Abstract
Background
ACC can occasionally undergo dedifferentiation also referred to as high-grade transformation (ACC-HGT). However, ACC-HGT can also undergo transformation to adenocarcinomas which are not poorly differentiated. ACC-HGT is generally considered to be an aggressive variant of ACC, even more than solid ACC. This study was aimed to describe the genetic changes of ACC-HGT in relation to clinico-pathological features, and to compare results to solid ACC.
Methods
Genome wide DNA copy number changes were analyzed by microarray CGH in ACC-HGT, four with transformation into moderately differentiated adenocarcinoma (MDA) and two into poorly differentiated carcinoma (PDC), and five solid ACC. In addition, Ki67 index and p53 immunopositivity was assessed.
Results
ACC-HGT carried fewer copy number changes compared to solid ACC. Two ACC-HGT cases harboured a breakpoint at 6q23, near the cMYB oncogene. The complexity of the genomic profile concurred with the clinical course of the patient. Among the ACC-HGT, p53 positivity significantly increased from the conventional to the transformed (both MDA and PDC) component.
Conclusion
ACC-HGT may not necessarily reflect a more advanced stage of tumor progression, but rather a transformation to another histological form in which the poorly differentiated forms (PDC) presents a genetic complexity similar to the solid ACC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Adenoid cystic carcinoma is a slow-growing tumor presenting a dual cellular composition, i.e., ductal (luminal) and myoepithelial cell differentiation and three major growth patterns: tubular, cribriform and solid [1]. The solid growth pattern has been considered to be an adverse prognosticator [2–4] and in a three-tired system for grading ACC, tumors having more than 30% of the solid component are classified as grade III or poorly differentiated. Grade I tumors are those with tubular and cribriform areas but without solid components whereas Grade II are pure or mixed cribriform with less than 30% of solid areas [3]. Grade III ACC has been associated with increased disease mortality and greater frequency of aneuploidy than grade I or II tumors [5] Furthermore, comparing low-grade foci with high-grade ones within the same tumor revealed a greater number of mutations at either the p53 or Rb genes in the latter [6].
ACC can occasionally undergo transformation into poorly differentiated adenocarcinoma or undifferentiated carcinoma. This phenomenon has been referred to as dedifferentiation or high-grade transformation (ACC-HGT), and there have been only 33 reported cases so far [7–16]. This process was first believed to occur in low-grade ACCs without morphological recognizable changes, as an abrupt transition, but recently cases have been described showing a gradual transformation of solid ACC into high-grade adenocarcinoma [12, 13].
ACC-HGT is generally considered to be an aggressive variant of ACC, even more than solid ACC [13]. However, recently it has been claimed that high-grade transformation of ACC may, in addition to poorly differentiated carcinomas, also result in adenocarcinomas with a moderate differentiation [16].
The pathogenesis of high-grade transformation of ACC is poorly understood, partly because few studies have been dedicated to this tumor type. Some molecular studies reported TP53 mutations, loss of heterozygosity at the TP53 locus [7, 9], and strong overexpression of p53 protein in high-grade components [7, 9, 11, 13], suggesting that p53 alterations may play a significant role in the pathogenesis of high grade transformation of ACC [9].
Genetic characterization of these lesions may give more insight in this complex matter. This study was aimed to compare the ACC-HGT and solid type ACC using genome-wide high resolution microarray CGH analysis. In addition, the genetic changes were correlated with clinical outcome.
2 Material and methods
2.1 Material
The present study included 11 paraffin embedded carcinoma samples of patients with ACC diagnosed between 1996 and 2007: six cases ACC-HGT and five cases solid ACC. Five cases of ACC-HGT were obtained from the archives of the Department of Pathology of the University of Campinas, Brazil, one case of ACC-HGT of the Department of Pathology of the Hospital Universitario Central de Asturias, Spain and five cases of solid ACC of the Department of Pathology, VU University Medical Center, The Netherlands. The solid ACC have previously been described as part of a larger series [17]. Hematoxylin-Eosin (H&E) stained slides from each tissue block were reviewed (Fig. 1) to confirm the pathological diagnosis. The transformed component was identified according to the criteria described by Seethala et al. [13] and all cases showed the following features: proliferation of tumor cells with at least a focal loss of myoepithelial cells surrounding tumor nests, nuclear size at least 2–3 times the size of tubular/cribriform ACC nuclei, thickened irregular nuclear membranes and prominent nucleoli in a majority of cells. In addition (Table 1), based on the degree of gland formation (differentiation), cellular pleomorphism and mitotic activity, the transformed components were classified into: moderately differentiated adenocarcinomas (MDA) when at least 2/3 exhibited gland differentiation and poorly differentiated carcinomas (PDC) those with scarce or absent gland differentiation [18]. The conventional component was classified in a three-tired system proposed by Szanto et al. [3], which is widely used in the literature.
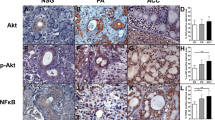
Expression of α-SMA in myoepithelial cells of conventional area (a) and transformed area (b) of ACC-HGT (Case F). The transformed component shows few positive myoepithelial cells (arrows) for α-SMA, demonstrating the loss of biphasic ductal-myoepithelial differentiation in this area. Ki-67 (Case C) and p53 (Case A) expression in conventional areas (c, e) and in transformed component (d, f). Original magnification 400× (a, b, c and d)
The ACC-HGT group consisted of one male and five female patients ranging from 44 to 65 years of age, with a median of 56 years. Two tumors occurred in the submandibular gland, two in paranasal sinus and one in palate and one in parotid gland. Four cases underwent transformation into MDA and two into PDC. The solid ACC group comprised of two male and three female patients ranging from 33 to 66 years of age, with a median of 52 years. Three tumors occurred in the parotid gland and one in the submandibular gland. The exact origin of case 2 could not be determined; it concerned a large mass located in the oropharynx and nasopharynx. The follow-up time was 7–144 months (mean 55 months) for the ACC-HGT and 7–81 months (mean 43 months) for the solid ACC. The clinical and pathologic data of all cases are summarized in Table 1.
2.2 Microdissection and DNA extraction
Tumor tissue of five solid ACC and six ACC-HGT was obtained from 10 paraffin sections of 10 μm. Regions of interest (conventional and transformed areas) of the tumors were carefully dissected manually, on the basis of H&E-stained slides. Tumor DNA was extracted using Qiagen extraction kits (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s recommendations. Special care was taken to obtain high quality DNA from the formaldehyde-fixed, paraffin-embedded tissues. DNA extracted from archival material can be partly degraded and cross-linked, the extent of which depends on the pH of the formaldehyde and the time of the fixation before paraffin embedding. To improve the quality of the isolated DNA, we have applied an elaborate extraction protocol especially for paraffin tissues, which includes thorough deparaffination with xylene, methanol washings to remove all traces of the xylene, and 24-h incubation in 1 mol/L sodium thiocyanate to reduce cross-links. Subsequently, the tissue pellet is dried and digested for 3 days in lysis buffer with high doses of proteinase K (final concentration 2 ug/uL, freshly added twice a day). Finally, the DNA was purified with Qiagen columns (QIAamp DNA mini-kit Qiagen GmbH, Hilden, Germany). With this protocol, most formaldehyde-fixed, paraffin-embedded tissue samples yielded DNA of relatively good quality, with A260/A280 values between 1,7 and 2.0 measured by Nanodrop (Thermo Scientific, Wilmington DE, USA) and lengths between 2,000 and 20,000 bp. Before performing microarray CGH, we performed an additional quality test using the ENZO Bioscore Screening and Amplification kit (Enzo Life Sciences, Lörrach, Germany). The assay consists of an isothermal whole genome amplification reaction using 100 ng of DNA, followed by a purification by QIAquick PCR Purification columns (Qiagen GmbH, Hilden, Germany) and measurement of the DNA concentration by Nanodrop (Thermo Scientific, Wilmington DE, USA). Only those samples that gave a total yield of 3.0 or more were used for microarray CGH analysis.
2.3 Microarray CGH
Microarray CGH analysis was performed as described previously by Buffart et al. [19]. Briefly, sample DNA and reference DNA (extracted and pooled from blood of 18 different healthy female donors) were differently labeled by using the Enzo Genomic DNA Labeling kit according to the manufacturer’s instructions (Enzo Life Sciences, Lörrach, Germany). Five hundred nanograms test and 500 ng pooled reference DNA were hybridized to a 180 k oligonucleotide array (SurePrint G3 Human CGH Microarray Kit 4 × 180 K, Agilent Technologies, Palo Alto CA, USA). Hybridization and washing took place in a specialized hybridization chamber (Agilent Technologies). Images were acquired using a Microarray scanner G2505B (Agilent Technologies, Amstelveen, Netherlands). Analysis and data extraction were quantified using feature extraction software (version 9.1, Agilent Technologies). Normalization of the calculated ratios was done against the mode of the ratios of all autosomes. Graphics were plotted using a moving average of log2 ratios of five neighboring clones. Gains and losses were defined as deviations of 0.2 or more from log2 ratio = 0.0. High-level amplification was considered when at least two neighboring clones reached a log2 ratio of 1.0 or higher. The locations of possible copy number variations (rather than copy number alterations) were verified with the database of genomic variants and mapped according the human genome build NCBI 36 (http://projects.tcag.ca/variation/). The microarray CGH analyses of the five solid ACC have been performed as part of a previous study, using a home-made microarray consisting of approximately 4.500 BAC-PAC clones [17].
2.4 Immunohistochemistry
One paraffin block from each case was chosen for the immunohistochemical study and the following antibodies (DAKO, Carpenteria, California) were used: Ki67 (MIB1-dilution 1:150), p53 (DO-7, dilution: 1:100) and alpha smooth muscle actin (α-SMA, 1A4—dilution 1:200), Fig. 2. The 5 μm sections were deparaffinized, hydrated and endogenous peroxidase activity was quenched by immersion of the slides in 3% hydrogen peroxide. Antigen retrieval (AR) was performed for Ki-67 and p53 by using Tris-EDTA (pH 9.0), heating 5 min in a pressure cooker. For α-SMA, AR was not done. Staining was done at room temperature on an automatic staining workstation (Autostainer; DAKO, Carpenteria, California). Subsequently, for all antibodies the sections were incubated with the primary antibody, and afterwards with the Envision peroxidase system (Envision Plus; DAKO, Carpenteria, California) and with 3.3′-diaminobenzidine tetrahydrochloride (DAB) chromogen used as the substrate (DAKO). Counterstaining with hematoxylin for 1 min was the final step. After staining, the slides were dehydrated through graded alcohols and mounted with a coverslip. Negative controls were run by omitting primary antibodies.
Immunostaining of alpha smooth muscle actin (α-SMA) and p63, the latter as part of a previous study [16], was done in all cases of ACC-HGT for detection of myoepithelial cells contributing the selection of the transformed area for DNA extraction. In Ki-67 and p53 stained sections, three hotspot areas were chosen for counting of positive cells at 40× magnification. To quantify positive and negative cells, images were obtained from three areas and analyzed with Imagelab analysis software (version 2.4, Softium informática LTDA-ME, São Paulo, Brasil). Ki-67 and p53 indexes were calculated as the percentage of positive cells in relation to all tumor cells in these three areas in each sample.
2.5 Statistical analysis
Possible correlations between genetic and clinico-pathological parameters were statistically analyzed by SPSS 12.0 software for Windows (SPSS® Inc. Illinois, USA), using the Fisher exact Chi2 test and Student’s t-test. Kaplan-Meier analysis was performed for estimation of survival, comparing distributions of survival through the logarithmic range test (log-rank test). P values below 0.05 were considered significant.
3 Results
3.1 Clinical follow up
All patients except the case 1 of solid ACC underwent radiotherapy after resection. Chemotherapy was not used in any case. During the follow-up period, three of six ACC-HGT and four of five solid ACC developed either a recurrence or a metastasis. The overall survival (Fig. 3a) of the six ACC-HGT was more favorable than the five solid ACC (mean 58 versus 42 months) and this was the same with regard to the disease-free survival (mean 52 versus 33 months), although this did not reach statistical significance (Fig. 3b).
3.2 Microarray CGH
Two of six ACC-HGT yielded a bad quality of DNA and had to be excluded from microarray CGH analysis (Table 2). Two ACC-HGT showed only one aberration; gain of whole chromosome 16 in case B and loss of 4q13.2-q22.3 in case D. Cases E and F harbored six and 11 changes, respectively. A detailed description of all copy number changes is given in Table 3 and Fig. 4. Two aberrations were recurrent, loss of 6q23.3-qter and gain of chromosome 8, both in cases E and F. These two cases also shared two transition points (where a change in the copy number begins or ends), which may indicate a translocation breakpoint. One lies in chromosomal band 6q23.3 at point 135.7 Mbp, and the second recurrent breakpoint in band 9p22.3 at point 14.1 Mbp (Fig. 5).
Overview of all copy number alterations of four ACC-HGT (cases B, D, E and F) and five solid ACC (cases 1–5). To the right of the pictogram of each chromosome, a scale is placed expressing the number of megabasepairs (Mpb) counting from pter to qter. Copy number losses are presented as bars left to the Mbp-scale and copy number gains to the right
The left panel shows the microarray CGH profile of chromosome 6 of cases E and F, both carrying a telomeric deletion that begins at the same 6q23.3 breakpoint, marked by an arrow. The right panel shows the microarray CGH profile of chromosome 9 of cases E, carrying a gain, and of case F carrying three distinct regions with copy number loss. The arrows mark the common 9p22.3 breakpoint
The genome wide profiles of the ACC-HGT differed much from the solid ACC, both in number of alterations (Table 2) and in the specific chromosomes involved in alterations (Fig. 4). The average number of alterations in the four ACC-HGT was 4.7 (three gains and 1.7 loss) whereas the solid ACC demonstrated on average 21.8 events (19 gains and 2.8 losses). The five solid ACC showed many recurrent events, of which the most striking were gains at 9q33-q34, 11q13, 11q25, 12q13, 16p13, 16q24, 19 and 22 and loss at 14q. Only few of the recurrent aberrations in solid ACC were also seen in the ACC-HGT (Fig. 4).
3.3 Immunohistochemistry
All ACC-HGT showed at least focal loss or absence of α-SMA and p63 [16] immunoreactivity for myoepithelial cells at the periphery of tumor nests in the transformed component, demonstrating the loss of biphasic ductal-myoepithelial differentiation in this area (Fig. 2).
The Ki-67 index showed a trend for higher expression in the transformed component (both MDA and PDC) compared to the conventional areas (mean 19.6 versus 34.2, p = 0,093) (Fig. 2 and Table 2). Among the ACC-HGT group, a correlation was found between the degree of differentiation (Table 1) of the transformed component and the Ki-67 index. The proliferation index was significantly lower in the MDA than in the PDC group (mean 24.7 versus 53.3, p = 0,028). A comparison of the transformed component of ACC-HGT with solid ACC, showed no significant difference in Ki-67 index (mean 34.2 versus 33.0, respectively; p = 0,917). When comparing the solid ACC to MDA and PDC group separately, no significant differences were found in Ki-67 index (mean solid 33.0 versus MDA 24.7 and PDC 53.3; p = 0,502 and p = 0,270). PDC did show a trend for a higher index but unfortunately there were only two cases in the series.
Neither did we find a significant difference between the solid conventional area of ACC-HGT (cases B-F, regardless of the degree of differentiation MDA or PDC) and the solid ACC, (mean 20.7 versus 33.0; p = 0,276).
In all cases, both components of ACC-HGT showed positive p53 staining (Fig. 2 and Table 2). The p53 expression was significantly higher in the transformed component (both MDA and PDC) than in the conventional area (mean 62.9 versus 38.9, p = 0,000), for group. No significant difference was found between the groups MDA and PDC (mean 63.4 versus 62.0; p = 0,713). Similar expression of p53 without significant differences was observed between ACC-HGT and solid ACC (mean 62.9 versus 64.0; p = 0,929) and also between MDA or PDC group and solid ACC (mean 63.4 (MDA) and 62.0 (PDC) versus 64.0; p = 0,968 and p = 0,929, respectively). The solid conventional areas (cases B-F) of the ACC-HGT group showed a trend towards lower p53 expression compared to the solid ACC group (mean 38.2 versus 64.0; p = 0,085).
3.4 Clinicopathologic-genetic correlations
The two cases of ACC-HGT with transformation into MDA (cases B and D) showed the lowest number of copy number abnormalities and one of the patients was a long-term survivor, who did not develop recurrence or metastasis. Conversely, the solid ACC group and ACC-HGT with transformation into PDC carried the highest number of abnormalities and had the worst clinical course; three out of five patients with solid type of ACC and one out of two with PDC died of disease (Tables 1 and 2).
4 Discussion
An important point of interest with ACC-HGT lies in their proposed poor prognosis, which is suggested to be comparable to or even worse than solid ACC. The median survival of the largest reported series of ACC-HGT, in which all cases were poorly differentiated carcinomas, was estimated at 12 months [13], while in solid ACC this is approximately 36–48 months [3]. In addition to recurrence and distant metastasis, a high propensity for lymph node metastasis has been observed, which would indicate a role for neck dissection in these patients [13]. However, ACC-HGT may encompass a wide spectrum of tumors in morphological appearance and probably in biological behavior as well [16].
To date, genetic studies on ACC-HGT have almost exclusively been restricted to protein expression studies with immunohistochemistry [7–9, 11–13, 15, 16]. Here, we applied a high resolution microarray CGH analysis in an attempt to uncover genes involved in high-grade transformation of ACC, supplemented by immunohistochemical analysis of Ki67 and p53 and clinico-pathological data. In addition, we contrasted our data to an existing set of microarray CGH data on solid ACC [17].
An interesting finding in our study was the correlation between the number of chromosomal aberrations and the degree of gland differentiation of the transformed component in the ACC-HGT group. The two MDA had relatively simple genomic profiles carrying one single abnormality, whereas the two PDC showed a higher number of alterations. Solid ACC exhibited even higher numbers of chromosomal aberrations.
ACC with many chromosomal aberrations have been reported to be more aggressive and associated with less favorable outcome than those with few alterations [17]. Also in our series of ACC-HGT the complexity of the genomic profile grossly concurred with the clinical course of the patient: case F with transformation into PDC showed the worst clinical course and the highest number of chromosomal abnormalities. This patient developed lymph node and distant metastasis, and died of disease 7 months after diagnosis. In contrast, case B (a MDA with a single chromosomal aberration) was a long-term survivor and did not present metastasis or local recurrence. However, owing to our small number of cases, this association between degree of gland differentiation of the transformed component, amount of chromosomal aberrations and clinical outcome needs further confirmation in a larger series.
Two recurrent chromosomal changes were found: deletion at 6q23.3-qter and gain of whole chromosome 8, both in the two cases of PDC. Both aberrations have been found previously by cytogenetic and LOH analyses in salivary gland tumors [20–24] and in ACC [25–28]. Rao and collaborators [26] reported that gain of chromosome 8 was significantly associated with ACC solid type. In our series, in the ACC-HGT with PDC, the conventional component was of the solid type. Therefore, we believe that this chromosomal aberration in PDC areas is residual of the parent ACC. In our solid ACC group four cases out of five also showed gains of chromosome 8 reinforcing its association with this subtype of ACC.
In the two PDC cases, especially interesting were breaks found at the 6q and 9p regions, because they both occurred at exactly the same localization, which could indicate unbalanced chromosomal translocations. Both 6q23 and 9p22 have previously been found involved in translocations in salivary gland tumors [20–24, 29], and in ACC [25–28]. Interestingly, recurrent translocations between 6q23 and 9p13-23 have been identified before in ACC [20, 21], and very recently, Persson et al. [30] identified the genes MYB at 6q23 and NFIB at 9p22 being the fusion partners of this translocation, leading to chimeric transcripts predominantly consisting of MYB and overexpression of MYB protein [30]. In our two cases, the two breaks at 9p22 were located within NFIB in one, and just 100 kb centromeric to NFIB in the other case. At 6q23.3-qter the two breakpoints were identical, lying within the gene AHI1, which is a close neighbour telomeric of cMYB. Hence it remains unclear if our results confirm the findings of Persson et al. [30]. Overexpression of the oncogene AHI-1 has been implicated in the tumorigenesis of cutaneous T-cell lymphoma [31] and chronic myeloid leukemia [32]. However, we found no studies on a role for AHI-1 in solid tumors.
Although TP53 mutations and/or loss of heterozygosity at the TP53 locus have been suggested to play a role in the pathogenesis of high grade transformation of ACC [9, 11], in the current series none of the cases of ACC with transformation showed chromosomal aberration at 17p13, the locus of TP53. However, positive immunostaining is indicative of mutations in TP53 [33] and all cases in the current series showed positive p53 protein immunoexpression, increasing in the transformed component, suggesting a pivotal role of TP53 in the transformation of ACC.
Finally, our findings do not lend support to the hypothesis that ACC-HGT as a single group or separately (MDA and PDC) is more aggressive than solid ACC [13]. Due to the low number of cases, we cannot conclude whether PDC are more aggressive than MDA. Our data do suggest that the clinical course in ACC-HGT is dependent of the amount of chromosomal abnormalities, in which the poorly differentiated forms (PDC) presents a genetic complexity similar to the solid ACC. Although our series of cases is too small for strong conclusions, we do believe our data are valid, because we found agreement between the genetic, morphology, proliferation index and clinical data. Perhaps ACC-HGT does not necessarily reflect a more advanced stage of tumor progression, but rather a transformation to another histological form, which encompasses a wide spectrum of carcinomas in terms of aggressiveness [16]. Therefore, the term ‘high grade transformation’ may not be adequate for cases of ACC with transformation into MDA. In addition, we propose that for prognostication of ACC-HGT, histopathological classification may be supplemented by genomic profiling by microarray CGH copy number analysis or other genome-wide analysis techniques.
References
A.K. El-Naggar, A.G. Huvos, Adenoid cystic carcinoma, in World Organization Classification of Tumors. Pathology & Genetics Head and Neck Tumours, ed. by L. Barnes, J.W. Everson, P. Reichart et al. (IARC Press, Lyon, 2005), pp. 221–222
K.H. Perzin, P. Gullane, A.C. Clairmont, Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer 42, 265–282 (1978)
P.A. Szanto, M.A. Luna, M.E. Tortoledo et al., Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 54, 1062–1069 (1984)
D.E. da Cruz Perez, F. de Abreu Alves, I. Nobuko Nishimoto et al., Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol 42, 139–146 (2005)
G. Franzén, S. Nordgård, M. Boysen et al., DNA content in adenoid cystic carcinomas. Head Neck 17, 49–55 (1995)
H. Papadaki, S.D. Finkelstein, S. Kounelis et al., The role of p53 mutation and protein expression in primary and recurrent adenoid cystic carcinoma. Hum Pathol 27, 567–572 (1996)
T. Nagao, T.A. Gaffey, H. Serizawa et al., Dedifferentiated adenoid cystic carcinoma: a clinicopathologic study of 6 cases. Mod Pathol 16, 1265–1272 (2003)
F. Ide, K. Mishima, I. Saito, Small foci of high-grade carcinoma cells in adenoid cystic carcinoma represent an incipient phase of dedifferentiation. Histopathology 43, 604–606 (2003)
Y. Chau, T. Hongyo, K. Aozasa et al., Dedifferentiation of adenoid cystic carcinoma: report of a case implicating p53 gene mutation. Hum Pathol 32, 1403–1407 (2001)
M.A. Moles, I.R. Avila, A.R. Archilla, Dedifferentiation occurring in adenoid cystic carcinoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88, 177–180 (1999)
W. Cheuk, J.K. Chan, R.K. Ngan, Dedifferentiation in adenoid cystic carcinoma of salivary gland: an uncommon complication associated with an accelerated clinical course. Am J Surg Pathol 23, 465–472 (1999)
K. Sato, Y. Ueda, A. Sakurai et al., Adenoid cystic carcinoma of the maxillary sinus with gradual histologic transformation to high-grade adenocarcinoma: a comparative report with dedifferentiated carcinoma. Virchows Arch 448, 204–208 (2006)
R.R. Seethala, J.L. Hunt, Z.W. Baloch et al., Adenoid cystic carcinoma with high-grade transformation: a report of 11 cases and a review of the literature. Am J Surg Pathol 31, 1683–1694 (2007)
A. Handra-Luca, D. Planchard, P. Fouret, Docetaxel-cisplatin-radiotherapy in adenoid cystic carcinoma with high-grade transformation. Oral Oncol 45, 208–209 (2009)
K.P. Malhotra, V. Agrawal, R. Pandey, High grade transformation in adenoid cystic carcinoma of the parotid: report of a case with cytologic, histologic and immunohistochemical study. Head Neck Pathol 3, 310–314 (2009)
V.L. Bonfitto, A.P. Demasi, A.F. Costa et al., High-grade transformation of adenoid cystic carcinomas may result in adenocarcinomas with wide spectrum of differentiation. A study of the expression of GLUT1 glucose transporter and of mitochondrial antigen in the transformed component. J Clin Pathol 63, 615–619 (2010)
H. Vékony, B. Ylstra, S.M. Wilting et al., DNA copy number gains at loci of growth factors and their receptors in salivary gland adenoid cystic carcinoma. Clin Cancer Res 13, 3133–3139 (2007)
B.M. Wening, Neoplasmas of the salivary glands, in Atlas of head and neck pathology, ed. by B.M. Wening, C.S. Heffess (China, Saunders Elsevier, 2008), pp. 628–631
T.E. Buffart, D. Israeli, M. Tijssen et al., Across array comparative genomic hybridization: a strategy to reduce reference channel hybridizations. Genes Chromosom Cancer 47, 994–1004 (2008)
G. Stenman, J. Sandros, R. Dahlenfors et al., 6q- and loss of the Y chromosome–two common deviations in malignant human salivary gland tumors. Cancer Genet Cytogenet 22, 283–293 (1986)
K. Higashi, Y. Jin, M. Johansson et al., Rearrangement of 9p13 as the primary chromosomal aberration in adenoid cystic carcinoma of the respiratory tract. Genes Chromosom Cancer 3, 21–23 (1991)
A. Nordkvist, J. Mark, H. Gustafsson et al., Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes Chromosom Cancer 10, 115–121 (1994)
L. Queimado, A. Reis, I. Fonseca et al., A refined localization of two deleted regions in chromosome 6q associated with salivary gland carcinomas. Oncogene 16, 83–88 (1998)
M. Kishi, M. Nakamura, M. Nishimine et al., Loss of heterozygosity on chromosome 6q correlates with decreased thrombospondin-2 expression in human salivary gland carcinomas. Cancer Sci 94, 530–535 (2003)
W. El-Rifai, S. Rutherford, S. Knuutila et al., Novel DNA copy number losses in chromosome 12q12–q13 in adenoid cystic carcinoma. Neoplasia 3, 173–178 (2001)
R.H. Rao, D. Roberts, Y.J. Zhao et al., Deletion of 1p32-p36 is the most frequent genetic change and poor prognostic marker in adenoid cystic carcinoma of the salivary glands. Clin Cancer Res 14, 5181–5187 (2008)
I. Stallmach, P. Zenklusen, P. Komminoth et al., Loss of heterozygosity at chromosome 6q23-25 correlates with clinical and histologic parameters in salivary gland adenoid cystic carcinoma. Virchows Arch 440, 77–84 (2002)
S. Rutherford, Y. Yu, C.A. Rumpel et al., Chromosome 6 deletion and candidate tumor suppressor genes in adenoid cystic carcinoma. Cancer Lett 236, 309–317 (2006)
J.M. Geurts, E.F. Schoenmakers, E. Röijer et al., Identification of NFIB as recurrent translocation partner gene of HMGIC in pleomorphic adenomas. Oncogene 16, 865–872 (1998)
M. Persson, Y. Andrén, J. Mark et al., Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA 106, 18740–18744 (2009)
E. Kennah, A. Ringrose, L.L. Zhou et al., Identification of tyrosine kinase, HCK, and tumor suppressor, BIN1, as potential mediators of AHI-1 oncogene in primary and transformed CTCL cells. Blood 113, 4646–4655 (2009)
L.L. Zhou, Y. Zhao, A. Ringrose et al., AHI-1 interacts with BCR-ABL and modulates BCR-ABL transforming activity and imatinib response of CML stem/progenitor cells. J Exp Med 205, 2657–2671 (2008)
T. Soussi, The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann NY Acad Sci 910, 121–137 (2000)
Acknowledgements
This work was financially supported by project EMER 07–048 and PI08-153 of Fondos de Investigación Sanitaria (FIS), and RD06/0020/0034 of Red Temática de Investigación Cooperativa en Cáncer (RTICC), Spain. Supported in Brazil by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 09/54377-2 and 10/51571-0; Programa de Mobilidade Internacional do Banco Santander/Universidade Estadual de Campinas (UNICAMP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 473641/2008-9. Special thanks to Bauke Ylstra of Microarray Laboratory of VU University Medical Center, Amsterdam, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is a reprint from ‘Genetic profile of adenoid cystic carcinomas (ACC) with high-grade transformation versus solid type, Ana Flávia Costa, Albina Altemani, Hedy Vékony, Elisabeth Bloemena, Florentino Fresno, Carlos Suárez, José Luis Llorente, Mario Hermsen’ originally published in Analytical Cellular Pathology/Cellular Oncology, Volume 33, number 5–6, 2010, pp. 217–228, IOS Press.
An erratum to this article can be found at http://dx.doi.org/10.1007/s13402-011-0057-1
Rights and permissions
About this article
Cite this article
Costa, A.F., Altemani, A., Vékony, H. et al. Genetic profile of adenoid cystic carcinomas (ACC) with high-grade transformation versus solid type. Cell Oncol. 34, 369–379 (2011). https://doi.org/10.1007/s13402-011-0037-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-011-0037-5