Abstract
Background
Oxaliplatin is frequently used in the treatment of metastatic colorectal cancer (CRC). Our previous work shows that oxaliplatin induces the pro-apoptotic protein Noxa in CRC cells. The Bcl2-inhibitor ABT-737 is particularly effective in cells with high Noxa levels. Therefore, we tested whether oxaliplatin and ABT-737 display synergy in killing CRC cells.
Methods
A panel of CRC cell lines was treated with oxaliplatin and ABT-737, either alone or in combination. Apoptosis was measured by FACS analysis of sub-G1 DNA content and by Western blot analysis of caspase-3 processing. Noxa expression was suppressed by lentiviral RNA interference.
Results
Oxaliplatin and ABT-737 displayed a strong synergistic apoptotic response, which was dependent on wildtype TP53 and oncogenic KRAS. TP53 and KRAS were required for drug-induced Noxa expression and this was essential for tumor cell apoptosis. Oxaliplatin, but not ABT-737, induced p53 accumulation, but both drugs stimulated Noxa expression. Combination treatment of mice with subcutaneous tumor xenografts drastically reduced tumor volume, while single drug treatment had no effect.
Conclusion
ABT-737 synergizes with oxaliplatin to kill colorectal cancer cells. This requires induction of Noxa by wildtype TP53 and oncogenic KRAS. Future studies should explore the anti-tumor efficacy of this drug combination in mouse models for spontaneous CRC development and in patient-derived tumor cell cultures and xenografts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Oxaliplatin and 5-fluorouracil (5-FU) are widely used chemotherapeutic drugs in the treatment of colorectal cancer patients. Although these drugs increase the lifespan of patients with metastatic disease, overall survival rarely exceeds 2 years [3]. Increasing the therapy response to prolong overall survival therefore remains a major challenge.
Previously, we showed that the efficient killing of colorectal cancer cells by oxaliplatin or by 5-FU was dependent on the presence of oncogenic KRAS and wild type TP53. This was caused by induction of the pro-apoptotic TP53 response gene Noxa [4].
Noxa belongs to the family of BH3-only proteins, which induce apoptosis by binding to and neutralizing survival proteins like Bcl-2, Bcl-XL, Bcl-w, Mcl-1 and Bfl1/A1. In doing so, these proteins allow the pro-apoptotic proteins Bax and Bak to form oligomers within the mitochondrial membrane which causes mitochondrial outer membrane depolarization, release of cytochrome C, formation of the apoptosome, and activation of effector caspases that mediate cell destruction [25]. In normal untransformed cells Noxa is not generally considered to be a potent inducer of cell death when compared to other members of the BH3-only proteins, like Puma and Bim. However, in tumor cells Noxa does play a major role in the induction of apoptosis [4, 17]. The capacity of Noxa to induce apoptosis is strongly enhanced when anti-apoptotic proteins that are not bound by Noxa, such as Bcl-2, Bcl-XL and Bcl-w, are neutralized [2]. A potent inhibitor that binds and neutralizes Bcl-2, Bcl-XL and Bcl-w (but not Mcl-1 or Bfl1/A1) is the small molecule inhibitor ABT-737 [15]. Interestingly, ABT-737 is particularly effective in cells in which the Mcl-1 survival protein is neutralized by high Noxa levels [6, 12, 28]. Based on our previous study in which oxaliplatin caused apoptosis in a Noxa-dependent fashion [4], we tested whether ABT-737 could synergize with oxaliplatin in stimulating apoptosis in CRC cells. Furthermore, we tested the contribution of mutant KRAS and wild type TP53 to Noxa induction and sensitization to apoptosis.
2 Materials and methods
2.1 Cell culture
The human CRC cell line HCT116G13D/wt and the isogenic cell line Hkh2ko/wt lacking the mutant KRASD13 allele were kindly provided by Dr Shirasawa [18]. The murine C26 and human DLD1, Ls174T and HT29 CRC cell lines were purchased from ATCC (Manassas, VA, USA). HCT116 p53wt/wt and p53−/− cells were kindly provided by Dr B Vogelstein. HCT116 Noxa knockdown and control cells were described before [4]. The C26 cells express a mutant Kras oncogene (G12D) [19]. C26 cells contain wildtype p53. This is based on the finding that C26 cells show induction of p21 following chemotherapy, like all other wildtype p53 cell lines. By contrast, all p53 mutant cell lines fail to induce p21. (DR, unpublished observations). All cells were cultured in Dulbecco’s modified Eagle’s medium (Dulbecco, ICN Pharmaceuticals, Costa Mesa, CA, USA) supplemented with 5% (v/v) fetal calf serum and 2 mM Glutamine both purchased from Cambrax (New Jersey, USA).
2.2 FACS analysis of the cell cycle profile and apoptosis
The cell cycle profile was determined to asses the percentage of apoptotic cells (sub-G1 population) and G2 arrest after treatment with oxaliplatin (Sanofi Aventis, Gouda, The Netherlands) and ABT-737 (kindly provided by ABBOT Laboratories, Illinois, USA) by FACS analysis using propidium ioide (Sigma Aldrich). Cells (2 × 105) were seeded in 6-well plates (Costar Corning, NY, USA) and incubated overnight at 37°C in a humidified atmosphere containing 5% CO2. The following day the cells were treated with either 25 μM oxaliplatin, 2.5 μM ABT-737 or the combination for 24 h (Ls174T were treated for 48 h). Floating and attached cells were harvested and fixed as single cells in ice-cold 70% Ethanol for 15 min at 4°C followed by an incubation of 30 min at 37°C with 20 μg/ml propidium iodide and 100 μg/ml RNase A (Roche Diagnostics, Basel, Switzerland). All samples were analysed by bivariate flow cytometry using Cell Quest software (Becton Dickinson, Breda, The Netherlands).
2.3 Determination of synergistic drug responses
Combination index (CI) values were calculated by Calcusyn software (Biosoft, Cambridge, UK) to determine synergy. To this end the cells were treated with both drugs at varying doses of a constant ratio: 50:5, 25:2.5, 12.5:1.25 and 6.25:0.625 μM oxaliplatin:ABT-737 respectively, in triplicate. FACS analysis of sub-G1 content was then used to determine the % apoptotic cells in all these cultures. These values were used in Calcusyn to asses the CI values. CI values < 0.30 indicate strong synergy. CI values > 0.80 indicate no synergy.
2.4 Western blotting and antibodies
Lysates of treated and untreated tumor cells were prepared in lysisbuffer (20 mM HEPES pH7.4, 1% NP40, 150 mM NaCl, 5 mM MgCl2, 10% glycerol). Equal amounts of protein were run out on SDS-PAA gels and were analyzed by Western blotting using antibodies directed against p53, p21 (DO-1, C19; Santa Cruz, CA, USA;), cleaved caspase-3, Puma (Cell Signaling Technology, Danvers, MA, USA), Noxa (114 C307.1; Imgenex, San Diego, CA, USA), Bcl-2 (Santa Cruz, CA, USA) and Mcl-1 (BD Pharmingen, San Diego, CA, USA). β-Actin (AC-15) was used to control for protein load and was obtained from Novus Biologicals, Littleton, CO, USA.
2.5 Xenograft tumor mouse model
All experiments were conducted in accordance with the guidelines of the Animal Welfare Committee of the University Medical Centre Utrecht, The Netherlands. C26 cells (1 × 106) were subcutaneously injected into the flanks of 8–10 weeks old male BALB/c mice (Charles River; Sulzfeld, Germany). Tumor volume (V) was calculated by \( {\text{V}} = {\text{A}}\, \times \,{{\text{B}}^2}\, \times \,0.{5236} \), whereas A indicates the largest diameter and B the diameter perpendicular to A. Tumors were grown for 7 days until a volume of approximately 50mm3 was reached. At day 7 the mice were injected intraperitonally (i.p.) with one dose of 5 mg/kg oxaliplatin followed by daily i.p. injections of 50 mg/kg ABT-737 in 30% propylene glycol (Sigma Aldrich), 5% Tween-80 (Merck, New Jersey, USA) and 65% 50 mg/ml dextrose (Sigma Aldrich) in water at pH4. Mice that did not receive oxaliplatin or ABT-737 received the same injections of the vehicles (PBS for oxaliplatin and PG/TX100/dextrose for ABT-737).
2.6 Statistical analysis
All in vitro experiments were performed at least three times independently. In vivo experiments consisted of 8 mice per group. Figures depict group means ± SEM. Statistical differences were analyzed by one-way ANOVA followed by a Bonferroni multiple comparison test using GraphPad Prism software (GraphPad, San Diego, CA). A p value < 0.05 was considered to be statistically significant (*). An unpaired student’s T test was used for comparing apoptosis percentages based on FACS analysis of sub-G1 DNA content.
3 Results
3.1 ABT-737 synergizes with oxaliplatin to kill colorectal cancer cells
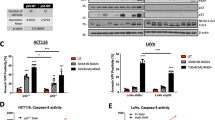
A panel of 5 colorectal cancer cell lines (C26, HCT116, LS174T, DLD1, HT29) was treated with oxaliplatin and ABT-737 either alone or in combination. Apoptosis was measured by FACS analysis of sub-G1 content. In three cell lines (C26, HCT116, LS174T) the combination treatment showed a strong synergistic apoptosis response, indicated by low combination indices (CI) (Fig. 1a), while in two others (DLD1, HT29) this effect was not observed. Interestingly, the synergistic response to combination treatment correlated with wild type TP53 status (Fig. 1a).
Synergistic p53-dependent killing of colorectal cancer cells by oxaliplatin and ABT-737. (a) Cells were treated with oxaliplatin and/or ABT-737 for 24 h (C26, HCT116, DLD1, HT29) or 48 h (Ls174T). Cells were then fixed in ethanol and stained with propidium iodide (PI). The sub-G1 fraction was determined by FACS analysis of PI-stained cells. Error bars represent SEM based on 3 independent experiments. Combination-index (CI) values were calculated using Calcusyn. CI values < 0.30 indicate strong synergy. CI values > 0.80 indicate no synergy. (b) Isogenic HCT116 p53 wt and knockout cells were treated as in A. Lysates were prepared and analyzed for the presence of p53, p21, cleaved caspase-3 and β-Actin by Western blotting. The sub-G1 fraction was determined by FACS analysis of PI-stained cells. *p < 0.05
3.2 TP53 is required for synergistic tumor cell killing by oxaliplatin and ABT-737
To test whether TP53 is required for synergistic tumor cell killing by oxaliplatin and ABT-737, we used HCT116 p53wt/wt and HCT116 p53−/− cells. These isogenic cell lines, only differing in p53 status, were treated with either drug alone or with the combination. FACS analysis of sub-G1 DNA content and Western blotting for active caspase-3 showed that HCT116 p53−/− cells were completely resistant to apoptosis induced by the combination treatment (Fig. 1b).
3.3 Noxa is required for caspase-3 processing by oxaliplatin and ABT-737
Since oxaliplatin induces Noxa to promote apoptosis [4] we next tested how the single and combination treatments affected expression levels of Noxa, the related protein Puma, and the Noxa/Puma-target Mcl1. Single treatment of TP53 wildtype cells with oxaliplatin or ABT-737 alone induced expression of Noxa, and this was strongly enhanced during combination treatment (Fig. 2a). Puma levels were unaffected by treatment with either drug alone, but were reduced by the combination treatment. Mcl1 was marginally induced by ABT-737, but this was not observed in cells treated with oxaliplatin alone or in cells treated with both drugs (Fig. 2a).
Oxaliplatin and ABT-737 induce Noxa in a TP53- and mutant KRAS-dependent manner. (a) HCT116 p53wt/wt and p53−/− cells were treated with oxaliplatin and/or ABT-737. Lysates were prepared and analyzed for the presence of Mcl-1, Puma, Noxa, cleaved caspase-3 and β-Actin by Western blotting. (b) As in (a) but using HCT116 KRASG13D/wt and Hkh2 KRASko/wt cells. The sub-G1 fraction was determined by FACS analysis of PI-stained cells. (c) As in A but using HCT116 Noxa-knockdown and control HCT116 cells expressing shRNA’s targeting firefly luciferase. The sub-G1 fraction was determined by FACS analysis of PI-stained cells. *p < 0.05
Apoptosis-resistant p53−/− cells were unable to induce Noxa expression following single or combined drug treatment, did not activate caspase-3, and were resistant to apoptosis (Figs. 1b and 2a). TP53-deficiency did not affect Puma levels but reduced Mcl1 levels by approximately 50% (Fig. 2a).
Our previous results had indicated that induction of Noxa by oxaliplatin not only depends on TP53 but also on oncogenic KRAS [4]. Therefore, we tested whether the synergistic induction of Noxa following combined treatment with oxaliplatin and ABT-737 required the presence of this oncogene. Indeed, mutant KRAS-deficient HCT116 cells were unable to induce Noxa, failed to process caspase-3 and were resistant to apoptosis following treatment with either drug alone or with the combination (Fig. 2b).
To assess the contribution of Noxa to apoptosis induced by the combination treatment we made use of HCT116 cells in which Noxa was stably suppressed by RNA interference [4]. Neither oxaliplatin alone nor the combination with ABT-737 increased Noxa levels under these conditions and caspase-3 processing and apoptosis were largely prevented (Fig. 2c).
3.4 Oxaliplatin and ABT-737 synergistically reduce tumor growth
Finally, we assessed whether oxaliplatin and ABT-737 could also affect tumor growth in a synergistic fashion. To this end BALB/c mice were injected subcutaneously with the syngenic colorectal cancer cell line C26. Treatment with oxaliplatin, ABT-737 or the combination started 7 days following tumor inoculation and the mice were sacrificed on day 15. Tumor growth was not significantly affected by oxaliplatin or ABT-737 alone at the doses used. However, treatment of the mice with both drugs strongly and significantly reduced tumor growth resulting in a 75% reduction of tumor volume on day 15 (Fig. 3).
Synergistic reduction of tumor growth by oxaliplatin and ABT-737. Mice carrying subcutaneous C26 tumors were treated with oxaliplatin on day 7 (arrow) and ABT-737, either alone or in combination. Control mice received both vehicles. Treatment started 7 days following subcutaneous tumor cell injection. Tumor growth was analyzed by caliper measurements. Observers were blinded to treatment. *p <0,05
4 Discussion
Our results show that ABT-737 and oxaliplatin kill colorectal tumor cells in a synergistic fashion, and that this depends on induction of the pro-apoptotic TP53 target gene Noxa. Oncogenic KRAS may further promote TP53-dependent Noxa induction by stimulating p53 Ser37 phosphorylation [4]. The increase in Noxa levels following oxaliplatin exposure is caused by the classical DNA damage – p53 pathway. ABT-737 selectively inhibits the anti-apoptotic proteins Bcl-2, Bcl-XL, and Bcl-W, but leaves the Noxa targets Mcl-1 and A1 unaffected [10, 15, 22]. Like oxaliplatin, ABT737 increased Noxa levels via an as yet unknown mechanism. We propose a model in which the cooperative induction of Noxa by oxaliplatin and ABT-737 effectively neutralizes the Mcl1/A1 branch of survival proteins, while ABT-737 targets the Bcl2 branch in addition (Fig. 4). Together, this is sufficient to neutralize all Bcl2 survival proteins, which ultimately results in capsase-3 activation and apoptosis.
In our previous study we showed that oxaliplatin alone was sufficient to induce apoptosis [4]. In that study oxaliplatin-induced apoptosis was marginal after 24 h (as in the present study), but steadily increased over a period of 3 days. In the present study we analyzed apoptosis following single and combination treatments after 24 h. Most likely, oxaliplatin-induced DNA damage accumulates over time but, at the concentration used in both the present and our previous study, it is not sufficient to induce massive apoptosis within 24 h.
Recently it was shown that Puma (but not Noxa) is sensitive to caspase-3-dependent proteolytic destruction [5]. In line with these results, we found that caspase-3 processing in cells treated with the drug combination was associated with strongly reduced levels of Puma, but not Noxa.
A number of studies have demonstrated the importance of the Mcl1/Noxa axis in determining the tumor cell response to ABT-737. This relationship was shown in renal cell carcinoma cells [28], small cell and non small cell lung cancer cells [6, 12, 21, 24, 26], lymphoma and acute lymphoblastic leukemia cells [7, 8, 27], acute and chronic myeloid leukemia cells [10, 11], prostate cancer cells [1, 16], melanoma cells [9, 13, 23], glioblastoma cells [20] and colorectal cancer cells [14]. Our study demonstrates that the widely used chemotherapeutic drug oxaliplatin induces Noxa expression, and that this makes cells highly sensitive to killing by ABT-737. In addition, we have identified two genetic determinants of synergistic tumor cell killing by oxaliplatin and ABT-737: wildtype TP53 and oncogenic KRAS. The molecular basis for this dependency is that both proteins are required for drug-induced Noxa expression. Further work using patient-derived colonospheres and xenografts is needed to asses the potential therapeutic benefit of oxaliplatin/ABT-737 combination therapy in (a subpopulation of) human colorectal tumors.
References
K. Bray, H.Y. Chen, C.M. Karp, M. May, S. Ganesan, V. Karantza-Wadsworth, R.S. DiPaola, E. White, Bcl-2 modulation to activate apoptosis in prostate cancer. Mol. Cancer Res. 7, 1487–1496 (2009)
L. Chen, S.N. Willis, A. Wei, B.J. Smith, J.I. Fletcher, M.G. Hinds, P.M. Colman, C.L. Day, J.M. Adams, D.C. Huang, Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 (2005)
D. Cunningham, W. Atkin, H.J. Lenz, H.T. Lynch, B. Minsky, B. Nordlinger, N. Starling, Colorectal cancer. Lancet 375, 1030–1047 (2010)
M.T. de Bruijn, D.A. Raats, F.J. Hoogwater, W.J. van Houdt, K. Cameron, J.P. Medema, I.H.M. Borel Rinkes, O. Kranenburg, Oncogenic KRAS sensitises colorectal tumour cells to chemotherapy by p53-dependent induction of Noxa. Br. J. Cancer 102, 1254–1264 (2010)
A. Hadji, C. Clybouw, M.T. Auffredou, C. Alexia, K. Poalas, A. Burlion, O. Feraud, G. Leca, A. Vazquez, Caspase-3 triggers a TPCK-sensitive protease pathway leading to degradation of the BH3-only protein puma. Apoptosis 2010
P. Hauck, B.H. Chao, J. Litz, G.W. Krystal, Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT-737. Mol. Cancer Ther. 8, 883–892 (2009)
L.M. High, B. Szymanska, U. Wilczynska-Kalak, N. Barber, R. O’Brien, S.L. Khaw, I.B. Vikstrom, A.W. Roberts, R.B. Lock, The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol. Pharmacol. 77, 483–494 (2010)
M.H. Kang, Z. Wan, Y.H. Kang, R. Sposto, C.P. Reynolds, Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J. Natl Cancer Inst. 100, 580–595 (2008)
A.M. Keuling, K.E. Felton, A.A. Parker, M. Akbari, S.E. Andrew, V.A. Tron, RNA silencing of Mcl-1 enhances ABT-737-mediated apoptosis in melanoma: role for a caspase-8-dependent pathway. PLoS ONE 4, e6651 (2009)
M. Konopleva, R. Contractor, T. Tsao, I. Samudio, P.P. Ruvolo, S. Kitada, X. Deng, D. Zhai, Y.X. Shi, T. Sneed, M. Verhaegen, M. Soengas, V.R. Ruvolo, T. McQueen, W.D. Schober, J.C. Watt, T. Jiffar, X. Ling, F.C. Marini, D. Harris, M. Dietrich, Z. Estrov, J. McCubrey, W.S. May, J.C. Reed, M. Andreeff, Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10, 375–388 (2006)
J. Kuroda, S. Kimura, M. Andreeff, E. Ashihara, Y. Kamitsuji, A. Yokota, E. Kawata, M. Takeuchi, R. Tanaka, Y. Murotani, Y. Matsumoto, H. Tanaka, A. Strasser, M. Taniwaki, T. Maekawa, ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. Br. J. Haematol. 140, 181–190 (2008)
X. Lin, S. Morgan-Lappe, X. Huang, L. Li, D.M. Zakula, L.A. Vernetti, S.W. Fesik, Y. Shen, ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene 26, 3972–3979 (2007)
L.A. Miller, N.B. Goldstein, W.U. Johannes, C.H. Walton, M. Fujita, D.A. Norris, Y.G. Shellman, BH3 mimetic ABT-737 and a proteasome inhibitor synergistically kill melanomas through Noxa-dependent apoptosis. J. Invest. Dermatol. 129, 964–971 (2009)
K. Okumura, S. Huang, F.A. Sinicrope, Induction of Noxa sensitizes human colorectal cancer cells expressing Mcl-1 to the small-molecule Bcl-2/Bcl-xL inhibitor, ABT-737. Clin. Cancer Res. 14, 8132–8142 (2008)
T. Oltersdorf, S.W. Elmore, A.R. Shoemaker, R.C. Armstrong, D.J. Augeri, B.A. Belli, M. Bruncko, T.L. Deckwerth, J. Dinges, P.J. Hajduk, M.K. Joseph, S. Kitada, S.J. Korsmeyer, A.R. Kunzer, A. Letai, C. Li, M.J. Mitten, D.G. Nettesheim, S. Ng, P.M. Nimmer, J.M. O’Connor, A. Oleksijew, A.M. Petros, J.C. Reed, W. Shen, S.K. Tahir, C.B. Thompson, K.J. Tomaselli, B. Wang, M.D. Wendt, H. Zhang, S.W. Fesik, S.H. Rosenberg, An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005)
B. Pandit, A.L. Gartel, New potential anti-cancer agents synergize with bortezomib and ABT-737 against prostate cancer. Prostate 70, 825–833 (2010)
T. Shibue, S. Suzuki, H. Okamoto, H. Yoshida, Y. Ohba, A. Takaoka, T. Taniguchi, Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO J. 25, 4952–4962 (2006)
S. Shirasawa, M. Furuse, N. Yokoyama, T. Sasazuki, Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science 260, 85–88 (1993)
N. Smakman, L.M. Veenendaal, P. van Diest, R. Bos, R. Offringa, I.H.M. Borel Rinkes, O. Kranenburg, Dual effect of Kras(D12) knockdown on tumorigenesis: increased immune-mediated tumor clearance and abrogation of tumor malignancy. Oncogene 24, 8338–8342 (2005)
K.E. Tagscherer, A. Fassl, B. Campos, M. Farhadi, A. Kraemer, B.C. Bock, S. her-Goeppinger, B. Radlwimmer, O.D. Wiestler, C. Herold-Mende, W. Roth, Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene 27, 6646–6656 (2008)
S.K. Tahir, X. Yang, M.G. Anderson, S.E. Morgan-Lappe, A.V. Sarthy, J. Chen, R.B. Warner, S.C. Ng, S.W. Fesik, S.W. Elmore, S.H. Rosenberg, C. Tse, Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 67, 1176–1183 (2007)
M.F. van Delft, A.H. Wei, K.D. Mason, C.J. Vandenberg, L. Chen, P.E. Czabotar, S.N. Willis, C.L. Scott, C.L. Day, S. Cory, J.M. Adams, A.W. Roberts, D.C. Huang, The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10, 389–399 (2006)
A. Weber, Z. Kirejczyk, S. Potthoff, C. Ploner, G. Hacker, Endogenous noxa determines the strong proapoptotic synergism of the BH3-mimetic ABT-737 with chemotherapeutic agents in human melanoma cells. Transl. Oncol. 2, 73–83 (2009)
E. Wesarg, S. Hoffarth, R. Wiewrodt, M. Kroll, S. Biesterfeld, C. Huber, M. Schuler, Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int. J. Cancer 121, 2387–2394 (2007)
S.N. Willis, J.M. Adams, Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17, 617–625 (2005)
H. Xu, G.W. Krystal, Actinomycin D decreases Mcl-1 expression and acts synergistically with ABT-737 against small cell lung cancer cell lines. Clin. Cancer Res. 2010
D. Yecies, N.E. Carlson, J. Deng, A. Letai, Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 115, 3304–3313 (2010)
H. Zall, A. Weber, R. Besch, N. Zantl, G. Hacker, Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the Noxa-dependent inactivation of Mcl-1 or A1. Mol. Cancer 9, 164 (2010)
Acknowledgements
This study was supported by grants from the Association for International Cancer Research (07–552 to MTdB), the Vanderes Foundation (07–177 to DAER) and the Dutch Cancer Society (2009–4367 to EJAS and 2009–4367 to BLE)
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniëlle A. E. Raats and Menno T. de Bruijn contributed equally to this manuscript
Rights and permissions
About this article
Cite this article
Raats, D.A.E., de Bruijn, M.T., Steller, E.J.A. et al. Synergistic killing of colorectal cancer cells by oxaliplatin and ABT-737. Cell Oncol. 34, 307–313 (2011). https://doi.org/10.1007/s13402-011-0026-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-011-0026-8