Abstract
Background
It has recently been found that 5-lipoxygenase (5-LO) and cytochrome P450-2J2 (CYP2J2), molecules capable of arachidonic acid (AA) metabolism, might promote cancer cell viability through several mechanisms similar to those of cyclooxygenase-2 (COX-2). We found that not only COX-2 expression, but also the expression of 5-LO and CYP2J2 is up-regulated in head and neck squamous cell carcinoma (HNSCC) cell lines. From these observations, we hypothesized that AA metabolism by 5-LO and/or CYP2J2 may lower the efficacy of anti-cancer effect by COX-2 inhibition.
Methods and Results
Although COX-2 was highly expressed in all cell lines tested, COX-2-specific inhibition showed little growth-inhibitory effect in some cell lines. Inhibition of COX-2 resulted in increased production of LTB4 and 14-15-DHET/EET, metabolites of 5-LO and CYP2J2, respectively. Combined knock-down of COX-2 and 5-LO or CYP2J2 by siRNA results in a decrease in cell proliferation and VEGF production. Furthermore, these results are dependent on 5-LO and CYP2J2 expression in cells.
Conclusion
Therefore, combined inhibition of COX-2 and 5-LO or CYP2J2 may be one way to overcome low efficacy of single inhibition of COX-2 in cancer cells. In addition, combined therapies should be chosen based on the expression of members of other AA metabolism pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Biologically active metabolites of arachidonic acid (AA) are emerging as key regulators of cancer cell growth and inflammation [1]. Agents that specifically target these lipid mediators are being intensively investigated as potential anticancer drugs. In response to certain stimuli AA is released from membrane phospholipids, and free AA is subsequently metabolized by three major enzymatic pathways: the cyclooxygenase, lipoxygenase and cytochrome P450 epoxygenase-dependent pathways [2].
Cyclooxygenase-2(COX-2)-induced-prostaglandins (PGs) promote carcinogenesis through up-regulation of cell proliferation, metastasis and angiogensis as well as inhibition of apoptosis and anti-cancer immunity [3, 4]. In addition to PGs by COX-2, 5-lipoxygenase (5-LO) metabolites (i.e. 5-HETE, LTB4 and CysLTs) and cytochrome P450-2J2 (CYP2J2) metabolites (i.e. EETs) have been recently reported to be related to carcinogenesis in some types of cancers. These metabolites increase cell viability through binding to novel receptors and stimulating related signal transduction pathways [5, 6]. Interestingly, 5-LO and CYP2J2 appear to have similar mechanisms to COX-2 in the regulation of cell viability, although they often utilize different signaling pathways. Furthermore, it was suggested that AA might be shunted from one pathway to the other when a particular pathway is inhibited in the cellular processes of cancer and inflammation [7–9].
From these findings, we can hypothesize that if COX-2 and other AA metabolites are highly expressed simultaneously inside the same cancer cell, combined inhibition of these pathways would likely be a more effective anti-cancer modality with less side-effect. Some groups have suggested the synergistic anti-cancer effects of combinatorial inhibition of COX-2 and 5-LO in some types of cancer [7, 8]. In this study, we suggest the possibility that COX-2 inhibition might fail to show potent anti-cancer effects, even in those cancer cells types in which COX-2 has been shown to play a carcinogenic role. It is possible that the shunting of AA between COX-2 and other pathways that utilize AA may bypass COX-2 inhibition. We addressed the interaction between the COX-2 pathway and the 5-LO and CYP2J2 pathways. By verifying our hypothesis, we expect to expand the range of cancer models applied to COX-2-targeted molecular therapy for cancer treatment.
2 Materials & methods
2.1 Cell culture
SNU-1041, 1066 and 1076 cells (human HNSCC cell lines) were obtained from the Korean Cell Line Bank (Seoul National University, Seoul, Korea), while PCI-1, 13 and 50 (human HNSCC cell lines) were obtained from the Pittsburgh Cancer Institute (University of Pittsburgh, PA) [10]. Cells were maintained at 37°C in a humidified, 5% CO2, 95% air atmosphere and routinely sub-cultured using trypsin-EDTA.
2.2 Reagents
NS-398 (COX-2 specific inhibitor) and REV5901 (5-LO specific inhibitor) were obtained from Cayman Chemical (Ann Arbor, MI). All chemicals were pre-tested and used according to the provided suggestions (IC50 from manufacturer’s information).
2.3 RNA extraction and RT-PCR analysis
Total RNA was extracted from cell lines using Trizol RNA extraction reagent (Life Technologies, Gaithersberg, MD) according to the manufacturer’s protocol. Total RNA (1 μg) was used to synthesize cDNA with Moloney murine leukemia virus reverse transcriptase (Perkin Elmer Corp., Foster City, CA) and random hexamers. The entire cDNA reaction mixture was used for PCR amplification according to manufacturer’s protocol. The sequence of primers and conditions of amplification were previous reported [10–12]. Amplification was performed using a Perkin-Elmer GeneAmp PCR System 9600.
2.4 Western blot analysis
Denatured protein lysates were resolved by 4–12% NuPAGE gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes (Schleicher & Schuell, Dachen, Germany). The membranes were incubated with anti-COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-5-LO (BD, Franklin Lakes, NJ) or anti-CYP2J2 (Abcam Inc., Cambridge, UK) for 2 h at room temperature or overnight at 4°C. Membranes were then washed (4 times) with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibody (Pierce, Rockford, IL) for 1 h. Immunoreactive proteins were visualized by developing with Lumi-light western blotting substrate (Roche Diagnostics GmbH, Mannheim, Germany) followed by exposure in a LAS-3000 (Fuji Film Co., Tokyo, Japan) according to the manufacturer’s instructions.
2.5 Transfection of siRNA
Individual siRNAs against COX-2 (D-004557-04), 5-LO (L-004530-00), CYP2J2 (L-008208-01) and non-targeting control (D-001210-01) were obtained from Dharmacon RNA Technologies (Lafayette, CO). The best conditions of siRNAs application (used doses and treatment time) were established beforehand by western blotting and EIA (Supplementary material-1 and 2). Cells were plated in 6-, 12- or 24-well plates and grown to 50–70% confluence. After 24 h, the cells were transfected with siRNA (100–200 nM) using Lipofectamine-2000 reagent (Invitrogen) for 48 h according to the manufacturer’s instructions.
2.6 Quantification of PGE2, LTB4, 14,15-DHET/EET and VEGF production
The amount of the desired factor released by the cells was determined using PGE2, LTB4 (Cayman Chemical, Ann Arbor, MI), 14,15-DHET/EET (Detroit R&D Inc., Detroit, MI) and VEGF enzyme immunoassay kits (EIA) (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
2.7 Cell proliferation assay
Cells were seeded in 96-well plates and incubated for 24 h at 37°C prior to treatment with specific drugs or siRNAs for the indicated time. After treatment, Cell Counting Kit-8 (Dojindo Lab., Tokyo, Japan) was used to measure cell proliferation according to the manufacturer’s instructions.
2.8 Transfection of COX-2, 5LO and CYP2J2 expressing plasmids
COX-2 cDNA was a gift from Dr. William L. Smith (Department of Biological Chemistry, University of Michigan), 5-LO from Dr. Thomas G. Brock (Department of Internal Medicine, University of Michigan Health System) and CYP2J2 from laboratory of Darryl C. Zeldin (Environmental Cardiopulmonary Disease Group, National Institute of Environmental Health Sciences-National Institute of Health). Using each cDNA, we established three pcDNA3.1 expressing vectors (pcDNA3.1-COX2, -5-LO and -CYP2J2). Expression and activity of expression plasmids was confirmed by western blotting and EIA, respectively. Cells were co-transfected with 0.5–1.5 μg of plasmid and 100–200 nM siRNAs against COX-2 or 5-LO or CYP2J2 using Lipofectamine-plus according to the manufacturer’s instructions (Life Technologies). After 4 h, medium containing 10% FBS was added and the cells were incubated for an additional 24–72 h.
2.9 Statistical analysis
Data are presented as the mean ± standard deviation (SD) of triplicates, or as a representative of 3 separate experiments. Statistical analysis was performed using one-way ANOVA analysis. In the case of a significant result in the ANOVA, Student’s t-test was used for dose–response results and Dunnett’s post-test for all other experiments. A P value less than 0.05 was considered statistically significant.
3 Results
3.1 The expression of COXs, LOs and CYPs in head and neck cancer cells.
In addition to COX-2, several factors known to be related to AA metabolism have been previously shown to be expressed in cancer cells, including 3 subtypes of LOs and 3 subtypes of CYPs [6, 13]. Therefore, we investigated whether the anti-cancer effect of COX-2 inhibition is related to the degree of expression of these other factors related to AA metabolism in HNSCC cells. In HNSCC, we sometimes observed that COX-2 inhibition showed little anti-cancer effects even if the tested cancer cells had high COX-2 activity and expression of the related downstream molecules (terminal PGs synthases and their receptors) and there is no previous suggestion on the abnormal expression and carcinogenic actions of LOs and CYP epoxygenases. Expression of COX-2, 5-LO and CYP2J2 was determined in several HNSCC cell lines by RT-PCR (Fig. 1a) and western blotting (Fig. 1b). In HNSCC cell lines, 5-LO as well as COX-2 was frequently up-regulated, however, the expression of CYP2J2 appeared to be low except for that in PCI-1 and PCI-50. Cell lines 1041, 1076 and 50 were chosen for further analysis of the interaction between COX-2 and 5-LO or CYP2J2.
Expression of COXs, LOs and CYPs in 6 head and neck cancer cells. The expression of various genes related to AA pathways was determined for the indicated HNSCC cell lines by PCR a and western blotting b with the indicated primers and antibodies, respectively. GAPDH and α-tubulin were used for loading control in PCR reactions and western blotting
3.2 Production of LTB4 and 14,15-DHET/EET is increased with inhibition of COX-2
We next investigated whether AA is shunted from the COX-2 pathway to 5-LO or CYP2J2 pathways upon inhibition of COX-2. We performed EIA assays for LTB4 or 14,15-DHET/EET, which are products synthesized by 5-LO and CYP2J2-epoxygenase, respectively. Both NS-398, a COX-2 specific inhibitor, and siRNA against COX-2 prevented COX-2-mediated PGE2 production (Fig. 2a). Both treatments resulted in increased 5-LO activity by 40–150% in all HNSCC tested (Fig. 2b). However, inhibition of COX2 resulted in a moderate increase in CYP2J2 activity in only one cell line, PCI-50 with detectable CYP2J2 protein and 14,15-DHET/EET (Fig. 2c).
Increased production of LTB4 and 14,15-DHET/EET by inhibition of COX-2. Cells were treated with NS-398, a COX-2 inhibitor, at the indicated doses (μM) for 48 h and COX-2 siRNA (100nM) was transfected at the indicated doses for 48 h. The siNC was used for siRNA negative control. PGE2 a, LTB4 b and 14,15-DHET/EET c level were determined using EIA. Results are expressed as percentage relative to control (% of control) (*, P < 0.05)
3.3 Improved anti-cancer effects with combined inhibition of COX-2 and 5-LO or CYP2J2
Given that inhibition of COX-2 results in a dramatic increase in 5-LO activity and a moderate increase in CYP2J2 activity, we next investigated whether combinational treatment might show synergistic anti-proliferative effects on cancer cell growth. Three different cell lines were treated with both COX-2 and 5-LO inhibitors (Fig. 3a) or siRNA against COX-2 and 5-LO (Fig. 3b). These inhibitions prevented PGE2 and LTB4 synthesis effectively. Inhibition of 5-LO enhanced the anti-proliferative effect of COX-2 inhibition by 20–40%, suggesting a role of 5-LO in cell proliferation of HNSCC (Fig. 4a and b). In contrast, co-inhibition of COX-2 and CYP2J2 showed little enhancement of anti-cancer effects (data not shown). We likely do not observe the enhanced effect with CYP2J2 and COX2 inhibition because the expression of CYP2J2 was low in these cells compared to 5-LO and COX-2 expression (Fig. 1a).
The inhibition of PGE2 and LTB4 production by COX-2 and 5-LO (co)inhibition in PCI-50. Cells were treated with singly or combined with NS-398 (a COX-2 inhibitor) and REV5907 (a 5-LO inhibitor) with the indicated concentrations (μM). At 48 h, cells were subjected to PGE2 and LTB4 EIA a. The siRNAs of COX-2 and 5-LO were transfected at 100 nM doses. The siNC was used for siRNA control. Each sample was subjected to PGE2 and LTB4 EIA b at 48 h. Results are expressed as percentage relative to control (% of control) (*, P < 0.05)
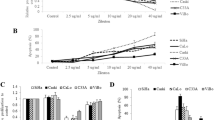
Improved anti-cancer effect with combined inhibition of COX-2 and 5-LO. Cells were treated with singly or combined with NS-398 (a COX-2 inhibitor) and REV5907 (a 5-LO inhibitor) with the indicated concentrations (μM). At 72 h, cells were subjected to cell proliferation assays a. Differences in cell proliferation after exposure to NS-398 and REV5907 separately and to their combination were determined using the one-way ANOVA test (*, P < 0.05). The siRNAs of COX-2 and 5-LO were transfected at 100 nM doses. The siNC was used for siRNA control. Each sample was subjected to cell proliferation assay b at 72 h and to VEGF EIA c at 48 h. Results are expressed as percentage relative to control (% of control). Differences in EIA after exposure to siCOX-2 and si5-LO separately and to their combination were determined using the one-way ANOVA test (**, P < 0.05)
3.4 Improved prevention of VEGF production with combined inhibition of COX-2 and 5-LO or CYP2J2
Because 5-LO as well as COX-2 is known to be related to the expression of VEGF (a key pro-angiogenic factor) in various types of cancers [13], we investigated whether combined inhibition of COX-2 and 5-LO might result in synergistic inhibition of VEGF synthesis. Cells were treated with both COX-2 and 5-LO siRNAs and VEGF production was determined (Fig. 4c). Inhibition of 5-LO enhanced the effect of COX-2 inhibition on VEGF production by 20–40%, suggesting a role of 5-LO in VEGF synthesis in HNSCC. In agreement with the cell growth studies, inhibition of CYP2J2 did not enhance the effect of COX-2 inhibition on VEGF synthesis (data not shown).
3.5 Increased production of LTB4 and 14,15-DHET/EET with COX-2 inhibition in cells transfected with plasmids expressing COX-2 and 5-LO or CYP2J2
From the above experiments, we have determined that shunting of AA to 5-LO and CYP2J2 is possible when COX-2 is inhibited in HNSCC. Additionally, we have determined that shunting of AA to the 5-LO pathway may by-pass the anti-cancer effects of COX-2 inhibition (i.e. the inhibition of cell-growth and VEGF production). Although in the cell lines we tested CYP2J2 was not able to compensate for loss of COX-2, this may be related to the low expression of CYP2J2 in these cells. We found that COX-2 inhibition increased the product of CYP-epoxygenase, 14,15-DHET/EET, in PCI-50 cells which had moderate levels of CYP2J2 expression. Therefore, we determined whether expression levels, alone, are responsible for the differences we see between the ability of the 5-LO pathway, but not the CYP2J2 pathway, to compensate for loss of COX-2. To this end, we expressed COX-2, 5-LO and CYP2J2 in 1041, 1076 and 50 cells. After transfecting plasmids expressing COX-2 + 5-LO or COX-2 + CYP2J2, we performed EIA assays for LTB4 or 14-15-DHET/EET to investigate whether AA can be shunted from the COX-2 pathway to 5-LO or CYP2J2 pathways when COX-2 is inhibited. We checked the correct change of PGE2 level by these co-treatments with expressing plasmids and siRNAs (Fig. 5a and b). COX-2 inhibition likely made more AA available to 5-LO in all three cell lines transfected with COX-2 and 5-LO expressing plasmids by about 60-100% (Fig. 6a). In addition, COX-2 inhibition increased the activity of CYP-epoxygenase by 40–100% in all three cell lines transfected with COX-2 and CYP2J2 expressing plasmids (Fig. 6b). Therefore, these data show that upon inhibition of COX-2, either the 5-LO or the CYP2J2 pathway can compensate in metabolizing AA. These data also suggest that the pathway utilized may be regulated by expression levels of 5-LO and CYP2J2.
The inhibition of PGE2 production by siRNA of COX-2 in cells transfected with COX-2 and 5-LO or CYP2J2. Plasmids (0.5 + 0.5 μg) expressing COX-2 and 5-LO a or COX-2 and CYP2J2 b were co-transfected into cells with siRNA (100 nM). LacZ expressing plasmid and siNC were used for controls. Forty-eight hours later cells were harvested and PGE2 EIA were performed. Results are expressed as percentage relative to control (% of control) (*, P < 0.05)
Increased production of LTB4 and 14,15-DHET by inhibition of COX-2. Plasmids (0.5 + 0.5 μg) expressing COX-2 and 5-LO (a) or COX-2 and CYP2J2 (b) were co-transfected into cells with siRNA (100 nM). LacZ expressing plasmid and siNC were used for controls. Forty-eight hours later cells were harvested and LTB4 (A) and 14,15-DHET/EET (B) EIA were performed. Results are expressed as percentage relative to control (% of control) (*, P < 0.05)
3.6 Improved prevention of VEGF production with combined inhibition of COX-2 and 5-LO or CYP2J2
When cells were individually transfected with COX-2, 5-LO or CYP2J2, we observed that CYP2J2 increased VEGF production as effectively as COX-2 and 5-LO, 200-300% (Fig. 7a). These results may suggest a role for COX-2, 5-LO and CYP2J2 in angiogenesis in HNSCC. Next, we investigated the synergistic effects of combined inhibition of COX-2 and 5-LO or CYP2J2 on VEGF synthesis by using siRNA. Combined inhibition of COX-2 and 5-LO or COX-2 and CYP2J2 enhanced the effect of COX-2 inhibition alone in SNU-1041 cells (Fig. 7b) and PCI-50 (Fig. 7c) transfected with COX-2 and 5-LO or COX-2 and CYP2J2. The change of PGE2, LTB4 and 14-15-DHET/EET level (Supplementary material-3) and expression in protein level (Supplementary material-4) by these co-treatments with expressing plasmids and siRNAs were confirmed. Our results clearly demonstrate crosstalk between the COX-2, CYP2J2 and 5-LO pathways in the production of VEGF.
Improved prevention of VEGF production with combined inhibition of COX-2 and 5-LO or CYP2J2. Cells were transfected with 0.5 μg of pcDNA3.1- COX-2, 5-LO or CYP2J2. Forty-eight hours after transfection culture media was subjected to VEGF EIA (a). The combined plasmids (0.5 + 0.5 μg) and siRNA (100 nM) were transfected into SNU-1041 (b) and PCI-50 (c). The plasmid expressing LacZ gene and siNC were used for negative control. Forty-eight hours after transfection culture media was subjected to VEGF EIA. Results are expressed as percentage relative to control (% of control) (*, P < 0.05)
4 Discussion
Our initial experiments revealed that COX-2 inhibition failed to show potent anti-cancer effects in HNSCC cells with high activity of COX-2. We had thought that this result may be due to loss of COX-2 pathway regulation in these cells by modification of the expression or activity of downstream terminal PGs synthases, PG receptors or related signal transduction pathways [14]. However, we next thought that perhaps we have to consider the interaction between COX-2 and other AA pathways as a possible reason for the failure of COX-2 inhibition on cancer cell viability.
The possible carcinogenic actions of other pathways involved in AA degradation are now being investigated. Among several subtypes of LOs, evidence suggests that 5-LO seems to be another AA degradation pathway that may play a role in carcinogenesis [15]. On the other hand, it has been suggested that 15-LO may be anti-carcinogenic [16, 17]. Also, CYP2J2, a subtype of CYPs, appears to be the most up-regulated subtype of CYPs in cancer cells and its metabolites seem to contribute to cell viability [18, 19]. Because 5-LO and CYP2J2 have different downstream signaling pathways but the same substrate (AA) and previous findings suggest they play a similar role as COX-2 in carcinogenesis, we investigated the relationship and the possible interaction between these pathways.
Having studied the carcinogenic roles of COX-2 in HNSCC for several years, we accumulated the significant experimental findings on COX-2 physiology in cancer development [20–22]. However, we realized that we overestimated the anti-cancer effects of COX-2 inhibitors because of unexpected COX-2-independent mechanisms. In some cases we observed no response to COX-2 inhibition in the cell lines tested even those with high COX-2 and PG expression. We also observed that up-regulation of 5-LO increased cell proliferation and VEGF synthesis in some HNSCC cells, suggesting that AA might be shunted from the COX-2 pathway to the 5-LO pathway when COX-2 is inhibited. We also found that AA could be shunted from the COX-2 pathway to the CYP2J2 pathway after COX-2 inhibition, but observed no direct relevance to cell viability. Though, in HNSCC cell models transfected with plasmids expressing COX-2 and CYP2J2, we confirmed the possibility that the AA shunting to CYP2J2 might reverse the decrease of VEGF synthesis by COX-2 inhibition. As far as we know, we originally suggest the possibility that CYP2J2 as well as 5-LO might play the carcinogenic roles through promoting VEGF production in HNSCC.
In this study, we show that AA shunting to the 5-LO or CYP2J2 pathway prevented the anti-proliferative and anti-angiogenic effects of COX-2 inhibition in HNSCC cells. Our findings suggest that combinatorial treatment with inhibitors of COX-2 and 5-LO or CYP2J2 may enhance anti-cancer effects. Therefore, we investigated the relevance of AA shunting to 5-LO and CYP2J2 after COX-2 inhibition because these pathways had already been suggested to promote cancer cell viability. Of note, the expression of other factors of AA metabolism (i.e. 12-LO, 15-LO and CYP2C9) was observed in our study, therefore, other pathways may participate in AA shunting by COX-2 inhibition and affect cancer cell viability.
We have addressed the role of other AA metabolizing pathways in HNSCC cells, however the hidden roles of COX-2, 5-LO and CYP2J2 should be investigated in other types of cancer cells which may identify unique molecular targets whose inhibition may enhance COX-2-inihibitory cancer therapy. In conclusion, we show that combined inhibition of COX-2 and other AA degrading pathways might maximize the anti-cancer effects of COX-2 inhibition in cancer cells with high COX-2 activity. Furthermore, these data indicate that determining the expression and activity of COX-2 and other AA metabolizing pathways would be beneficial for cancer patients in identifying pathways whose inhibition may enhance COX-2 inhibition for more effective treatments.
Abbreviations
- siRNA:
-
small interfering RNA
- CYP2J2:
-
cytochrome P450 family 2-subfamily J-polypeptide 2
- PGE2 :
-
prostaglandin E2
- LTB4 :
-
Leukotriene B4
- DHET:
-
dihydroxyeicosatrienoic acid
- EET:
-
epoxyeicosatrienoic acid
- VEGF:
-
vascular endothelial growth factor
- 5-HETE:
-
5-Hydroxyeicosatetraenoic acid
- cytLTs:
-
cysteinyl-leukotrienes
References
H. Harizi, J.B. Corcuff, N. Gualde, Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol. Med. 14, 461–469 (2008)
S.P. Levick, D.C. Loch, S.M. Taylor, J.S. Janicki, Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J. Immunol. 178, 641–646 (2007)
Y. Zheng, J.D. Ritzenthaler, X. Sun, J. Roman, S. Han, Prostaglandin E2 stimulates human lung carcinoma cell growth through induction of integrin-linked kinase: the involvement of EP4 and Sp1. Cancer Res. 69, 896–904 (2009)
N. Arber, Cyclooxygenase-2 inhibitors in colorectal cancer prevention: point. Cancer Epidemiol. Biomarkers Prev. 17, 1852–1857 (2008)
W.G. Tong, X.Z. Ding, M.S. Talamonti, R.H. Bell, T.E. Adrian, LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem. Biophys. Res. Commun. 335, 949–956 (2005)
J.G. Jiang, Y.G. Ning, C. Chen, D. Ma, Z.J. Liu, S. Yang, J. Zhou, X. Xiao, X.A. Zhang, M.L. Edin, J.W. Card, J. Wang, D.C. Zeldin, D.W. Wang, Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 67, 6665–6674 (2007)
Y.N. Ye, W.K. Wu, V.Y. Shin, I.C. Bruce, B.C. Wong, C.H. Cho, Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis 26, 827–834 (2005)
F. Cianchi, C. Cortesini, L. Magnelli, E. Fanti, L. Papucci, N. Schiavone, L. Messerini, A. Vannacci, S. Capaccioli, F. Perna, M. Luli, V. Fabbroni, G. Perigli, P. Bechi, E. Masini, Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol. Cancer Ther. 5, 2716–2726 (2006)
M. Barry, R.A. Cahill, G. Roche-Nagle, T.G. Neilan, A. Treumann, J.H. Harmey, D.J. Bouchier-Hayes, Neoplasms escape selective COX-2 inhibition in an animal model of breast cancer. Ir. J. Med. Sci. 178, 201–208 (2009)
S.W. Park, S.G. Lee, S.H. Song, D.S. Heo, B.J. Park, D.W. Lee, K.H. Kim, M.W. Sung, The effect of nitric oxide on cyclooxygenase-2 (COX-2) overexpression in head and neck cancer cell lines. Int. J. Cancer 107, 729–738 (2003)
C. Vincent, R. Fiancette, M. Donnard, D. Bordessoule, P. Turlure, F. Trimoreau, Y. Denizot, 5-LOX, 12-LOX and 15-LOX in immature forms of human leukemic blasts. Leuk. Res. 32, 1756–1762 (2008)
T.C. Delozier, G.E. Kissling, S.J. Coulter, D. Dai, J.F. Foley, J.A. Bradbury, E. Murphy, C. Steenbergen, D.C. Zeldin, J.A. Goldstein, Detection of human CYP2C8, CYP2C9, and CYP2J2 in cardiovascular tissues. Drug Metab. Dispos. 35, 682–688 (2007)
A. Catalano, A. Procopio, New aspects on the role of lipoxygenases in cancer progression. Histol. Histopathol. 20, 969–975 (2005)
V.B. O’Donnell, B. Maskrey, G.W. Taylor, Eicosanoids: generation and detection in mammalian cells. Methods Mol. Biol. 462, 5–23 (2009)
L.G. Melstrom, D.J. Bentrem, M.R. Salabat, T.J. Kennedy, X.Z. Ding, M. Strouch, S.M. Rao, R.C. Witt, C.A. Ternent, M.S. Talamonti, R.H. Bell, T.A. Adrian, Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin. Cancer Res. 14, 6525–6530 (2008)
H. Zhu, W. Glasgow, M.D. George, K. Chrysovergis, K. Olden, J.D. Roberts, T. Eling, 15-lipoxygenase-1 activates tumor suppressor p53 independent of enzymatic activity. Int. J. Cancer 123, 2741–2749 (2008)
Q. Yang, Y. Feng, C.J. Schultz, X.A. Li, H. Wu, D. Wang, Synergistic effect of 15-lipoxygenase 2 and radiation in killing head-and-neck cancer. Cancer Gene Ther. 15, 323–330 (2008)
J.G. Jiang, X.N. Fu, C.L. Chen, D.W. Wang, Expression of cytochrome P450 arachidonic acid epoxygenase 2J2 in human tumor tissues and cell lines. Chin. J. Cancer 28, 93–96 (2009)
J.G. Jiang, C.L. Chen, J.W. Card, S. Yang, J.X. Chen, X.N. Fu, Y.G. Ning, X. Xiao, D.C. Zeldin, D.W. Wang, Cytochrome P450 2 J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 65, 4707–4715 (2005)
J.L. Roh, M.W. Sung, S.W. Park, D.S. Heo, D.W. Lee, K.H. Kim, Celecoxib can prevent tumor growth and distant metastasis in postoperative setting. Cancer Res. 64, 3230–3235 (2004)
M.W. Sung, J.L. Roh, B.J. Park, S.W. Park, T.K. Kwon, S.J. Lee, K.H. Kim, Bile acid induces cyclo-oxygenase-2 expression in cultured human pharyngeal cells: a possible mechanism of carcinogenesis in the upper aerodigestive tract by laryngopharyngeal reflux. Laryngoscope 113, 1059–1063 (2003)
S.W. Park, M.W. Sung, D.S. Heo, H. Inoue, S.H. Shim, K.H. Kim, Nitric oxide upregulates the cyclooxygenase-2 expression through the cAMP-response element in its promoter in several cancer cell lines. Oncogene 24, 6689–6698 (2005)
Acknowledgements
We thank Dr. William L. Smith, Dr. Thomas G. Brock and Darryl C. Zeldin for kindly providing us with the cDNA of COX-2, 5-LO and CYP2J2.
This study was supported by a grant from the national R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea [Grants no. 0620250] and BK21 project for Medicine, Dentistry and Pharmacy, Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Supplementary material 1
(DOC 142 kb)
Supplementary material 2
(DOC 76 kb)
Supplementary material 3
(DOC 108 kb)
Supplementary material 4
(DOC 153 kb)
Supplementary material 5
(DOC 148 kb)
Rights and permissions
About this article
Cite this article
Park, SW., Heo, DS. & Sung, MW. The shunting of arachidonic acid metabolism to 5-lipoxygenase and cytochrome p450 epoxygenase antagonizes the anti-cancer effect of cyclooxygenase-2 inhibition in head and neck cancer cells. Cell Oncol. 35, 1–8 (2012). https://doi.org/10.1007/s13402-011-0051-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-011-0051-7