Abstract
Introduction
The progressive growth of malignancies is accompanied by a decline in the immune response through mechanisms which are poorly understood. Apoptosis and induction of inflammation by tumor released cytokines as tumor escape mechanisms have been proposed to play an important role in colorectal carcinogenesis.
Methods
Expression of Tumor necrosis factor-alpha (TNF-α) was analyzed in colorectal cancer specimen and the cancer cell line HT-29 by immunohistochemistry and RT-PCR. TNF-α expression on protein and mRNA level were correlated with clinical characteristics and impact on survival. TNFR-1 was co-labelled with TNF-α and CD8+ cytotoxic T cells in immunofluorescence double staining experiments. Results: 94% (n = 98/104) of the patients with CRC expressed TNF-α. High TNF-α expression was significantly associated with positive lymph node stage and recurrence of the tumor. Multivariate analysis revealed high TNF-α expression as an independent prognostic factor. Immunohistochemistry was correlated with RT-PCR results (т = 0.794). Immunofluorescence double staining experiments revealed increased TNFR-1 expression by CD8+ cells.
Conclusions
TNF-α expression by tumor cells may be an efficient immunological escape mechanism by inflammation-enhanced metastases and probably by induction of apoptosis in tumor-infiltrating CD8+ immune cells resulting in a down regulation of the tumoral immune response. Our data support the role of tumor-derived TNF-α expression as an important promoter of tumoral immune escape mechanisms and malignant progression, and suggest that analysis on either protein (immunohistochemistry) or RNA level (RT-PCR) can be used effectively in this respect. Targeting TNF-α may be a promising option, especially in cases with high TNF-α expression and positive lymph node metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related death worldwide. Between 40 and 50% of the patients die within 5 years after diagnosis although great advances have been made in the diagnosis [11]. Surgery is the mainstay of treatment for all colorectal cancer cases, aiming at complete (R0) resection of the tumor-infiltrated part of the colon, and removal of its lymphatic drainage. Nodal positive patients (UICC stage III) are a well-recognized risk population, and adjuvant treatment is administered in these patients. Although the role of lymphadenectomy seemed well-established over years, with tumor involvement of lymph nodes clearly recognized as one of the strongest independent prognostic factor, its role with respect to survival benefit has only recently been questioned: One group in Germany around the biostatistician D. Hölzel [24, 25] has suggested from indirect lines of evidence based on epidemiologic data, that lymph node metastastases are not able to metastasize. Although this is merely a hypothesis, there seems surprisingly little evidence in the literature for its ad hoc falsification, extensively stimulating basic research regarding lymphatic spread.
While many studies recently improved our understanding of tumor initiation and progression, many questions regarding the phenomenon of an increasingly ineffective tumor immune response during tumor growth still remains to be clarified [15, 16]. In fact, during cancer progression, colorectal tumor cells acquire various characteristics which allow them to evade immunological surveillance [28, 31, 51]. Particularly with regard to therapeutic modulation of the immune system, understanding of tumor-specific immunological responses and mechanisms leading to induction or suppression of the immune system are required. In this respect it has been hypothesized that tumor progression in immunocompetent individuals may reflect a failure of the immune system to recognize tumor antigen or may result from subversion of anti-tumor responses [20, 26]. Innate and adaptive immune responses can be induced against tumors and the protective effector cells include CD8+ cytotoxic T cells, Interferon (IFN)-γ producing CD4+ and CD8+ T cells, natural killer (NK) cells and macrophages. Although T cells usually constitute a main immune cell population attracted to the tumor site, they are often ineffective in their anti tumoral effects. Our recent evidence suggests that this may result from cytokine-induced apoptosis CD8+ T cells which is obviously one of these crucial tumor immune escape mechanisms [20, 21]. Cytokines are messengers of the immune system that can be also released from tumor cells. They are functioning in an autocrine and/or paracrine manner and are able to enhance or suppress immunity.
TNF-α is a cytokine ligand of the TNF family interacting with different receptors of the TNF receptor superfamily, which can induce apoptosis and may be one of the reasons for the progression of colorectal cancer [41]. TNF-α is produced by macrophages as well as tumor cells and has multiple effects on cell function by binding to specific, high-affinity cell surface receptors. Beside apoptotic mechanisms, TNF-α may even promote tumor growth at lower levels during cancer progression [54, 55]. Interestingly, during cancer progression tumor cells from CRC have been described to lose their susceptibility for the induction of apoptosis [5]. In a previous study we demonstrated significantly increased TNF-α expression in cancer patients detected by cytocine-measurements, indicating a potential role during tumor progression [20]. TNF-α acts via two distinct receptors [50]. Although the affinity for TNF receptor 2 (TNFR-2) is five times higher than that for TNFR-1 [48], the latter initiates the majority of the biological activities of TNF-α. TNFR-1 (p60) is expressed on all cell types, while TNFR-2 (p80) expression is mainly confined to immune cells [2]. The major difference between the two receptors is the death domain (DD) of TNFR-1 that is absent in TNFR-2. For this reason, TNFR-1 is an important member of the death receptor family that shares the capability of inducing apoptotic cell death [3]. Therefore we focused on TNFR-1 in our study. Besides this apoptotic signalling, TNFR-1 is widely studied because it is a dual role receptor: next to induction of apoptosis, it also has the ability to mediate inflammation signals. Although signalling pathways are well defined nowadays, the life-death signalling regulation is still poorly understood [4, 35].
However, the clinical impact of TNF-α expression by cancer cells in patients with colorectal cancer remains unanswered. Therefore we looked for the expression of this inflammatory cytokine in tumor tissues by immunohistochemistry and RT-PCR. Results of protein and RNA level were correlated. We furthermore analyzed protein expression results with tumor stages and patients’ survival to determine the role of TNF-α expression by cancer cells in tumor progression.
2 Material and methods
2.1 Tumor patients
Patients with histologically proven colorectal cancer (n = 104) undergoing curative surgical resection in our Department between 2001 and 2005 were included in the study [9]. The histological stage of the tumor was determined according to the Union International Contre le Cancer (UICC)-TNM staging system [19, 47]. Tumors localization, UICC stages, and tumor differentiation (grading) according to WHO [22] were documented in a prospectively maintained database. Data concerning age, gender, depth of infiltration, and lymph node metastases were collected. Regular medical visits of the patients after curative therapy were performed at intervals according to the governmental guidelines for tumor patients. Follow-up data were obtained from our local tumor registry of Lower Frankonia/Germany. Follow-up was complete (100%) for all patients. Mean follow-up accounted for (32 months ± 20.5 standard deviation). None of the patients that were chosen for analysis had undergone neoadjuvant treatment, with any preoperative antineoplastic protocol (neither chemotherapy nor radiochemotherapy). Patients with rectal cancer having undergone neoadjuvant treatment were excluded. Only patients in whom complete (R0) resections had been achieved were included in our study. Tumor tissue samples as well as normal colon tissues from the patients were collected with informed consent before surgical resection, frozen instantly in liquid nitrogen, and stored at −80°C until analyzed. Normal colon tissues from healthy individuals served as controls (n = 10). For stratification, immunohistochemical analysis confirmed that none of the analyzed tumors was positive for expression of hMLH1 and hMSH2 indicative for microsatellite instability (positive mismatch repair (MMR) status). The mucinous phenotype of colorectal carcinoma could be associated with false positive subcellular reactions by immunohistochemistry and was therefore excluded from our study. Clinical characteristics of the study population are summarized in Table 1.
2.2 Cell culture
We analyzed TNF-α expression in cells (1 × 104) from the human colorectal colon cell line HT-29 (Promochem, Wesel, Germany) in cytospins. HT-29 cells were cultured in RPMI-1640 medium supplemented with 10% newborn calf serum, 80 U/mL penicillin and 100 U/mL streptomycin in humidified atmosphere (90% relative humidity) with 5% CO2 at 37°C. The culture media were changed every 2 days.
2.3 Immunohistochemical staining procedure
First we assessed H&E sections from each tumor tissue to differentiate between tumor cell areas, stromal areas and infiltrating immune cells. We then stained for TNF-α in additional serial sections. The staining of TNF-α was performed on serial cryostat sections of the 104 snap-frozen colorectal cancer specimens in early-stage tumors (UICC I/II) and late-stage tumors (UICC III/IV) with neighbouring normal colon tissue and 10 normal colon specimens. For immunohistochemical analysis, unconjugated monoclonal mouse anti-human TNF-α was purchased from R&D systems (Wiesbaden-Nordenstadt, Germany). The unconjugated mouse isotype control antibody was obtained from eBioscience (San Diego, USA). A second unconjugated monoclonal mouse anti-human TNF-α clone was purchased from BD Pharmingen (Heidelberg, Germany) and was used for confirmation of TNF-α expression measured by the staining with anti-TNF-α antibody from R&D systems in a choice of 28 colorectal cancer specimens. In this choice, we stained for CD8 (Dako, Hamburg, Germany) and TNFR-1 (Abcam, Cambrige, UK). Additionally, we analyzed TNF-α expression in control cancer cell line HT-29 as positive control in cytospins. All tumors stained positive for cytokeratin-20 (CK-20) (Dako) and negative for cytokeratin-7 (CK-7) (Dako), a pattern characteristic of colonic adenocarcinoma [42]. Serial cryostat sections (5 μm) were mounted on glass slides and fixed in acetone for 10 min and then dried for 5 min. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Subsequently, the slides were incubated with the primary antibody or control antibody diluted in TBS plus 0.5% bovine serum albumine (BSA) overnight at 4°C in a humidified chamber and with horseradish peroxidise (HRP)-conjugated AffiniPure Donkey anti-mouse IgG at a 1:200 dilution (Jackson ImmunoResearch Laboratories Inc., Suffolk, England) for 30 min at room temperature in a humidified chamber. Slides were subsequently incubated for 5 min in DAB (3,3′-diaminobenzidine) (Biogenex, San Ramon, USA), counterstained with Haemalaun and mounted with Glycergel (Dako) and analyzed using a Zeiss camera (Jena, Germany) and imported into Microsoft Office Picture Manager.
2.4 Immunofluorescence double staining experiments (IF)
The sequential immunofluorescence (IF) double staining (co-expression) was analysed for TNFR-1 with TNF-α and CD8 expression. The secondary antibody used for immunofluorescence double staining of TNF-α and CD8 was a fluoresceinisothiocyanat (FITC)-conjugated AffiniPure donkey-anti-mouse IgG (Jackson ImmunoResearch) or Cy3-conjugated AffiniPure donkey-anti-mouse IgG (Jackson ImmunoResearch), used at 1:200 dilution with 30 min incubation time at room temperature in a humidified chamber. The secondary antibody for TNFR-1 was a fluoresceinisothiocyanat (FITC)-conjugated AffiniPure donkey-anti-rabbit IgG (Jackson ImmunoResearch) or Cy3-conjugated AffiniPure donkey-anti-rabbit IgG (Jackson ImmunoResearch), used at 1:200 dilution with 30 min incubation time at room temperature in a humidified chamber. Slides were counterstained with DAPI (4',6-Diamidino-2- phenylindoldihydrochlorid) (Sigma-Aldrich), covered with Polyvinyl-alcohol mounting medium (DABCO) (Sigma-Aldrich) and analysed using a Zeiss camera (Jena, Germany). The photographed images using the Metamorph software package (Visitron Systems, Puchheim, Germany) were imported into the Microsoft Office Picture Manager.
2.5 Quantification of Immunohistochemistry (IHC)
Quantification of TNF-α immunoenzymatic staining of tumor cells was performed analyzing six defined representative individual high power fields (×400) for each staining sample. Scoring was done by means of cell counting. The results were expressed as percentages (number of positive cells within 100 counted tumor cells,%). Cases with less than 10% positive cells were regarded as negative. Sections were evaluated by two independent blinded investigators for clinical data separately and, in case of discrepancies, both evaluated the slides simultaneously and made an agreement. We must, however, consider the fact that two investigators scores could be highly correlated with one another but show little agreement. Therefore, beside Kendall’s tau (т) correlation coefficient, the Intraclass Correlation Coefficient (ICC) was calculated, which is a measure to perform reliability of measurements or ratings [45]. In this purpose a distinction was made between two preliminary study models: First, TNF-α-expression was rated by the same investigators and second TNF-α-expression was rated by a different, second rater. In both models, the ICC was always a measure for absolute agreement because systematic differences were regarded as relevant.
2.6 Real-time quantitative reverse transcription-PCR analysis (RT-PCR)
To analyze gene expression of TNF-α by RT-PCR, we extracted total cellular RNA using RNeasy Minikit from Qiagen (Hilden, Germany). Areas of interest for each tissue section were manually microdissected with a scalpel from serial cryostat sections (5 μm) of colorectal cancer specimens in early-stage tumors (UICC I/II) and late-stage tumors (UICC III/IV). For both groups (early and advanced tumor stages) equal amounts of tumor tissue areas were assessed. RNA extraction was performed according to the manufacturer’s instructions. The lysate was loaded onto the RNeasy silica membrane (“RNeasy Mini spin column”, RNeasy Mini Kit Qiagen, Hilden, Germany). RNA binds, and all contaminants were efficiently washed away. Pure, concentrated RNA was eluted in water and stored at −70°C until further analyzes. The amount of total RNA was determined by measuring absorbance at 260 nm. The purity of the total RNA was established by confirming that the 260 nm: 280 nm ratio was within a 1.8-2.0 range, indicating that the RNA preparations were free of protein contaminants. Primer sets of short segments (50–150 base pairs) from Qiagen (Hilden/Germany) were used. Optimum primer concentration was determined by titration. Human colon matched cDNA was purchased from Pharmingen (Heidelberg, Germany) as control. The housekeeping gene Glyceraldehyde-3- phosphate dehydrogenase (GAPDH) was used for relative quantification and cDNA quality control. All PCR reactions were carried out with a DNA Engine Opticon 2 System (MJ Research, Biozym, Oldendorf, Germany). Reverse transcription from RNA to cDNA was carried out by using iScript cDNA Synthesis Kit (BioRad, Hercules, CA). Each PCR reaction was performed in 23 μl volume containing 11.5 μl of the LightCycler-DNA Master SYBR Green I mix (Applied Biosystems, Darmstadt, Germany), 10 pmol/μl forward primer, 10 pmol/μl reverse primer, 3 μl template DNA and 7.5 μl RNase free water. Initial denaturation at 95°C for 15 min was followed by 39 cycles of a denaturation step at 95°C for 15 s, an annealing step at 57.5°C for 30 s, and an extension step at 72°C for 30 s. To confirm amplification specificity, the PCR products from each primer pair were subjected to a melting curve analysis. The quantification data were analyzed with the LightCycler analysis software. Reproducibility was confirmed by independent PCR repeated twice. The average threshold cycle (Ct) value was calculated as the cycle number at which the fluorescence of the reporter reaches a fixed threshold. The difference (ΔCt) between the average Ct values of the samples in the target wells and those of the housekeeping gene GAPDH was assessed, followed by the calculation of the difference between the average ΔCt values of the tumor samples for each target and the ΔCt value of the normal tissues for that target (ΔΔCt). The relative quantification value, fold difference (mean) is expressed as 2-∆∆Ct.
2.7 Statistical analysis
Statistical analysis was performed with MedCalc Software (Mariakerke, Belgium). All values were expressed as median ± interquartil Range (IQR) because D’Agostino-Pearson test did not show a normal distribution of gene and protein expression. Overall survival was defined as the time period from surgical tumor resection to death or tumor specific death of the patient. The overall survival time in association with TNF-α expression was estimated using the Kaplan-Meier method [27]. Median cut-off value for either high or low expressors was set at 26% for TNF-α expression in all CRCs (n = 104); univariate analysis of significance for TNF-α expression differences in survival curves was evaluated with the log rank test. Multivariate analysis by using the Cox Proportional Hazards Model was performed on all parameters that were found to be significant on univariate factors [12]. The relation between TNF-α expression and clinicopathologic characteristics was tested via Pearson’s chi-square test analysis. Fisher’s exact test was used to ask whether there is any relation between two categorical variables where sample sizes were small (association between tumor recurrence and lymph node metastases).
Data were analyzed using the non-parametric Mann–Whitney U test. P values of less than 0.05 were regarded statistically significant. Correlation of immunohistochemistry and RT-PCR analysis was performed by the non-parametric Kendall’s tau (т) correlation coefficient because this coefficient does not assume a linear relationship between both methods/variables.
3 Results
3.1 Intra- and Interobserver reliability
A preliminary study was carried out to assess the intra- and interobserver reliability. There were significant correlations between the first and the second assessment for intraobserver reliability (TNF-α expression: т = 0.812, p < 0.0001, 95% CI 0.742 to 0.866; ICC for average measures was 0.9804, 95% CI 0.9700 to 0.9870) and also between two observers (TNF-α expression: т = 0.9593, p < 0.0001, 95% CI 0.9404 to 0.9722; ICC for average measures was 0.9777; 95% CI 0.9672 to 0.9849).
3.2 TNF-α expression is associated with tumor progression of colorectal adenocarcinomas
TNF-α was not expressed in normal colon tissue. TNF-α expression in the stroma was considerably weak and strongly associated with cancer cells but only particularly with infiltrating stromal cells. 94% (n = 98/104) of the patients with CRC expressed TNF-α. Stainings from the HT-29 colon cancer cell line in cytospins served as control for tumoral TNF-α expression and showed 70-80% positive tumor cells.
Furthermore, we analyzed positivity of all counted cells according to early (UICC I/II) and advanced (UICC III/IV) tumor stages. Compared to early (UICC I/II) tumor stages (n = 46, Median 15%, Interquartile range 8% - 33%; 95% CI 13% - 20%) TNF-α expression was significantly upregulated in advanced (UICC III/IV) tumor stages (n = 58, Median 30%, Interquartile range 28% - 34%; 95% CI 28% - 32%; p < 0.0001; Table 1).
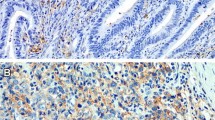
Figure 1a and b demonstrate a representative example of TNF-α expression in early (UICC I/II) and advanced (UICC III/IV) tumor stages. TNF-α staining results of six distant liver metastases were comparable with related primary tumors of advanced (UICC III/IV) tumor stages and showed no differences in increased TNF-α expression.
Immunohistochemical staining of TNF-α. Representative images of immunohistochemical expression of TNF-α by tumor cells during tumor progression (cytoplasmic staining pattern, brown, arrows). TNF-α is increased on tumor cells in early stage tumors (UICC I/II) a. In advanced stage tumors (UICC III/IV) TNF-α expression is increased compared to early stage tumors (UICC I/II) b. Adjacent stromal cells (asterisks) stained negative for TNF-α. DAB brown color, Haemalaun blue color, nuclear counterstaining. Original magnification: left x 100, right x 200
3.3 Correlation of TNF-α protein and gene expression
To confirm the results of the immunohistochemical staining, gene expression of TNF-α in 31 microdissected cases of CRC was assessed and correlated with immunohistochemical results. Compared to early (UICC I/II) tumor stages (n = 14, Median 1.70 fold-difference to normal tissue, Interquartile range 0.54 – 4.49; 95% CI 1.30 to 3.43) TNF-α expression was significantly upregulated in advanced (UICC III/IV) tumor stages (n = 17, Median 4.60 fold-difference to normal tissue, Interquartile range 2.16 – 5.10; 95% CI 3.91 to 4.80; p < 0.0001). We found a strong correlation of TNF-α protein and gene expression (т = 0.794, Fig. 2). The results confirmed elevated TNF-α protein and gene expression by cancer cells.
3.4 CD8+ infiltrating immune cells are associated with TNFR-1 expression
In a choice of 28 tumor samples, we associated CD8+ infiltrating immune cells with TNFR-1 expression. Figure 3a and b demonstrate a representative example of CD8+ and TNFR-1 expression in a serial section of an early tumor stage (UICC I/II). Immunofluorescence double staining showed TNF-α expression by tumor cells with adjacent TNFR-1 expressing by tumor infiltrating immune cells (Fig. 4). Most of these cells were shown to have a CD8+ phenotype. This suggests that CD8+ T cells are expressing TNFR-1 measured in immunhistochemical serial sections above (Fig. 5).
Immunohistochemical staining using antibodies for CD8 and TNFR-1: Immunohistochemistry shows representative images of CD8 and TNFR-1 expression in tumor infiltrating cells (membranous staining pattern, brown). In serial sections, increased expression of CD8 a and TNFR-1 b is found adjacent to the tumor (asterisks) in early tumor stages (UICC I/II). DAB brown color, Haemalaun blue color nuclear counterstaining. Original magnification: left x 100, right x 200
Co-expression of TNF-α with TNFR-1 by an immunofluorescent double staining: Immunofluorescent double staining images demonstrate a representative example of TNF-α expression by tumor cells (asterisks) with adjacent TNFR-1 expressing tumor infiltrating immune cells (arrows) in patients with CRC with (bottom) and without (top) nuclear counterstaining. Indication line shows tumor border in adjacent to infiltrating immune cells. FITC green Fluoresceinisothiocyanat, Cy3 red, and DAPI 4′,6-Diamidino-2- phenylindoldihydrochlorid blue. Calibration bar represents 25 μm
Co-expression of CD8 with TNFR-1 by an immunofluorescent double staining: Immunofluorescent double staining images demonstrate a representative example of CD8 co-expression with TNFR-1 in patients with CRC (arrows). FITC green Fluoresceinisothiocyanat, Cy3 red, and DAPI 4′,6-Diamidino-2- phenylindoldihydrochlorid blue. Calibration bar represents 25 μm
3.5 High TNF-α expression is strongly associated with tumor recurrence in patients with positive lymph node metastases
Analysis of TNF-α expression profiles within the tumors with clinicopathologic parameters demonstrated a strong association between tumor recurrence in patients with lymph node metastases (p = 0.000002716, Fisher’s exact test). Second, we analyzed a significant elevated TNF-α expression in patients within patients of tumor recurrence compared to patients without tumor recurrence (TNF-α expression in patients without tumor recurrence: n = 63, Median 20%, Interquartile range 10% - 33%; 95% CI 15% to 27%; TNF-α expression in patients with tumor recurrence: n = 41, Median 30%, Interquartile range 15% - 35%; 95% CI 28% to 33%; p = 0.0002). Subgroup analysis revealed a significantly elevated TNF-α expression in patients with tumor recurrence and positive lymph node metastases (n = 33, Median 30%, Interquartile range 28% - 34%; 95% CI 29% - 34%) compared to patients without tumor recurrence and positive lymph node metastases (n = 21, Median 23%, Interquartile range 20% - 32%; 95% CI 20% - 31%; p = 0.015; Fig. 6). No difference of TNF-α expression was found when comparing TNF-α expression in patients with and without tumor recurrence and negative lymph node metastases.
High TNF-α expression is strongly associated with tumor recurrence in patients with positive lymph node metastases: Significant elevated TNF-α expression was found within patients of tumor recurrence compared to patients without tumor recurrence (p = 0.0002). Subgroup analysis demonstrates a significantly elevated TNF-α expression in patients with tumor recurrence and positive lymph node metastases compared to patients without tumor recurrence and positive lymph node metastases (p = 0.015). No difference of TNF-α expression is found by comparing TNF-α expression in patients with and without tumor recurrence and negative lymph node metastases
3.6 Prognostic value of TNF-α in CRC
To analyze differences in the overall/tumor related survival among patients after successful (R0) curative surgical resection for CRC patients were divided into two subgroups as described above (dichotomous variables). Lymph node metastases (N+, p < 0.0001, HR = 0.1098, 95% CI = 0.05565 to 0.2167), pT stage (pT3/4, p < 0.0001, HR = 0.1089, 95% CI = 0.05397 to 0.2199) and grading (G3/4, p < 0.0001, HR = 0.2418, 95% CI = 0.1157 to 0.5055) were shown to be predictive factors of poorer survival in univariate analysis of all (n = 104) CRCs. To analyze differences in tumor related survival dependant on the TNF-α-expression by immunohistochemistry in CRC we divided the patients in at least two subgroups as described above (dichotomous variables). Survival in the subgroup with high TNF-α expression within their tumors (x > 26%) in CRC (n = 52, p = 0.0002, HR = 0.2835, 95% CI = 0.1476 to 0.5447), was significantly poorer in comparison to the subgroup of patients with low expression of TNF-α (Fig. 7). Data show that TNF-α expression in CRC is associated with clinical pathological features which may predict worse clinical outcome (Table 1). Multivariate analysis using the Cox Proportional Hazards Model demonstrate positive lymph node metastases, pT stage (pT3/4), and high TNF-α expression but not grading (G3/4) as independent prognostic factors in all (n = 104) cancer samples (LN positive: Exp (b) 6.4391; 95% CI of Exp (b) 2.6649 to 15.5585; p < 0.0001. pT3/4: Exp (b) 3.8363; 95% CI of Exp (b) 1.8550 to 7.9341; p = 0.0003086. High TNF-α expression: Exp (b) 3.1064; 95% CI of Exp (b) 1.4686 to 6.5705; p = 0.003174)).
4 Discussion
In the present study we focused on the putative role of inflammatory TNF-α in tissue invasion and lymph node metastases. Once, the development of cancer was categorized in six essential alterations in cell physiology that collectively dictate malignant growth. These important findings were described as ‘hallmarks of cancer’ [23, 34] and were characterized as self-sufficiency in growth signals, insensitivity to growth-inhibitory (antigrowth) signals, evasion of programmed cell death (apoptosis), unlimited replicative potential, sustained angiogenesis, and tissue invasion and metastases. Several experimental and epidemiological evidence indicate that, irrespective of the trigger for the development (chronic infection/inflammation or genetic alteration), a ‘smouldering’ inflammation suggested as the seventh hallmark of cancer is associated with the most of, if not all, tumors and supports their progression [10, 30].
The metastatic process consists of tumor cell detachment, local invasion, angiogenesis and survival in the circulation, adhesion to endothelial cells, extravasation and regrowth in different organs. In each step, causative molecules have been identified: these include cell adhesion molecules, various growth factors, matrix degradation enzymes and motility factors [28, 31, 51]. Inflammation by TNF-α-signalling has been shown to be involved in this process. Our finding of increased TNF-α gene and protein expression in cancer progression and metastasis is in line with results in other cancer entities [1, 32, 33, 36, 37, 43, 49, 52] and show high expression levels of this cytokine to be associated with an increased likelihood of tumor recurrence among patients with positive lymph node metastases.
The mode of tumor spread remains an enigma and has received great attention in recent years, as metastastatic spread remains the major cause of cancer mortality. The complex and highly selective metastatic cascade does not only depend on the intrinsic properties of tumor cells but also the microenvironment that they derive from. An inflammatory micorenvironment consisting of secretory cytokines, chemokines and growth factors has been suggested to contribute significantly to the invasive and metastatic traits of cancer cells [40, 49]. Our findings of increased TNF-α expression can bee seen in line with this perspective.
During inflammation, a number of proteins can be up-regulated to allow immune cells to migrate to sites of inflammation [6, 7, 10, 30]. Tumors use these same processes to invade adjacent structures. TNF is a potent pro-inflammatory cytokine that can be utilized by tumors to induce other downstream molecules involved in the metastatic process [29, 38, 39]. Blocking of TNF-α melanoma model reduced the number of metastatic lung tumors indicating that some tumors may intrinsically use TNF within their microenvironment to aid metastasis [14]. This suggests that human TNF may also promote metastasis in human tissue [53]. Our results regarding TNF-α expression in colorectal cancer specimen and the colorectal cancer cell line HT-29 indicate that TNF-α expression is associated with cancer progression and reduced tumor-specific survival among patients with high TNF-α expression compared to patients with low TNF-α expression. TNF-α was expressed by colorectal cancer cells but was rarely detected in stromal cells which represent the other part of the inflammatory microenvironment. From our immunohistochemical results we conclude that the TNF-α mediated inflammatory microenvironment is tumor-derived and in a lesser extent related to tumor-infiltrating leukocytes. The following failure of the immune system may result from subversion of anti-tumor responses in cancer patients [26]. Based on our immunofluorescence double staining experiments in patients with CRC we suggest an inflammatory microenvironment or even apoptotic depletion of CD8+ cells by a TNF-α/TNFR-1 mechanism. This may result in promoting metastasis or further recruitment of tumor infiltrating cells [35].
It was initially thought that the majority of the effects of TNF on cancers were beneficial enhancing immunological rejection of cancers via immune responses. However, the clinical trials using TNF to treat cancer were disappointing due to the high toxicity caused by large amounts of cytokine [8, 13, 17, 44]. Therefore, the use of TNF as an anti-cancer agent has clear limitations due to its toxicity and may even be deleterious in the long term, as it can lead to re-growth of resistant tumors and, in the case of melanoma, more aggressive strains [56].
Today, there is no doubt about the link between inflammation and cancer [6, 7, 10, 30]. TNF-α may play a key role in this process [5], which make new TNF-related antineoplastic therapies attractive. The use of anti-TNF-α biologicals as therapeutic approach has been successful in patients suffering from Rheumatoid arthritis (RA) [46]. On the other hand, it was stated that the use of anti-TNF-α agents may induce cancer because of TNF-α inhibition released by immune cells leading to apoptosis in cancer cells. This possible additional increased risk for lymphoma associated with TNF blockers was based on few cases and needs confirmation [18]. TNF-α does not only act as pro-inflammatory cytokine contributing to the wide spectrum of human diseases including inflammatory diseases, but can also induce tumor development. Therefore, the use of TNF-α inhibitors with a focus in cancer treatment has been taken into account [54]. With regard to our results of increased TNF-α expression by cancer cells and its role in other diseases we hypothesize the inflammatory mechanism of action by this cytokine as an example for the seventh ‘hallmark of cancer’ in CRC. However, the role of TNF-α-expression and its therapeutic implication in cancer patients remains a double-edged sword.
TNF-α protein and gene expression was significantly elevated in cancer samples. Strong correlation of immunohistochemistry and RT-PCR results were associated with tumor progression. Expression of TNF-α by tumor cells during cancer progression may sustain multi-step carcinogenesis, further tumor growth by several tumor escape mechanisms, such as recruitment and education of immune cells, inflammation-enhanced metastases, and induction of apoptosis in infiltrating CD8+ cells. These findings may contribute to a further understanding in the pathogenesis of colorectal cancer. The use of anti-TNF-α agents may be a promising option especially in cases with high TNF-α expression and positive lymph node metastases.
Abbreviations
- CRC:
-
colorectal carcinoma
References
F. Adami, A. Guarini, M. Pini, F. Siviero, R. Sancetta, M. Massaia, L. Trentin, R. Foa, G. Semenzato, Serum levels of tumour necrosis factor-alpha in patients with B-cell chronic lymphocytic leukaemia. Eur J Cancer 30A(9), 1259–1263 (1994)
B.B. Aggarwal, Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3(9), 745–756 (2003)
A. Ashkenazi, V.M. Dixit, Death receptors: signalling and modulation. Science 281(5381), 1305–1308 (1998)
S.J. Baker, E.P. Reddy, Modulation of life and death by the TNF receptor superfamily. Oncogene 17(25), 3261–3270 (1998)
F. Balkwill, Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 13(2), 135–141 (2002)
F. Balkwill, A. Mantovani, Inflammation and cancer: back to Virchow? Lancet 357(9255), 539–545 (2001)
F. Balkwill, L.M. Coussens, Cancer: an inflammatory link. Nature 431(7007), 405–406 (2004)
T.D. Brown, P. Goodman, T. Fleming, J.S. Macdonald, E.M. Hersh, T.J. Braun, A phase II trial of recombinant tumor necrosis factor in patients with adenocarcinoma of the pancreas: a Southwest Oncology Group study. J Immunother 10(5), 376–378 (1991)
M. Bueter, M. Gasser, N. Schramm, T. Lebedeva, G. Tocco, C. Gerstlauer, M. Grimm, E. Nichiporuk, A. Thalheimer, A. Thiede, D. Meyer, G. Benichou, A.M. Waaga-Gasser, T-cell response to p53 tumor-associated antigen in patients with colorectal carcinoma. Int J Oncol 28(2), 431–438 (2006)
F. Colotta, P. Allavena, A. Sica, C. Garlanda, A. Mantovani, Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30(7), 1073–1081 (2009)
C.C. Compton, L.P. Fielding, L.J. Burgart, B. Conley, H.S. Cooper, S.R. Hamilton, M.E. Hammond, D.E. Henson, R.V. Hutter, R.B. Nagle, M.L. Nielsen, D.J. Sargent, C.R. Taylor, M. Welton, C. Willett, Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124(7), 979–994 (2000)
D.R. Cox, Regression models and life tables. J R Stat Soc 34, 1987–2001 (1972)
E.T. Creagan, J.S. Kovach, C.G. Moertel, S. Frytak, L.K. Kvols, A phase I clinical trial of recombinant human tumor necrosis factor. Cancer 62(12), 2467–2471 (1988)
S. Cubillos, B. Scallon, M. Feldmann, P. Taylor, Effect of blocking TNF on IL-6 levels and metastasis in a B16-BL6 melanoma/mouse model. Anticancer Res 17(3C), 2207–2211 (1997)
J. Finke, S. Ferrone, A. Frey, A. Mufson, A. Ochoa, Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today 20(4), 158–160 (1999)
A.B. Frey, N. Monu, Signalling defects in anti-tumor T cells. Immunol Rev 222, 192–205 (2008)
W.L. Furman, D. Strother, K. McClain, B. Bell, B. Leventhal, C.B. Pratt, Phase I clinical trial of recombinant human tumor necrosis factor in children with refractory solid tumors: a Pediatric Oncology Group study. J Clin Oncol 11(11), 2205–2210 (1993)
P. Geborek, A. Bladstrom, C. Turesson, A. Gulfe, I.F. Petersson, T. Saxne, H. Olsson, L.T. Jacobsson, Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis 64(5), 699–703 (2005)
F.L. Greene, TNM staging for malignancies of the digestive tract: 2003 changes and beyond. Semin Surg Oncol 21(1), 23–29 (2003)
Grimm M, Kim M, von Rahden B, Meier E, Tsaur I, Rosenwald A, Germer CT, Gasser M and W.-G. AM., Tumour-mediated TRAIL-Receptor expression indicates effective apoptotic depletion of infiltrating CD8+ immune cells in clinical colorectal cancer, Eur J Cancer accepted (2010).
Grimm M, Gasser M, Bueter M, Otto C, Strehl J, Wang J, Nichiporuk E, Germer CT, Meyer D, Waaga-Gasser AM and T. A., Immunological escape mechanisms of colorectal hepatic metastases, BMC Cancer accepted (2010).
A.L. Hamilton SR, Pathology and genetics of tumours of the digestive system. Tumours of small intestine (IARC, Lyon, 2000), pp. 69–91
D. Hanahan, R.A. Weinberg, The hallmarks of cancer. Cell 100(1), 57–70 (2000)
D. Holzel, J. Engel, U. Lohrs, Elective lymph node dissections–still a standard in cancer surgery? Zentralbl Chir 133(6), 582–589 (2008)
D. Holzel, R. Eckel, J. Engel, Colorectal cancer metastasis. Frequency, prognosis, and consequences. Chirurg 80(4), 331–340 (2009)
A.G. Jarnicki, J. Lysaght, S. Todryk, K.H. Mills, Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol 177(2), 896–904 (2006)
E.L. Kaplan, P. Meier, Nonparametric estimation from incomplete observations. J Am Stat Assoc 75, 457–487 (1958)
S. Lankiewicz, E. Rother, S. Zimmermann, C. Hollmann, F. Korangy, T.F. Greten, Tumour-associated transcripts and EGFR deletion variants in colorectal cancer in primary tumour, metastases and circulating tumour cells. Cell Oncol 30(6), 463–471 (2008)
S.T. Malik, M.S. Naylor, N. East, A. Oliff, F.R. Balkwill, Cells secreting tumour necrosis factor show enhanced metastasis in nude mice. Eur J Cancer 26(10), 1031–1034 (1990)
A. Mantovani, Cancer: Inflaming metastasis. Nature 457(7225), 36–37 (2009)
W.E. Mesker, G.J. Liefers, J.M. Junggeburt, G.W. van Pelt, P. Alberici, P.J. Kuppen, N.F. Miranda, K.A. van Leeuwen, H. Morreau, K. Szuhai, R.A. Tollenaar, H.J. Tanke, Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol 31(3), 169–178 (2009)
V. Michalaki, K. Syrigos, P. Charles, J. Waxman, Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 90(12), 2312–2316 (2004)
D.W. Miles, L.C. Happerfield, M.S. Naylor, L.G. Bobrow, R.D. Rubens, F.R. Balkwill, Expression of tumour necrosis factor (TNF alpha) and its receptors in benign and malignant breast tissue. Int J Cancer 56(6), 777–782 (1994)
C.P. Morales, R.F. Souza, S.J. Spechler, Hallmarks of cancer progression in Barrett’s oesophagus. Lancet 360(9345), 1587–1589 (2002)
J.R. Muppidi, J. Tschopp, R.M. Siegel, Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity 21(4), 461–465 (2004)
J. Nakashima, M. Tachibana, M. Ueno, A. Miyajima, S. Baba, M. Murai, Association between tumor necrosis factor in serum and cachexia in patients with prostate cancer. Clin Cancer Res 4(7), 1743–1748 (1998)
M.S. Naylor, G.W. Stamp, W.D. Foulkes, D. Eccles, F.R. Balkwill, Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest 91(5), 2194–2206 (1993)
P. Orosz, B. Echtenacher, W. Falk, J. Ruschoff, D. Weber, D.N. Mannel, Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med 177(5), 1391–1398 (1993)
Z. Qin, S. Kruger-Krasagakes, U. Kunzendorf, H. Hock, T. Diamantstein, T. Blankenstein, Expression of tumor necrosis factor by different tumor cell lines results either in tumor suppression or augmented metastasis. J Exp Med 178(1), 355–360 (1993)
S. Rajput, A. Wilber, Roles of inflammation in cancer initiation, progression, and metastasis. Front Biosci (Schol Ed) 2, 176–183 (2010)
J.C. Reed, Apoptosis-targeted therapies for cancer. Cancer Cell 3(1), 17–22 (2003)
M.J. Sack, S.A. Roberts, Cytokeratins 20 and 7 in the differential diagnosis of metastatic carcinoma in cytologic specimens. Diagn Cytopathol 16(2), 132–136 (1997)
H.I. Sati, M. Greaves, J.F. Apperley, R.G. Russell, P.I. Croucher, Expression of interleukin-1beta and tumour necrosis factor-alpha in plasma cells from patients with multiple myeloma. Br J Haematol 104(2), 350–357 (1999)
P. Selby, S. Hobbs, C. Viner, E. Jackson, A. Jones, D. Newell, A.H. Calvert, T. McElwain, K. Fearon, J. Humphreys et al., Tumour necrosis factor in man: clinical and biological observations. Br J Cancer 56(6), 803–808 (1987)
P.E. Shrout, J.L. Fleiss, Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2), 420–428 (1979)
J.S. Smolen, D. Aletaha, Developments in the clinical understanding of rheumatoid arthritis. Arthritis Res Ther 11(1), 204 (2009)
Sobin LH and W. Ch., UICC. TNM Classification of Malignant Tumors, 6th edition (2002).
L.A. Tartaglia, D.V. Goeddel, Two TNF receptors. Immunol Today 13(5), 151–153 (1992)
S. Trompet, A.J. de Craen, S. Mooijaart, D.J. Stott, I. Ford, N. Sattar, W. Jukema, R.G. Westendorp, High innate production capacity of proinflammatory cytokines increases risk for death from cancer: results of the PROSPER study. Clin Cancer Res 15(24), 7744–7748 (2009)
P. Vandenabeele, W. Declercq, R. Beyaert, W. Fiers, Two tumour necrosis factor receptors: structure and function. Trends Cell Biol 5(10), 392–399 (1995)
L.M. Veenendaal, O. Kranenburg, N. Smakman, A. Klomp, I.H. Borel Rinkes, P.J. van Diest, Differential Notch and TGFbeta signalling in primary colorectal tumors and their corresponding metastases. Cell Oncol 30(1), 1–11 (2008)
K. Warzocha, P. Ribeiro, N. Renard, J. Bienvenu, C. Charlot, B. Coiffier, G. Salles, Expression of genes coding for the tumor necrosis factor and lymphotoxin ligand-receptor system in non-Hodgkin’s lymphomas. Cancer Immunol Immunother 49(9), 469–475 (2000)
Y. Wu and B. P. Zhou, TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion, Br J Cancer.
I. Zidi, S. Mestiri, A. Bartegi, N. B. Amor, TNF-alpha and its inhibitors in cancer, Med Oncol (2009).
K. Zins, D. Abraham, M. Sioud, S. Aharinejad, Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res 67(3), 1038–1045 (2007)
C.C. Zouboulis, K. Schroder, C. Garbe, K. Krasagakis, S. Kruger, C.E. Orfanos, Cytostatic and cytotoxic effects of recombinant tumor necrosis factor-alpha on sensitive human melanoma cells in vitro may result in selection of cells with enhanced markers of malignancy. J Invest Dermatol 95(6 Suppl), 223S–230S (1990)
Acknowledgements
The authors thank the assistance of Mrs. Mariola Dragan and Mrs. Sabine Mueller for their technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is a reprint from ‘Tumor necrosis factor-α is associated with positive lymph node status in patients with recurrence of colorectal cancer indications for anti-TNF-α agents in cancer treatment, M. Grimm, M. Lazariotou, S. Kircher, A. Hfelmayr, C.T. Germer, B.H.A. von Rahden, A.M. Waaga-Gasser and M. Gasser’ originally published in Analytical Cellular Pathology/Cellular Oncology, Volume 33, number 3-4, 2010, pp. 151-163, IOS Press.
M. Grimm and M. Lazariotou contributed equally to this work.
An erratum to this article can be found at http://dx.doi.org/10.1007/s13402-011-0057-1
Rights and permissions
About this article
Cite this article
Grimm, M., Lazariotou, M., Kircher, S. et al. Tumor necrosis factor-α is associated with positive lymph node status in patients with recurrence of colorectal cancer—indications for anti-TNF-α agents in cancer treatment. Cell Oncol. 34, 315–326 (2011). https://doi.org/10.1007/s13402-011-0027-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-011-0027-7