDNA3.1-Bcl-2 lacking the 3′ UTR sequence, and miR-429. Data are expressed as the mean ± SD from three independent experiments. * P < 0.05 compared to the control group
3.7 Expression of SP-1 restores miR-429 anti-migration function
We found that co-transfection of the miR-429 agomir and pcDNA3.1-SP-1 led to an increased expression of SP-1, and abrogated the effects of the miR-429 agomir on SP-1 expression (Fig. 8a). In the trans-well assays we found that co-transfection of pcDNA3.1-SP-1 and the miR-429 agomir increased the average number of migrating cells (P < 0.05; Fig. 8b). From these results we conclude that SP-1 expression restores the miR-429 anti-migration function.
Expression of SP-1 abrogates the anti-migratory functions of miR-429. a Transfection with pcDNA3.1-SP-1 and the miR-429 agomir increased SP-1 expression. b Transfection with pcDNA3.1-SP-1 and the miR-429 agomir increased the average number of migrating cells, abrogating the effects of the miR-429 agomir on decreasing cell numbers. Blank, non-transfected cells; NC, cells transfected with scrambled miR-429 negative control; miR-429, cells transfected with miR-429 agomir. * P < 0.05 compared to control
4 Discussion
MicroRNAs have been estimated to regulate up to 30 % of all human genes, and to control a variety of cellular processes [31–33]. Recent studies have also shown that miRNAs are deregulated in various cancers, and that their expression is relevant to the diagnosis and prognosis of a diverse array of tumors [17, 32]. Previous work has indicated that miR-429 is down-regulated in many cancers [18, 19]. These findings nicely coincide with our results, indicating that miR-429 is significantly down-regulated in human EC tissues. It has also been reported that miR-429 is up-regulated in endometrial carcinoma and bladder cancer [34, 35] and that higher expression levels of miR-429 correlate with a poor prognosis in patients with serious ovarian carcinoma [36]. Not only does the expression level of miR-429 differ among cancers, but also its function depends on the context. This variability is possibly due to the fact that miRNAs can down-regulate numerous targets, including oncogenes and tumor suppressor genes.
In the present study we found, for the first time, that miR-429 is down-regulated in human EC tissues. Additionally, we found that altered miR-429 expression levels are associated with the occurrence of lymph node metastases and the differentiation status and TNM stage in EC. Up-regulation of miR-429 in EC9706 and KYSE30 cells was found to suppress the proliferative and invasive capacities of these EC cells, and at the same time to promote apoptosis. The observed aberrant expression of miR-429 suggests the existence of a close correlation with EC development, progression and metastasis. It has been established that miR-429 belongs to the miR-200 family. This miR-200 family has been shown to inhibit the initiation of metastasis, along with the inhibition of epithelial-mesenchymal transition (EMT). This inhibition is accomplished by maintaining the epithelial phenotype through direct targeting of transcriptional repressors of E-cadherin, ZEB1 and ZEB2 [37, 38]. These findings nicely correlate with our results, thereby substantiating the role of miR-429 as a tumor suppressor in EC.
SP1 is a member of the SP/KLF family of transcription factors [24] and is thought to function as a metastasis promoter [25–29]. Previous data indicate that SP1 is over-expressed in breast, thyroid, hepatocellular, pancreatic, colorectal and gastric cancers [39–41]. Lack of SP1 expression was found to significantly inhibit the invasive and metastatic potential of SGC-7901 cells in vitro [42]. In the current study, we confirmed that up-regulation of miR-429 may negatively regulate the expression of SP1. Concomitantly, miR-429 was found to suppress the metastatic and the invasive capacities of the EC cell lines EC9706 and KYSE30. We also showed that SP1 acts as a direct functional target of miR-429 using Western blot, luciferase reporter and rescue assays. Taken together, our findings indicate that miR-429 may act as a metastasis suppressor by targeting SP1.
Bcl-2 is an anti-apoptotic protein that promotes cell survival [43]. Previous studies have confirmed that over-expression of Bcl-2 in cells of various malignant tumors may result in resistance to apoptosis. When Bcl-2 expression is suppressed, tumors are pushed to undergo apoptosis [44]. Bcl-2 has also been related to the early development of EC, with its expression levels increasing as the degree of EC differentiation decreases [45]. Previous research has shown that over-expression of Bcl-2 occurs in various types of human tumors, including EC. In our study, we found that up-regulated miR-429 expression negatively regulates the expression of Bcl-2. Concurrently, apoptosis of EC9706 and KYSE30 cells was found to be enhanced as a result of increased miR-429 expression. In addition, we confirmed that Bcl-2 acts as a target of miR-429 using Western blot and luciferase reporter assays, and verified that miR-429 influences the apoptotic and metastatic capacities of EC. These results indicate that miR-429 likely promotes apoptosis by abrogating the anti-apoptotic Bcl-2 protein.
In conclusion, we have shown that miR-429 is down-regulated in EC. We have also shown that in EC-derived cell lines the up-regulation of miR-429 inhibits proliferative and invasive capacities and promotes apoptosis. Based on these findings, we propose that miR-429 may serve as a therapeutic agent in EC.
References
M. Abdelrahim, R. Smith 3rd, R. Burghardt, Safe S Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 64(18), 6740–6749 (2004)
J. Brennecke, S.M. Cohen, Towards a complete description of the microRNA complement of animal genomes. Genome Biol 4(9), 228 (2003)
G.A. Calin, C.M. Croce, MicroRNA signatures in human cancers. Nat. Rev. Cancer 6(11), 857–866 (2006)
J.A. Chan, A.M. Krichevsky, K.S. Kosik, MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 65(14), 6029–6033 (2005)
W.S. Dynan, R. Tjian, The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell 35(1), 79–87 (1983)
P.C. Enzinger, R.J. Mayer, Esophageal cancer. N. Engl. J. Med. 349(23), 2241–2252 (2003)
M. Esteller, Non coding RNAs in human disease. Nat. Rev. Genet. 12(12), 861–874 (2011)
Y. Gao, Z. Chen, L. Zhang, F. Zhou, S. Shi, X. Feng, B. Li, X. Meng, X. Ma, M. Luo, K. Shao, N. Li, B. Qiu, K. Mitchelson, J. Cheng, J. He, Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma. Cancer Res. 68(1), 26–33 (2008)
Y. Han, J. Chen, X. Zhao, C. Liang, Y. Wang, L. Sun, Z. Jiang, Z. Zhang, R. Yang, J. Chen, Z. Li, A. Tang, X. Li, J. Ye, Z. Guan, Y. Gui, Z. Cai, MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS One 6(3), e18286 (2011)
H. He, K. Jazdzewski, W. Li, S. Liyanarachchi, R. Nagy, S. Volinia, G.A. Calin, C.G. Liu, K. Franssila, S. Suster, R.T. Kloos, C.M. Croce, A. de la Chapelle, The role of microRNA genes in papillary thyroid carcinoma. Proc. Natl. Acad. Sci. U. S. A. 102(52), 19075–19080 (2005)
M.V. Iorio, M. Ferracin, C.G. Liu, A. Veronese, R. Spizzo, S. Sabbioni, E. Magri, M. Pedriali, M. Fabbri, M. Campiglio, S. Ménard, J.P. Palazzo, A. Rosenberg, P. Musiani, S. Volinia, I. Nenci, G.A. Calin, P. Querzoli, M. Negrini, C.M. Croce, MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65(16), 7065–7070 (2005)
M.V. Iorio, R. Visone, L.G. Di, V. Donati, F. Petrocca, P. Casalini, C. Taccioli, S. Volinia, C.G. Liu, H. Alder, G.A. Calin, S. Ménard, C.M. Croce, MicroRNA signatures in human ovarian cancer. Cancer Res. 67(18), 8699–8707 (2007)
R.H. Jensen, M. Tiirikainen, L. You, D. Ginzinger, B. He, K. Uematsu, Z. Xu, P. Treseler, F. McCormick, D.M. Jablons, Genomic alterations in human mesothelioma including high resolution mapping of common regions of DNA loss in chromosome arm 6q. Anticancer Res 23(3B), 2281–2289 (2003)
I.K. Kim, Y.K. Jung, D.Y. Noh, Y.S. Song, C.H. Choi, B.H. Oh, E.S. Masuda, Y.K. Jung, Functional screening of genes suppressing TRAIL-induced apoptosis: distinct inhibitory activities of Bcl-XL and Bcl-2. Br. J. Cancer 88(6), 910–917 (2003)
L.M. Kong, C.G. Liao, F. Fei, X. Guo, J.L. Xing, Z.N. Chen, Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 101(6), 1463–1470 (2010)
A. Kozomara, S. Griffiths Jones, miRBase: integrating microRNA annotation and deep Sequencing data. Nucleic Acids Res. 39, D152–157 (2011)
B.P. Lewis, C.B. Burge, D.P. Bartel, Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 120(1), 15–20 (2005)
J.S. Mattick, I.V. Makunin, Small regulatory RNAs in mammals. Hum. Mol. Genet. 14(Spec No1), R121–R132 (2005)
E.J. Nam, H. Yoon, S.W. Kim, H. Kim, Y.T. Kim, J.H. Kim, J.W. Kim, S. Kim, MicroRNA expression profiles in serous ovarian carcinoma. Clin. Cancer Res 14(9), 2690–2695 (2008)
P.N. Kelly, A. Strasser, The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ 18(9), 1414–1424 (2011)
D.M. Parkin, F. Bray, J. Ferlay, P. Pisani, Global cancer statistics, 2002. CA Cancer J. Clin 55(2), 74–108 (2005)
M. Sarbia, F. Bittinger, R. Porschen, P. Verreet, P. Dutkowski, R. Willers, H.E. Gabbert, bcl-2 expression and prognosis in squamous cell carcinomas of the esophagus. Int. J. Cancer 69, 324–328 (1996)
M. Torzewski, M. Sarbia, H. Heep, P. Dutkowski, R. Willers, H.E. Gabbert, Expression of Bcl-X(L), an antiapoptotic member of the Bcl-2 family, in esophageal squamous cell carcinoma. Clin. Cancer Res 577(4), 577–583 (1998)
D.D. Patel, J.M. Bhatavdekar, P.R. Chikhlikar, Y.V. Patel, N.G. Shah, N. Ghosh, T.P. Suthar, D.B. Balar, Clinical significance of p53, nm23, and bcl-2 in T3-4N1M0 oesophageal carcinoma: an immunohistochemical approach. J. Surg. Oncol 65(2), 111–116 (1997)
S. Safe, M. Abdelrahim, Sp transcription factor family and its role in cancer. Eur. J. Cancer 41(16), 2438–2448 (2005)
A.J. Schetter, S.Y. Leung, J.J. Sohn, K.A. Zanetti, E.D. Bowman, N. Yanaihara, S.T. Yuen, T.L. Chan, D.L. Kwong, G.K. Au, C.G. Liu, G.A. Calin, C.M. Croce, C.C. Harris, MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA 299(4), 425–436 (2008)
R. Schickel, B. Boyerinas, S.M. Park, M.E. Peter, MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene 27(45), 5959–5974 (2008)
Q. Shi, X. Le, J.L. Abbruzzese, Z. Peng, C.N. Qian, H. Tang, Q. Xiong, B. Wang, X.C. Li, K. Xie, Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 61(10), 4143–4154 (2001)
J. Snowdon, X. Zhang, T. Childs, V.A. Tron, H. Feilotter, The microRNA-200 family is upregulated in endometrial carcinoma. PLoS One 6(8), e22828 (2011)
U.T. Sankpal, P. Maliakal, D. Bose, O. Kayaleh, D. Buchholz, R. Basha, Expression of specificity protein transcription factors in pancreaticcancer and their association in prognosis and therapy. Curr. Med. Chem. 19(22), 3779–86 (2012)
T. Sun, C. Wang, J. Xing, D. Wu, MiR-429 modulates the expression of c-myc in human gastric carcinoma cells. Eur. J. Cancer 47(17), 2552–2559 (2011)
G. Suske, E. Bruford, S. Philipsen, Mammalian SP/KLF transcription factors: bring in the family. Genomic 85(5), 551–556 (2005)
R. Nagadia, P. Pandit, W.B. Coman, J. Cooper-White, C. Punyadeera, miRNAs in head and neck cancer revisited. Cell Oncol 36(1), 1–7 (2013)
S.F. Tavazoie, C. Alarcón, T. Oskarsson, D. Padua, Q. Wang, P.D. Bos, W.L. Gerald, J. Massagué, Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451(7175), 147–152 (2008)
J. Tie, D. Fan, Big roles of microRNAs in tumorigenesis and tumor development. Histol. Histopathol 26(10), 1353–1361 (2011)
S. Uhlmann, J.D. Zhang, A. Schwäger, H. Mannsperger, Y. Riazalhosseini, S. Burmester, A. Ward, U. Korf, S. Wiemann, O. Sahin, MiR-200bc/429 cluster targets PLCgamma1 and differentially regulates proliferation and EGF-driven invasion than miR-200a/141 in breast cancer. Oncogene 29(30), 4297–4306 (2010)
M. Van Kouwenhove, M. Kedde, R. Agami, MicroRNA regulation by RNA binding proteins and its implications for cancer. Nat. Rev. Cancer 11(9), 644–656 (2011)
K. Manav, Y. Kang, The emerging role of miR-200 family of microRNAs in epithelialmesenchymal transition and cancer metastasis. RNA Biol. 5(3), 115–119 (2008)
P. Sun-Mi, A.B. Gaur, E. Lengyel, M.E. Peter, The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 22, 894–907 (2008)
L. Wang, D. Wei, S. Huang, Z. Peng, X. Le, T.T. Wu, J. Yao, J. Ajani, K. Xie, Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 9(17), 6371–6380 (2003)
B. Wightman, I. Ha, G. Ruvkun, Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75(5), 855–862 (1993)
N. Yanaihara, N. Caplen, E. Bowman, M. Seike, K. Kumamoto, M. Yi, R.M. Stephens, A. Okamoto, J. Yokota, T. Tanaka, G.A. Calin, C.G. Liu, C.M. Croce, C.C. Harris, Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9(3), 189–198 (2006)
L.Q. Yang, D.C. Fang, R.Q. Wang, S.M. Yang, Effect of NF-κB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J. Gastroenterol 10(1), 22–25 (2004)
J.C. Yao, L. Wang, D. Wei, W. Gong, M. Hassan, T.T. Wu, P. Mansfield, J. Ajani, K. Xie, Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 10(12 Pt 1), 4109–4117 (2004)
P.D. Zamore, B. Haley, Ribo-gnome: the big world of small RNAs. Science 309(5740), 1519–1524 (2005)
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 81272188).
Conflict of interest
The authors have declared that no competing interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Li, M., Zang, W. et al. MiR-429 up-regulation induces apoptosis and suppresses invasion by targeting Bcl-2 and SP-1 in esophageal carcinoma. Cell Oncol. 36, 385–394 (2013). https://doi.org/10.1007/s13402-013-0144-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-013-0144-6