Abstract
Purpose
Recently, the detection of circulating tumor cells (CTCs) in peripheral blood has become an important tool for the non-invasive assessment of micrometastases and to predict clinical outcome. The objective of this study was to investigate if the presence of CTCs in peripheral blood influences the prognosis in colorectal cancer (CRC) patients without distant organ metastases.
Methods
The GCC mRNA and CK20 mRNA levels in peripheral blood and the serum levels of CEA of 92 CRC patients without distant organ metastasis were analyzed by quantitative RT-PCR and ELISA, respectively. Its associations with overall survival (OS) and disease-free survival (DFS) rates were analyzed.
Results
Univariate analyses showed that lower OS and DFS rates were significantly associated with GCC and CK20 mRNA levels, the presence of lymph node metastases, the presence of mesenteric root lymph node metastases, and the presence of tumor emboli in vessels (p < 0.05), but not with CEA levels. Multivariate analyses showed a significant association between 1) OS and GCC mRNA levels and differentiation types and 2) DFS and the presence of tumor emboli in the vessels. Kaplan-Meier curves showed that DFS was significantly associated with the presence of poorly differentiated cells, the presence of mesenteric root lymph node metastases having received prior chemotherapy, and the presence of tumor emboli in vessels.
Conclusion
The detection of CTCs in peripheral blood may be useful for the prediction of clinical outcome in CRC patients without distant organ metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Despite significant progress in reducing mortality rates and improving survival rates in patients, cancer is still the leading cause of death worldwide [1–3]. Colorectal cancer (CRC) is the second leading cause of death in the United States and has a mortality rate of almost 62 % [2]. A major factor for this high mortality rate is the development of metastases from the primary tumor site to distant organs via relatively poorly understood mechanisms. The process of metastasis involves the shedding of tumor cells from the primary mass and their subsequent dissemination into extracellular spaces via the blood stream or the lymphatic system. The presence of these disseminated tumor cells has been suggested to be a prognostic factor after surgical resection of the primary tumor [4, 5].
The detection of circulating tumor cells (CTCs) in peripheral blood has recently emerged as an important tool for the non-invasive assessment of micro-metastases and for the predicon of clinical outcome [6]. Identification of CTCs is primarily done using either nucleic acid based or cytometric approaches [7–9]. Although RT-PCR based assays are extremely sensitive, there has been a recent shift towards the use of the Cell Search System, which is the only FDA-approved cytometric assay to estimate the number of CTCs in whole blood [10, 11]. However, the prognostic relevance of CTCs in CRC patients with early- and middle-stage disease without metastases remains unclear and needs further investigation.
A number of tumor markers, such as guanylyl cyclase (GCC), cytokeratins CK19 and CK20, and carcinoembryonic antigen (CEA), have been shown to be specifically and stably expressed in primary and metastatic CRC cells and, as such, have been used for the efficient detection of CTCs in peripheral blood [12–14]. GCC, is an intestine-specific member of the guanylyl cyclases family [13, 15–18] and has been used to identify occult colorectal micro-metastases in peripheral blood of patients [19, 20]. CK20 is a member of the cytokeratin family of proteins and is expressed in primary colorectal tumors and their metastases [14, 21, 22]. GCC and CK20 mRNA levels in peripheral blood have previously been shown to serve as CTC-associated factors in CRC patients [11, 23] and have been used as tumor markers to detect disseminated tumor cells and occult metastases in peripheral blood of CRC patients, and to improve the early detection of distant organ metastases [11, 24]. However, since some of the markers used to detect CTCs are not cell type-specific, recent studies have suggested that the assessment of a combination of CTC markers could increase the efficiency of CTC detection, as compared to the analysis of single markers [25].
In this study, we investigated if the presence of CTCs in peripheral blood influences the prognosis in CRC patients without distant organ metastases.
2 Methods
2.1 Patient information
We recruited a total of 92 patients with colorectal carcinomas who underwent surgical treatment at the Surgical Department of Colorectal Cancer of the Zhejiang Cancer Hospital, Hangzhou, China between November 2007 and March 2012. Patients with a known second neoplastic disease or benign intestinal tumors were excluded from the study. Routine pathological examinations were used to examine 92 tumor samples from 92 patients. The mean age was 56.4 years (SD = 12.2). The study population comprised 60 (65.2 %) males and 32 (34.8 %) females. There were 24 (26.1 %) colon carcinomas and 68 (73.9 %) rectal carcinomas. Based on the UICC Classification of Colorectal Cancer, 18 patients (19.6 %) were classified as stage I, 34 patients (37.0 %) as stage II and 40 patients (43.5 %) as stage III. Stage II and III patients at risk for metastasis were treated with standard venous or oral chemotherapy regimens. Altogether, 41 patients (44.5 %) received only surgical treatment, while 19 patients (20.7 %) received oral chemotherapy and 32 patients (34.8 %) received venous chemotherapy post-surgery. All study protocols were approved by the Institutional Review Board and informed consent was obtained from all study participants.
2.2 Blood samples and tumor markers
Blood samples were drawn simultaneously for 1) the analysis of serum tumor markers and 2) the determination of GCC and CK20 mRNA levels. Peripheral venous blood was obtained at the time of primary staging, before surgery. The first 2 ml aliquot of blood was discarded in order to minimize the possibility of false-positives and 5 ml of blood was then collected into EDTA-containing vacutainer tubes. All samples were processed within 2 h of collection, immediately stored in cryovials, shock frozen in liquid nitrogen and stored at −80 °C until further processing. CEA was detected using an ARCHITECT i2000 kit (Abbott Diagnostics; Abbott Park, IL, USA) for routine enzyme immunoassays. Based on the manufacturer’s recommendations, CEA levels of >5 ng/ml were considered positive.
2.3 Quantitative real-time RT-PCR detection of CK20 and GCC mRNA
RNA was extracted from peripheral venous blood using Trizol (Invitrogen, USA) according to the manufacturer’s instructions. GCC mRNA and CK20 mRNA were prepared from 2 μg of total RNA using GCC mRNA and CK20 mRNA quantitative PCR kits (Shanghai Jiusheng medical supplies Co. Ltd.) according to the manufacturer’s instructions. Primers were designed as previously described [26]. The primer sequences for GCC mRNA were:
-
upstream primer 5′ TACGGCTCAATCGCCTTGAC 3′;
-
downstream primer 5′ ATCGTAAGGCTAGCCAGTA 3 ′;
-
Taqman probe 5′ -FAM-TCATGCACCGTAACGTAGC-TAMRA-3′.
-
The primer sequences for CK20 mRNA were: upstream primer 5′ CAGGTCAGTGTGGAGGTGGAT 3 ′;
-
downstream primer 5′ TTCGCATGTCACTCAGGATCTT 3 ′;
-
Taqman probe 5′ -FAM-CCGCTCCGGGCACCGATCT-TAMRA-3′.
The PCR amplifications included an initial denaturation step of 2 min at 93 °C followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The concentrations of GCC and CK20 mRNA (unit: gene copy number/μl) were calculated using the formula: A (copy number/μg total RNA) = B (copy number/μl cDNA)/OD260 value of sample RNA x 5/6. Quantitative Real-Time RT-PCR detection was performed using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems; Foster City, CA, U.S.A). We selected a cut-off value of 500 copies for GCC mRNA and CK20 mRNA based on 1) the manufacturer’s instructions and internationally used cut-off levels, 2) previous results, which showed a reaction efficiency of >92 %, a coefficient of variation of <5 %, a linearity of >6 orders of magnitude and a limit of quantification of >25 copies of GCC cRNA [20]. We also calculated the distribution curve (patients versus copy number). The selected 500 copies as the cutoff value for GCC mRNA showed that there were two major groups. In the first group, the highest GCC mRNA copy number was 364 (n = 40 with 0 copies). In the second group, the minimum copy number was 569. The selected 500 copies as the cutoff value for CK20 mRNA also showed that there were two major groups. In the first group, the highest CK 20 mRNA copy number was 362 (n = 45 with 0 copies). In the second group, the minimum copy number was 573 (supplementary Figure 1).
2.4 Follow-up
All patients were followed up by periodic intervals at the Surgical Department of Colorectal Cancer of the Zhejiang Cancer Hospital. This follow-up was performed by letter, telephone and self-comprehensive review, in order to make sure that the patients were alive and to evaluate whether they had developed local recurrences or distant organ metastases. The median follow-up time was 43.3 months (range from 6 to 52 months) and the follow-up was completed on March 30, 2012. Overall survival (OS) was defined as the duration between disease diagnosis and death or until the last follow-up. Disease-free survival (DFS) was defined as the time after surgical treatment during which the patient survived with no signs of the cancer. The end of follow-up was defined as the date at which the patient died due to tumor recurrence or metastasis during the research period. “Censored date” was defined as the date at which the patient died of other causes, or the termination of observation when the patient was still alive.
2.5 Statistical analysis
Subjects’ demographics and clinical characteristics were represented as n (%). Dispersion of GCC mRNA levels, CK20 mRNA levels, and CEA levels were summarized as n (%) for a given subject’s demographics and clinical characteristics. Non-parametric methods such as the Mann–Whitney U test or the Kruskall Wallis test were used to compare ordinal data such as GCC mRNA levels, CK20 mRNA levels, and CEA levels. Univariate Cox-regression model analysis was performed to assess associations of OS and DFS with GCC mRNA levels, CK20 mRNA levels, CEA levels, and the subject’s demographics and clinical characteristics. Variables with a P value <0.2 in univariate Cox-regression model analysis were put into multivariate Cox-regression model analysis equivalent to Backward Stepwise (Conditional LR). Kaplan-Meier curves with Log-rank test were also performed to evaluate OS or DFS for a given GCC mRNA level, CK20 mRNA level, or CEA level. P < 0.05 was considered statistically significant. All statistical analyses were performed using PASW statistics software version 18.0 (SPSS Inc, Chicago, IL, USA).
3 Results
A total of 92 subjects was recruited for this study. Of these, 26 (28.3 %) were younger than 50 years and 66 (71.7 %) were older than 50 years. Patient demographics and associations between clinical characteristics and GCC mRNA levels, CK20 mRNA levels, and CEA levels are listed in Table 1. The observed power was 78.9 % based on a mortality rate equivalent to 3.50 % (2/57) observed for the 57 patients with GCC mRNA with ≤500 GCC mRNA copies and 25.71 % (9/35) observed for the 35 patients with >500 GCC mRNA copies (The type I error probability associated with this test of null hypothesis was 0.05).
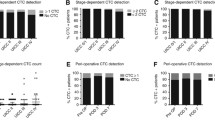
We found a significant association between GCC mRNA and CK20 mRNA levels and the presence of tumor emboli (P = 0.004 and P = 0.039, respectively), while CEA levels were significantly associated with depth infiltration, TNM staging, and lymph node metastasis (P = 0.022, 0.022, and 0.007, respectively).
More than 50 % of the study participants were alive during the follow-up period. The average survival time was 43.3 months (range 6 to 52). Of the remaining patients, 11 patients (12 %) died during the follow-up period. The 6-month, 1-year, 3-year, and 4-year survival rates were 98.9 %, 97.8 %, 89.1 %, and 88 %, respectively (data not shown). We found that OS as well as DFS were significantly associated with GCC mRNA levels and CK20 mRNA levels (both P < 0.05) but not with CEA levels (Table 1).
We used Univariate Cox-regression model analysis to assess associations of OS and DFS with GCC mRNA levels, CK20 mRNA levels, CEA levels, and subjects’ demographics and clinical characteristics (Table 2). Lower OS was significantly associated with high levels of GCC mRNA (HR = 7.92, 95 % CI = 1.71 to 36.70, P = 0.008) and CK20 mRNA (HR = 5.50, 95 % CI = 1.46 to 20.74, P = 0.012). Lower OS was also significantly associated with the presence of mesenteric root lymph node metastases (HR = 6.78, 95 % CI = 1.98 to 23.21, P = 0.002) and the presence of tumor emboli in vessels (HR = 5.08, 95 % CI = 1.48 to 17.36, P = 0.010). DFS was significantly associated with GCC mRNA levels (HR = 2.73, 95 % CI = 1.18 to 6.30, P = 0.019), CK20 mRNA levels (HR = 2.68, 95 % CI = 1.17 to 6.11, P = 0.019), presence of lymph node metastases (HR = 2.74, 95 % CI = 1.16 to 6.46, P = 0.022), presence of mesenteric root lymph node metastases (HR = 3.52, 95 % CI = 1.30 to 9.49, P = 0.013), having received prior chemotherapy (HR = 2.47, 95 % CI = 1.02 to 5.96, P = 0.044), and the presence of tumor emboli in vessels (HR = 5.27, 95 % CI = 2.27 to 12.21, P < 0.001) (Table 2).
Based on previous data [27], we selected variables which had a P value <0.05 in our univariate Cox-regression model analysis, and analyzed them using a multivariate Cox-regression model method equivalent to Backward Stepwise (Conditional LR) analysis, in order to evaluate the combined effects related to OS and DFS. The variables analyzed included GCC mRNA levels, CEA levels, and differentiation type. Although our univariate analysis indicated that CK20 mRNA was associated with OS and DFS, we did not include this in our multivariate analysis because our statistical data showed that OS or DFS exhibited a more significant correlation with GCC mRNA levels and CEA levels than with CK20 mRNA levels.
By doing so, we found a significant association between OS and GCC mRNA levels (HR = 8.68, 95 % CI = 1.88 to 10.62, P = 0.006) and differentiation types (HA = 3.98, 95 % CI = 1.03 to 15.46, P = 0.046) (Table 3). We also observed a significant association between DFS and the presence of tumor emboli in the vessels (HR = 4.15, 95 % CI = 1.69 to 10.17, P = 0.002) (Table 4). Using pair-wise combinations, we found no significant associations between combinations of GCC mRNA, CK20 mRNA or CEA levels with OS or DFS (data not shown).
We used Kaplan Meier survival curves to evaluate the relationship between 4 year OS and DFS in our CRC study group and the following six prognosis related factors; 1) tumor emboli in vessels, 2) peripheral blood GCC mRNA levels, 3) peripheral blood CK20 mRNA levels, 4) differentiation type, 5) mesenteric root lymph node metastases and 6) postoperative chemotherapy (Figs. 1 and 2). A log-rank test showed a significant difference in cumulative survival rates and DFS rates at different GCC mRNA, CK20 mRNA and CEA levels (all P < 0.05).
4 Discussion
In this study, we show that the presence of CTCs in peripheral blood, the presence of tumor emboli in vessels and mesenteric lymph node metastases 1) are all risk factors for metastasis in CRC patients without distant organ metastases and 2) may predict prognosis in these patients. A number of studies have previously assessed the prognostic value of CTCs to guide post-surgery management in colorectal cancer patients, and have used peripheral blood GCC and CK20 mRNA levels to detect CTCs [12, 28]. However, the association between GCC and CK20 mRNA levels and patient survival has not been previously reported.
Dissemination of tumor cells from the primary tumor to distant sites is thought to occur early in the disease process [29]. The efficiency of detection of these CTCs is dependent on sensitive methods using CTC-specific markers [30]. CEA and Carbohydrate Antigen 199 (CA199) have been widely used as serum markers to screen for colorectal cancer and as tumor-specific therapeutic targets [31, 32]. However, although high levels of serum CEA and CA199 often correlate with the development of metastases and poor prognoses after surgical removal of primary colorectal tumors [33, 34], they do not accurately reflect the presence of CTCs in peripheral blood. In this study, we show that there is no significant association between serum CEA levels and 3 year DFS in CRC patients (P >0.05).
Of the different CTC markers, CK20 has been used alone and in combination with other markers, although its use was limited by the fact that 1) it is expressed in the intestinal tract as well as parenteral tissues and 2) its expression failed to show prognostic value in some studies [14, 21, 25, 35]. GCC is expressed in apical membranes of intestinal epithelial cells [13, 19], primary and metastatic colorectal tumors [15, 19, 20] and may, therefore, qualify as an efficacious mucosa-restricted therapeutic target in colorectal cancer [36, 37]. Quantification of GCC mRNA levels in lymph node tissues has previously been used as a means to evaluate metastasis in CRC patients [20]. Based on the fact that CK20 is an epithelial tissue-specific marker and GCC is an intestinal tissue-specific marker, we determined the expression levels of both GCC and CK20 in peripheral blood of CRC patients. Our data are in agreement with previous reports [19, 38] and showed that 33.78 % and 31.08 % of the CRC patients were positive for GCC and CK20 mRNA, respectively. Our data also agreed with previous reports showing that high GCC and CK20 mRNA levels served as risk factors for metastatic progression and as useful predictors for poor prognosis [39]. Survival rates of gastric cancer, pancreatic cancer and CRC patients who were positive for CK20 mRNA have been found to be significantly shorter compared to CK20-negative patients [40]. However, unlike previous reports [34, 41, 42], we found that the combined prognostic power of the GCC and CK20 markers was not superior to the power of either GCC or CK20 alone to predict OS and DFS (supplementary Figure 2). This could be due to 1) the detection methods used or 2) a decreased sensitivity of detection of the GCC/CK20 mRNA combination compared to detection of each marker separately. Our data suggest an advantage of determining the expression of tissue-specific markers combined with tumor-specific markers in order to improve the efficiency of CTC detection in peripheral blood samples.
Our Kaplan Meier survival data agree with previous studies [11, 23, 43] and show that the presence of poorly differentiated cells, mesenteric root lymph node metastases and tumor emboli in vessels are all significantly associated with a reduced DFS. As yet, the association between duration of chemotherapy and survival is not well understood and evaluation of gene expression patterns has been used as a tool to predict prognosis and to identify early-stage patients who may be managed without chemotherapy [44–46]. Our study suggests that middle-stage patients have a worse prognosis than early-stage patients, even if they received reasonable chemotherapy.
In conclusion, our study shows a significant association between 1) GCC mRNA levels and OS and 2) presence of tumor emboli in vessels and DFS. We also show that peripheral blood GCC and CK20 mRNA levels act as independent prognostic factors for 3 year DFS with or without stratified stage analysis. Our data suggest that the detection of CTCs may become a valuable tool in routine examinations to diagnose micrometastases to blood, liver, lung and bone marrow. The advantages of evaluating the presence of intestinal tissue-associated tumor markers such as GCC and CK20 mRNA levels during preoperative diagnosis, intraoperative examination and postoperative follow-up visits are evident. Our data suggest that the detection of CTCs may influence the final tumor staging, determine therapeutic regimens and, most importantly, predict survival.
References
A. Jemal, R. Siegel, E. Ward, Y. Hao, J. Xu, T. Murray, M.J. Thun, Cancer statistics, 2008. CA Cancer J. Clin. 58(2), 71–96 (2008). doi:10.3322/CA.2007.0010
A. Jemal, R. Siegel, J. Xu, E. Ward, Cancer statistics, 2010. CA Cancer J. Clin. 60(5), 277–300 (2010). doi:10.3322/caac.20073
O.W. Brawley, Avoidable cancer deaths globally. CA Cancer J. Clin. 61(2), 67–68 (2011). doi:10.3322/caac.20108
U. Guller, P. Zajac, A. Schnider, B. Bosch, S. Vorburger, M. Zuber, G.C. Spagnoli, D. Oertli, R. Maurer, U. Metzger, F. Harder, M. Heberer, W.R. Marti, Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann. Surg. 236(6), 768–775 (2002). doi:10.1097/01.SLA.0000036267.30107.B9. discussion 775–766
X. Bessa, V. Pinol, S. Castellvi-Bel, E. Piazuelo, A.M. Lacy, J.I. Elizalde, J.M. Pique, A. Castells, Prognostic value of postoperative detection of blood circulating tumor cells in patients with colorectal cancer operated on for cure. Ann. Surg. 237(3), 368–375 (2003). doi:10.1097/01.SLA.0000055223.27623.F3
M.G. Krebs, J.M. Hou, T.H. Ward, F.H. Blackhall, C. Dive, Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther. Adv. Med. Oncol. 2(6), 351–365 (2010). doi:10.1177/1758834010378414
G. Peach, C. Kim, E. Zacharakis, S. Purkayastha, P. Ziprin, Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br. J. Cancer 102(9), 1327–1334 (2010). doi:10.1038/sj.bjc.6605651
S. Riethdorf, H. Wikman, K. Pantel, Review: biological relevance of disseminated tumor cells in cancer patients. Int. J. Cancer 123(9), 1991–2006 (2008). doi:10.1002/ijc.23825
H. Iinuma, K. Okinaga, H. Egami, K. Mimori, N. Hayashi, K. Nishida, M. Adachi, M. Mori, M. Sasako, Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int. J. Oncol. 28(2), 297–306 (2006)
M. Cristofanilli, G.T. Budd, M.J. Ellis, A. Stopeck, J. Matera, M.C. Miller, J.M. Reuben, G.V. Doyle, W.J. Allard, L.W. Terstappen, D.F. Hayes, Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351(8), 781–791 (2004). doi:10.1056/NEJMoa040766
S.J. Cohen, C.J. Punt, N. Iannotti, B.H. Saidman, K.D. Sabbath, N.Y. Gabrail, J. Picus, M. Morse, E. Mitchell, M.C. Miller, G.V. Doyle, H. Tissing, L.W. Terstappen, N.J. Meropol, Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26(19), 3213–3221 (2008). doi:10.1200/JCO.2007.15.8923
J. Friederichs, R. Gertler, R. Rosenberg, M. Dahm, H. Nekarda, B. Holzmann, J.R. Siewert, Correlation of CK-20-positive cells in peripheral venous blood with serum CEA levels in patients with colorectal carcinoma. World J. Surg. 31(12), 2329–2334 (2007). doi:10.1007/s00268-007-9149-5
K.A. Lucas, G.M. Pitari, S. Kazerounian, I. Ruiz-Stewart, J. Park, S. Schulz, K.P. Chepenik, S.A. Waldman, Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 52(3), 375–414 (2000)
A. Gradilone, P. Gazzaniga, I. Silvestri, O. Gandini, L. Trasatti, S. Lauro, L. Frati, A.M. Agliano, Detection of CK19, CK20 and EGFR mRNAs in peripheral blood of carcinoma patients: correlation with clinical stage of disease. Oncol. Rep. 10(1), 217–222 (2003)
R. Birbe, J.P. Palazzo, R. Walters, D. Weinberg, S. Schulz, S.A. Waldman, Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum. Pathol. 36(2), 170–179 (2005). doi:10.1016/j.humpath.2004.12.002
S.L. Carrithers, M.T. Barber, S. Biswas, S.J. Parkinson, P.K. Park, S.D. Goldstein, S.A. Waldman, Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc. Natl. Acad. Sci. U. S. A. 93(25), 14827–14832 (1996)
G. Chen, C.M. McIver, M. Texler, J.M. Lloyd, N. Rieger, P.J. Hewett, D. Sen Wan, J.E. Hardingham, Detection of occult metastasis in lymph nodes from colorectal cancer patients: a multiple-marker reverse transcriptase-polymerase chain reaction study. Dis. Colon Rectum 47(5), 679–686 (2004). doi:10.1007/s10350-003-0118-2
K.A. Steinbrecher, E.A. Mann, R.A. Giannella, M.B. Cohen, Increases in guanylin and uroguanylin in a mouse model of osmotic diarrhea are guanylate cyclase C-independent. Gastroenterology 121(5), 1191–1202 (2001)
G.S. Frick, G.M. Pitari, D.S. Weinberg, T. Hyslop, S. Schulz, S.A. Waldman, Guanylyl cyclase C: a molecular marker for staging and postoperative surveillance of patients with colorectal cancer. Expert. Rev. Mol. Diagn. 5(5), 701–713 (2005). doi:10.1586/14737159.5.5.701
S. Schulz, T. Hyslop, J. Haaf, C. Bonaccorso, K. Nielsen, M.E. Witek, R. Birbe, J. Palazzo, D. Weinberg, S.A. Waldman, A validated quantitative assay to detect occult micrometastases by reverse transcriptase-polymerase chain reaction of guanylyl cyclase C in patients with colorectal cancer. Clin. Cancer Res. 12(15), 4545–4552 (2006). doi:10.1158/1078-0432.CCR-06-0865
S.A. Bustin, V.G. Gyselman, S. Siddiqi, S. Dorudi, Cytokeratin 20 is not a tissue-specific marker for the detection of malignant epithelial cells in the blood of colorectal cancer patients. Int. J. Surg. Investig. 2(1), 49–57 (2000)
S.J. Cohen, R.K. Alpaugh, S. Gross, S.M. O’Hara, D.A. Smirnov, L.W. Terstappen, W.J. Allard, M. Bilbee, J.D. Cheng, J.P. Hoffman, N.L. Lewis, A. Pellegrino, A. Rogatko, E. Sigurdson, H. Wang, J.C. Watson, L.M. Weiner, N.J. Meropol, Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 6(2), 125–132 (2006). doi:10.3816/CCC.2006.n.029
T.A. Fava, R. Desnoyers, S. Schulz, J. Park, D. Weinberg, E. Mitchell, S.A. Waldman, Ectopic expression of guanylyl cyclase C in CD34+ progenitor cells in peripheral blood. J. Clin. Oncol. 19(19), 3951–3959 (2001)
R. Solmi, P. De Sanctis, C. Zucchini, G. Ugolini, G. Rosati, M. Del Governatore, D. Coppola, T.J. Yeatman, L. Lenzi, A. Caira, S. Zanotti, M. Taffurelli, P. Carinci, L. Valvassori, P. Strippoli, Search for epithelial-specific mRNAs in peripheral blood of patients with colon cancer by RT-PCR. Int. J. Oncol. 25(4), 1049–1056 (2004)
A. Gervasoni, R.M. Monasterio Munoz, G.S. Wengler, A. Rizzi, A. Zaniboni, O. Parolini, Molecular signature detection of circulating tumor cells using a panel of selected genes. Cancer Lett. 263(2), 267–279 (2008). doi:10.1016/j.canlet.2008.01.003
U.Y. Li, D.-C. Li, J.-G. Feng, Detection of the expression of CK19, CK20 mRNA in peripheral blood of colorectal cancer patients by fluorescence quantitative polymerase chain reaction. Chin. J. Exp. Surg. 21(7), 3 (2004)
A. Kilic, J.V. Conte, A.S. Shah, D.D. Yuh, Orthotopic heart transplantation in patients with metabolic risk factors. Ann. Thorac. Surg. 93(3), 718–724 (2012). doi:10.1016/j.athoracsur.2011.11.054
G. Khair, J.R. Monson, J. Greenman, Epithelial molecular markers in the peripheral blood of patients with colorectal cancer. Dis. Colon Rectum 50(8), 1188–1203 (2007). doi:10.1007/s10350-006-0875-9
C. Wittekind, M. Neid, Cancer invasion and metastasis. Oncology 69(Suppl 1), 14–16 (2005). doi:10.1159/000086626
M.S. Wicha, D.F. Hayes, Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J. Clin. Oncol. 29(12), 1508–1511 (2011). doi:10.1200/JCO.2010.34.0026
R.C. Bast Jr., P. Ravdin, D.F. Hayes, S. Bates, H. Fritsche Jr., J.M. Jessup, N. Kemeny, G.Y. Locker, R.G. Mennel, M.R. Somerfield, 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J. Clin. Oncol. 19(6), 1865–1878 (2001)
P.D. Khare, L. Shao-Xi, M. Kuroki, Y. Hirose, F. Arakawa, K. Nakamura, Y. Tomita, Specifically targeted killing of carcinoembryonic antigen (CEA)-expressing cells by a retroviral vector displaying single-chain variable fragmented antibody to CEA and carrying the gene for inducible nitric oxide synthase. Cancer Res. 61(1), 370–375 (2001)
M. Basbug, Z. Arikanoglu, N. Bulbuller, Z. Cetinkaya, E. Aygen, S. Akbulut, O. Satici, Prognostic value of preoperative CEA and CA 19–9 levels in patients with colorectal cancer. Hepatogastroenterology 58(106), 400–405 (2011)
H. Iinuma, T. Watanabe, K. Mimori, M. Adachi, N. Hayashi, J. Tamura, K. Matsuda, R. Fukushima, K. Okinaga, M. Sasako, M. Mori, Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J. Clin. Oncol. 29(12), 1547–1555 (2011). doi:10.1200/JCO.2010.30.5151
S.A. Bustin, V.G. Gyselman, N.S. Williams, S. Dorudi, Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br. J. Cancer 79(11–12), 1813–1820 (1999). doi:10.1038/sj.bjc.6690289
P. Li, S.A. Waldman, Corruption of homeostatic mechanisms in the guanylyl cyclase C signaling pathway underlying colorectal tumorigenesis. Cancer Biol. Ther. 10(3), 211–218 (2010)
J.E. Lin, P. Li, G.M. Pitari, S. Schulz, S.A. Waldman, Guanylyl cyclase C in colorectal cancer: susceptibility gene and potential therapeutic target. Future Oncol. 5(4), 509–522 (2009). doi:10.2217/fon.09.14
S.L. Kong, M. Salto-Tellez, A.P. Leong, Y.H. Chan, E.S. Koay, Discordant quantitative detection of putative biomarkers in nodal micrometastases of colorectal cancer: biological and clinical implications. J. Clin. Pathol. 58(8), 839–844 (2005). doi:10.1136/jcp. 2004.023853
S. Braun, B. Naume, Circulating and disseminated tumor cells. J. Clin. Oncol. 23(8), 1623–1626 (2005). doi:10.1200/JCO.2005.10.073
E. Soeth, I. Vogel, C. Roder, H. Juhl, J. Marxsen, U. Kruger, D. Henne-Bruns, B. Kremer, H. Kalthoff, Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res. 57(15), 3106–3110 (1997)
N.J. Shimwell, W. Wei, S. Wilson, M.J. Wakelam, T. Ismail, T. Iqbal, P.J. Johnson, A. Martin, D.G. Ward, Assessment of novel combinations of biomarkers for the detection of colorectal cancer. Cancer Biomark. 7(3), 123–132 (2010). doi:10.3233/CBM-2010-0155
M. Conzelmann, U. Linnemann, M.R. Berger, Detection of disseminated tumour cells in the liver of colorectal cancer patients. Eur. J. Surg. Oncol. 31(1), 38–44 (2005). doi:10.1016/j.ejso.2004.09.005
L.R. Jiao, C. Apostolopoulos, J. Jacob, R. Szydlo, N. Johnson, N. Tsim, N.A. Habib, R.C. Coombes, J. Stebbing, Unique localization of circulating tumor cells in patients with hepatic metastases. J. Clin. Oncol. 27(36), 6160–6165 (2009). doi:10.1200/JCO.2009.24.5837
L. Reggiani Bonetti, C. Di Gregorio, C. De Gaetani, A. Pezzi, G. Barresi, V. Barresi, L. Roncucci, M. Ponz de Leon, Lymph node micrometastasis and survival of patients with Stage I (Dukes’ A) colorectal carcinoma. Scand. J. Gastroenterol. 46(7–8), 881–886 (2011). doi:10.3109/00365521.2011.571708
R. Salazar, P. Roepman, G. Capella, V. Moreno, I. Simon, C. Dreezen, A. Lopez-Doriga, C. Santos, C. Marijnen, J. Westerga, S. Bruin, D. Kerr, P. Kuppen, C. van de Velde, H. Morreau, L. Van Velthuysen, A.M. Glas, L.J. Van’t Veer, R. Tollenaar, Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J. Clin. Oncol. 29(1), 17–24 (2011). doi:10.1200/JCO.2010.30.1077
A.I. Neugut, M. Matasar, X. Wang, R. McBride, J.S. Jacobson, W.Y. Tsai, V.R. Grann, D.L. Hershman, Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J. Clin. Oncol. 24(15), 2368–2375 (2006). doi:10.1200/JCO.2005.04.5005
Acknowledgments
This work was funded by a grant of the Medicine and Health Science Research Fund of Zhejiang Province (No 2008B021)
Competing interesting
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Calculation of the distribution (patients versus copy number) of GCC mRNA levels and CK20 mRNA levels. (A) distribution of GCC mRNA levels and (B) distribution of CK20 mRNA levels. (JPEG 20 kb)
Supplementary Figure 2
Kaplan-Meier curve of OS time (A) and DFS time (B) for combining GCC mRNA and CK20 mRNA levels, that group1 [GCCmRNA(−), CK20mRNA(−)], group2 [GCCmRNA(−), CK20mRNA(+)], group3 [GCCmRNA(+), CK20mRNA(−)], group4 [GCCmRNA(+),CK20mRNA(+)]. GCCmRNA(−): GCCmRNA ≤ 500 copies; GCCmRNA(+): GCCmRNA > 500 copies; CK20mRNA(−): CK20mRNA ≤ 500 copies; CK20mRNA(+): CK20mRNA > 500 copies. (A) The mortality rates for group 1, 2, 3, and 4 were derived as 4.2 % (2/48), 7.7 % (1/13), 0 % (0/10), and 38.1 % (8/21), respectively. The Log-rank test shows that the OS rates were significantly different among groups (P < .001). (B) The DFS rates for group 1, 2, 3, and 4 were derived as 14.6 % (4/48), 23.1 % (3/13), 20 % (2/20), and 52.4 % (11/21), respectively. The Log-rank test shows the DFS rates were significantly different among groups (P = 0.027). Kaplan-Meier curve of OS time (A) and DFS time (B) for combining GCCmRNA and CK20mRNA levels, that group1 [GCCmRNA(−), CK20mRNA(−)], group2 [GCCmRNA(−), CK20mRNA(+)], group3 [GCCmRNA(+), CK20mRNA(−)], group4 [GCCmRNA(+), CK20mRNA(+)]. GCCmRNA(−): GCCmRNA ≤ 500 copies; GCCmRNA(+): GCCmRNA > 500 copies; CK20mRNA(−): CK20mRNA ≤ 500 copies; CK20mRNA(+): CK20mRNA > 500 copies. (A) The mortality rates for group 1, 2, 3, and 4 were derived as 4.2 % (2/48), 7.7 % (1/13), 0 % (0/10), and 38.1 % (8/21), respectively. The Log-rank test shows that the OS rates were significantly different among groups (P < .001). (B) The DFS rates for group 1, 2, 3, and 4 were derived as 14.6 % (4/48), 23.1 % (3/13), 20 % (2/20), and 52.4 % (11/21), respectively. The Log-rank test shows that the DFS rates were significantly different among groups (P = 0.027). (JPEG 29 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Qian, J., Feng, JG. et al. Detection of circulating tumor cells in peripheral blood of colorectal cancer patients without distant organ metastases. Cell Oncol. 36, 43–53 (2013). https://doi.org/10.1007/s13402-012-0112-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-012-0112-6