Abstract
Excessive cadmium (Cd) in soils is a serious threat to food safety. Combinations of fungal endophytes and biochar have been shown to restrict Cd accumulation in crop plants, but the interactions of Paecilomyces variotii extracts (ZNC) and biochar on the remediation of alkaline Cd-contaminated soil remain unclear. This work aimed to study the Cd immobilization ability by amendment with ZNC and biochar via column experiments. The results indicated that ZNC alleviated the inhibition of Cd on photosynthesis of maize, thus supporting Cd resistance by growth promotion. Biochar promoted the conversion of exchangeable Cd into a less-available form, which reduced the content of DTPA-extractable Cd by 4.93 ~ 21.28% and − 0.83 ~ 19.51% in the 0–10- and 10–20-cm soil layers, respectively. The combination of biochar and ZNC substantially reduced Cd concentrations in maize plants, with more significantly lower grain Cd concentrations in higher biochar dosage treatments. These findings indicate that the combination of ZNC and biochar has significant potential to promote plant growth by altering Cd bioavailability and Cd tolerance in alkaline Cd-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil heavy metal contamination, especially cadmium (Cd), is a serious environmental issue of global concern. In China, a nationwide survey indicated that over 7.0% of farmland soils are polluted with Cd [1]. Excessive accumulation of Cd in plant tissues can cause growth retardation by causing negative physiological processes, accompanied by the restriction of the net photosynthetic rate and available nutrient absorption [2, 3]. Furthermore, Cd can readily accumulate in animals and the human body via food chains [4]. Therefore, effective strategies must be taken to mitigate Cd pollution and restrict excessive Cd entry into the food chain.
Biochar is a carbonaceous material produced by the pyrolysis of organic feedstock under oxygen-limited conditions [5]. The extraordinary physicochemical properties of biochar, such as porous structures, larger surface areas, large pore volumes, and active oxygen-containing functional groups, make it an ideal material for Cd remediation [6]. In addition to the direct sorption of Cd, the changed soil properties with biochar amendment also played essential roles in altering Cd polluted soils [7]. However, the effectiveness of the Cd amelioration ability of biochar significantly depends upon its properties, dosage, soil properties, environmental conditions, and soil microbial community [8]. In addition, the results and mechanisms for Cd immobilization were also inconsistent. For instance, some studies have demonstrated the effective immobilization of Cd in soil via surface complexation, electrostatic attraction, ion exchange, and co-precipitation with biochar [9, 10]. However, other studies have shown that the Cd immobilizing mechanisms of biochar were mainly attributed to the changed properties of soil (i.e., pH value, CEC, and redox potential) [11]. These different responses of Cd-polluted soil remediation to biochar suggest that further analysis is still needed.
Microbe-assisted remediation with microbial strains, which have vigorous Cd tolerance or sequestration abilities, is another promising measure to alleviate Cd stress [12]. Many results have found that the existence of endophytes in the rhizosphere restricts plant Cd absorption via chelation systems, metal sequestration abilities, and physiological metabolism [13, 14]. In addition, the genes encoding the metabolic pathways of these endophytes, which participate in the synthesis of phytohormones, also enable plants to alleviate Cd stress. However, the application of endophytes in the remediation of Cd-contaminated soil is still limited because specific microorganism needs suitable conditions to obtain the optimal performance [15]. Recently, a novel endophytic fungus (Paecilomyces variotii) extract, named ZNC, has been highlighted as an outstanding candidate for the promotion of plant growth in multistress conditions without the abovementioned limitations [16, 17], but its effectiveness on cereal crop growth is still unknown in Cd-polluted soils. Compared with other plant growth regulators, ZNC has the functionality to promote plant growth when applied at the concentrations as low as 1–10 ng/mL.
The combination of biochar and microorganisms has been considered an optimal strategy to recover soil functions contaminated by heavy metals [18, 19]. However, to the best of our knowledge, no research has been conducted concerning the interactions of biochar and ZNC on Cd remediation and plants grown in alkaline soil. Therefore, to fill the research gaps mentioned above, a column experiment with maize growth was conducted to (1) evaluate the effects of ZNC on the alleviation of the inhibition of Cd on photosynthesis; (2) illustrate the effectiveness of biochar on Cd immobilization in alkaline soil; and (3) provide insight into the interactive effects and mechanism between ZNC and biochar. This study will provide new insight into the remediation of Cd-polluted soil in China.
2 Materials and methods
2.1 Preparation of biochar and ZNC
Rice hull was collected from a grain processing factory in Yutai city, Shandong province, China. After being milled by using a pestle and mortar, the treated rice hulls were transferred into a tube furnace (SKQ-30-12A, Longkou Furnace Co., Ltd, China) which equipped with a quartz boat in the presence of N2. The material was pyrolyzed at a rate of 5 °C/min and then held for 3 h when the temperatures reached 450 °C. After the pyrolysis was complete, the biochar was naturally cooled and ground to pass through a 10-mesh sieve, and stored with plastic seal bags in a dry container for further use.
The extracts from the endophytic fungus were provided by Pengbo Biology Technology Co. Ltd. (Tai’an, Shandong province, China). Briefly, Paecilomyces variotii (CGMCCNO.10114) was first inoculated onto PDA cultivation media, incubated at 25℃ for 6 days, transferred into 50-mL seed culture media and shaken at 180 r min−1 for 3 days. After that, 10% of the above mediums were inoculated into 150 mL of fermentation media and shaken at 180 r min−1 for 5 days. The mycelium was smashed and extracted with ethyl alcohol 3 times. Finally, the mixtures were ultrasonically concentrated for 1 h and filtered to obtain ZNC. The amino acids existed in the form of granules, leaves, and flakes in ZNC were speculated to be the main active substances [20].
2.2 Soil column experiments
To determine the interactions of ZNC and biochar on soil Cd remediation and plant growth, a column experiment with maize growth was conducted at the Environment and Soil Experiment Station at Liaocheng University (N 36° 43′ 45″, E 116° 00′ 09″), Shandong province, China. The basic properties of the Typic Ochri-Aquic Cambosol soil in Chinese soil taxonomy and the biochar used are listed in Table 1.
Before experiments, in situ soil samples in 0–100 cm layers were dug out, and 18 PVC tubes (diameter 25 cm, height 110 cm, and thickness 0.5 cm) were buried in the site. CdCl2 solution (2.0 g L−1) was well mixed with 0–10 cm soil to obtain target Cd-contaminated soil (1.0 mg kg−1) and aged for 4 weeks before the incorporation of biochar at 60% saturated moisture content. The air-dried soil (10–50 and 50–90 cm) was backfilled directly in the same layer order and watered several times for consolidation to the initial bulk density. After that, mixtures of Cd-contaminated soil and biochar (0, 2%, and 5% w/w, respectively) were backfilled in each tube. Two seeds of maize were planted into each column and thinned to one per column at the V2 stage. Half of the maize was well sprayed with ZNC (20 ng mL−1) solution at the seeding, VT (tasseling) and R3 (milking) stages, while in the remaining half of the maize plants were absent [21]. Other agricultural management, including irrigation, fertilization, insects, and weed control of all treatments, were the same as needed. All treatments were described as BC0 (without biochar and ZNC), BC2 (2% w/w biochar), BC5 (5% w/w biochar), ZNC + BC0 (ZNC but without biochar), ZNC + BC2 (2%w/w biochar and ZNC), and ZNC + BC5 (5%w/w biochar and ZNC).
2.3 Characterization of biochar
The Barrett-Joyner-Halender (BJH) method was used to calculate the pore size distribution of the biochar. The surface morphology of the biochar was characterized using SEM (ZEISS G300, Germany). FTIR spectroscopy (Nicolet iS5 FTIR spectrometer, USA) was used to evaluate the chemical structure of biochar and ZNC powder within a KBr pellet. The surface mineral crystallographic composition of the biochar was measured using XRD (D8 ADVANCE, Bruker Co., Germany).
2.4 Soil–plant measurement
After maize was harvested, soil samples were collected from the 0–70 cm layer at 10 cm intervals. The available Cd was determined with DTPA extractable Cd as described by Hamid et al. [22]. The contents and fractions of Cd in the 0–70 cm soil were analyzed following the BCR sequential extraction method according to Ure et al. (1993) [23], which divided the fractions into the HOAc-extractable (exchangeable, soluble, and carbonate fractions), reducible (iron and manganese oxyhydroxide fractions), oxidizable (organically and sulfide bound fractions), and residual fractions. The detailed process for the measurement of Cd contents and fractions is shown in the supplementary materials.
The net photosynthetic rate (Pn) of maize was measured at the seeding, jointing, V12, silking, and maturity stages. The fully expanded upper leaves were recorded between 9:00 and 11:00 a.m. by an LI-6800 portable photosynthesis system (LI-Cor, Lincoln, NE, USA) with an average leaf temperature of 25 ± 1 °C, photosynthetic activity of 800 μmol m−2 s−1, and CO2 concentration of 400 μmol CO2 mol−1.
After maize harvested, plant samples were collected and separated into biomass (including stems, leaves, and cobs) and grains. The dried samples were weighed, ground, and passed through a 100-mesh sieve for Cd determination. Approximately 0.2 g of plant samples was digested in a 5-mL 3:1 (v/v) mixture of HNO3 and HClO4 at 180℃ for 4 h. The Cd concentrations of plants were measured using ICP–MS (Perkin Elmer 600X, USA).
2.5 Data analysis
Data were expressed as the standard deviations with the average value. Statistical analyses were performed by SPSS Statistics 19.0 software. ANOVA tests by least-significant difference (LSD) were conducted to verify the different letters (P < 0.05). Excel 2010 and Origin 9.5 were used for basic processing of the obtained data and to plot the graphs.
3 Results and discussion
3.1 Characterization of biochar
The biochar used was an alkaline material with high ash content (Table 1), which was mainly caused by the existence of alkaline compounds and inorganic minerals. The high ash components and inorganic anions, such as Mg2+, Fe3+, CO32−, and PO43−, released from biochar are positively correlated with Cd remediation in soil [24], which promotes sorption or precipitation reactions, ultimately limiting the mobility and toxicity of Cd.
In addition, FTIR showed a high functional group intensity of biochar (Fig. 1a), including -OH stretching vibration at 3430 cm−1, aromatic C-H bending at 892 cm−1, aromatic C = C or carboxylic C = O bonds in the broad spectrum between 1429 and 1565 cm−1, and C‒H stretching at 2934 cm−1. The existence of these functional groups indicates that the interaction of Cd2+-π on the aromatic structure of biochar [25] and the complexation of Cd2+ with oxy-containing functional groups are vital for the retention of Cd [26]. The XRD patterns of biochar at 2θ = 25.64°, 28.39°, and 30.14°corresponded to the (222), (200), and (220) lattice planes, respectively (Fig. 1b), which also verified the potential of biochar to immobilize Cd through complexation by the formation of intermolecular and intramolecular hydrogen bonds [27].
The sorption isotherms of biochar are categorized as type IV according to the IUPAC classification (Fig. 1c), which has H2-type hysteresis loops. The BET surface area, average pore size, and pore volume of biochar were calculated to be 3.42 m2 g−1, and 19.54 nm of 0.786 cm3 (STP) g−1, respectively. Meanwhile, the pore size distribution of biochar was in the range of 2–22 nm (Fig. 1d). These results are also consistent with the biochar SEM images (Fig. 2), which showed coarse surface and porous structures with some mineral particles. All the traits mentioned above of biochar exhibited great potential in facilitating the immobilization of Cd.
The growth-promoting mechanism of ZNC is difficult to identify due to its multiple bioactive components which may contribute to specific effects on plants. Therefore, the material composition and chemical structure of ZNC were verified. The FTIR results of ZNC indicate the existence of carbohydrate structures and aliphatics in ZNC (Fig.S1). In addition, ZNC had many amino acids, especially Thr, Gly, Glu, Gly, and Mann (Fig.S2). These amino acids were speculated to be the main active substances in ZNC for plant growth [21].
3.2 Changes in Cd bioavailability and fraction in soil
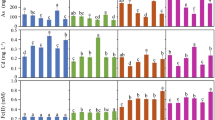
The DTPA-extractable Cd (available Cd) in rhizosphere soil is highly active and can be easily absorbed by plants [4]. The available Cd contents were highest in 0–10-cm soil profile, followed by the 10–20-cm profile, and were nearly the same in the soil layers below 20 cm (Fig. 3). This could be explained by the fact that there were more organic matter and root exudates in the surface soil, which could prompt the formation of a stable organic matter-Cd complex, thus restricting the downward migration of Cd [28]. In our study, the available Cd in the 0–10 cm soil decreased by 4.92 ~ 14.59% and 12.28 ~ 21.28% at the 2% and 5% doses, respectively, whereas the available Cd decreased by 2.3 ~ 4.7% and 8.9 ~ 12.4% in the 10–20 cm soil with the application of biochar. The effectiveness of biochar in limiting the availability of Cd was more dramatic at higher dosages, indicating that biochar amendment is an effective measure to renovate soil functions contaminated by Cd. Fellet et al. [29] also found that manure biochar applied at 1.5% and 3% w/w was able to reduce the contents of DTPA-Cd by 27.9% and 70.7%, respectively, in mine tailing soil. However, Janus et al. [30] reported that the amendment of 2% w/w biochar did not significantly decrease the bio-accessible Cd content in slightly alkaline soil. This may be due to the efficiency of biochar in Cd remediation being affected by soil traits, environmental conditions, pollutant types and concentrations, and biochar properties.
The use of endophytic microorganisms is another effective measure to remediate Cd-polluted soil. However, in our study, the use of ZNC only slightly decreased the available Cd contents by 0.68 ~ 8.57% in the 0–10 cm soil layer and 3.32 ~ 14.67% in the 10–20 cm soil layer compared with those in the control (Fig. 3). The high available Cd contents in the rhizosphere are toxic to plants. Our previous results found that ZNC enhanced plant growth by improving stress tolerance and resistance, accompanied by the promotion or reduction of biochemical activities (i.e., SOD, CAT, MDA, and NR) and alleviated the damage to cell membranes [31]. In addition, the application of ZNC could enable crops to take up more nutrients by promoting root morphological characteristics and vitality [32]. Thus, more root exudates, which can chelate with Cd, may exist in the rhizosphere and are helpful of the inhibition on Cd bioavailability. However, some researchers hold the view that the organic exudates excreted from roots, which are rich in hydroxyl, carboxylate, and other oxygen-containing functional groups, can chelate Cd and further improve its mobility and bioavailability [33]. Therefore, more investigations need to be completed that investigate the molecular mechanisms to solidify the theoretical foundations of ZNC-assisted pollutant remediation.
The fraction of Cd influences its potential toxicity and the hazard level of contaminated soil. Since the amendment of biochar and ZNC alleviates the bioavailable of Cd, the speciation variations in Cd were measured using BCR sequential extraction (Fig. 4). HOAc-extractable Cd, which can easily absorbed by plant roots, is highly mobile and sensitive to environmental changes [34], while the residual fraction is considered to be a stable form and difficult to extract [35]. HOAc-extractable and reducible Cd were the main fractions in the 0–10-cm soil layer, while the fraction was not significantly affected in deeper soil layers (Fig. 4 and Fig.S3). HOAc-extractable, reducible, oxidizable, and residual Cd in the 0–10 cm soil changed by − 8.09 ~ − 16.96%, 1.03 ~ 10.70%, − 13.99 ~ 14.64%, and 8.79 ~ 45.94%, respectively, with biochar amendments. The decreases in available soil Cd with biochar application were more pronounced than those in HOAc-extractable Cd, indicating that parts of water-soluble Cd have immobilized into CO32− or organic matter [36]. Some researchers believe that restricts the bioavailability of Cd, which is mainly associated with soil pH [37]. Houben and Sonnet [37] suggested that biochar amendment shifted the Cd exchangeable fraction to the insoluble pool due to an increasing soil pH value. However, in our study, the amendment of biochar did not increase or even slightly decrease the rhizosphere soil pH values compared with the corresponding control (Fig.S4), possibly due to the buffering effect of alkaline soil.
Changes of Cd contents (a and c) and Cd fractions (b and d) after maize harvested. Data points represent the means ± SD. BC0, BC2, and BC5 denote the soil samples with 0%, 2%, and 5%w/w biochar, respectively. ZNC denote endophytic fungus (Paecilomyces variotii) extract. Lowercase letters above the error bars indicate significant differences among different treatments in same soil layer analyzed by Duncan test (p < 0.05)
3.3 Plant growth and biomass accumulation
Excess Cd in mesophyll cells can induce stomatal closure, reduce transpiration and intrinsic CO2 assimilation, and restrict stomatal or nonstomatal ions [38]. Meanwhile, ultrastructural damage in the chloroplast, destruction of thylakoid membranes, and disturbance in electron transport chains of the photosystem caused by Cd stress can also be reasonable for the net photosynthetic rate (Pn) restrictions [39]. In the present study, the treatment effects on Pn varied with growth stage and were lowest in the CK treatment in all growth stages (Fig. 5). With the same biochar dosage, the use of ZNC alleviated the inhibition of Pn caused by Cd stress. However, since ZNC does not supply available nutrients for maize growth, the possible reason for the activated photosynthetic physiology was the stimulated secretion of auxin in the root. Our previous research has found that ZNC induces the expression of genes associated with auxin biosynthesis, such as YUC3 and YUC9, thus promoting auxin accumulation in root tips [32]. In addition, this increased Pn is also reasonable for enhancing maize biomass and grain under Cd toxicity.
Changes of net photosynthetic rates (Pn) during maize growth. Data points represent the means ± SD. BC0, BC2, and BC5 denote the soil samples with 0%, 2%, and 5%w/w biochar, respectively. ZNC denote endophytic fungus (Paecilomyces variotii) extract. Lowercase letters above the error bars indicate significant differences among different treatments in same soil layer analyzed by Duncan test (p < 0.05)
Compared with the corresponding control, biochar enhanced the Pn values of maize, especially during the jointing and filling stages (Fig. 5). In addition to the positive effects of biochar in reducing soil moisture infiltration, inhibiting soil water evaporation, increasing soil volumetric water content, and improving soil water holding capacity [40], nutrients released from biochar can also absorbed by roots, thus promoting the growth of maize. Similar results were observed by Kamran et al. [41], who found that biochar significantly increased the Pn of pak choi (Brassica chinensis L.) by 18 ~ 46% under 20 mg kg−1 Cd stress. The negative relationship between Cd contents in maize plants and these characteristics (Fig.S5) also strengthened our findings that the application of biochar could minimize Cd accumulation in plant tissues and thereby improve the physiological parameters of maize.
3.4 Plant Cd concentration and accumulation
In the present study, the Cd contents in the biomass (stem, leaf, and cob) and grains of maize were significantly reduced by 9.55 ~ 19.52% and 14.58 ~ 26.78%, respectively, with biochar application (Fig. 6). This suggests that biochar is effective in preventing excess Cd accumulation in maize grain. Similar results also reported that the Cd contents in rice grains were reduced by 57–61% with biochar application [10]. In addition to the larger specific surface area and higher CEC, the alkaline substances and the dissolved organic matter released from biochar can also decrease the bioavailability of Cd [42], thus reducing Cd accumulation in plants. However, the inconsistent result between the reductions in grain Cd contents (approximately 9.55 ~ 26.67%) and the dinky changes in soil Cd bioavailability (7.31 ~ 21.28% and − 0.83 ~ 10.11% in 0–10 and 10–20 cm soil, respectively) also indicates that the Cd content reduces in maize with biochar amendment not only by limiting Cd bioavailability in the soil [43]. In addition, biochar in the rhizosphere can also act as a natural barrier to Cd uptake by maize roots (Fig. 7). Biochar also helps to shape specific soil microorganism communities, especially bacteria, actinomycete, and fungal taxes which are associated with growth promotion and Cd resistance [33], ultimately resulting in lower Cd contents in plant tissues.
Plant growth and Cd uptake of maize at different treatments: (a) biomass; (b) grain yield; (c) Cd concentration in biomass; (d) Cd concentration in grain; (e) total Cd accumulation in biomass, and (f) total Cd accumulation in grain. Data points represent the means ± SD. BC0, BC2, and BC5 denote the soil samples with 0%, 2%, and 5%w/w biochar, respectively. ZNC denote endophytic fungus (Paecilomyces variotii) extract. Lowercase letters above the error bars indicate significant differences among different treatments in same soil layer analyzed by Duncan test (p < 0.05)
The application of ZNC reduced the concentration of Cd in grain by 2.71 ~ 5.79%. Nevertheless, it did not significantly decrease the Cd content in the biomass, indicating that ZNC induced a reduction in Cd translocation from the biomass (stem, leaf) to the grain in maize. The main pathways for reducing Cd concentration in grains may be due to the inhibition of Cd entering into plant cells via Cd-related transporters and channels, thus enhancing sequestration of Cd into the vacuole [44]. In addition, decreasing the Cd concentration in grains could also contribute to the decreased Cd translocation from roots to shoots by improving the fixation of Cd in cell vacuoles and/or reducing the xylem loading of Cd. Similarly, some studies also found that exogenous hormones have the potential to decrease Cd accumulation in plants and restrict Cd transport from plants to grain [45]. Since ZNC does not contain nutrients [21], it is possible that the accumulation of biomass was activated by the induced expression of genes associated with auxin biosynthesis, such as YUC3 and YUC9, which enhanced nutrient uptake and biomass accumulation [32]. However, the valid reasons for ZNC why Cd reduces accumulation in maize grains are still not well studied.
Combination of biochar and ZNC decreased the Cd concentration in grains and alleviated the growth inhibition in maize under Cd stress, which cannot be explained simply by the decreased Cd bioavailability in soils. In addition to the separate benefits of biochar in restricting Cd bioavailability and ZNC in enhancing plant Cd tolerance, the synergy between biochar and ZNC could also be understood in the context of two mechanisms (Fig. 7). That is, biochar acts as an ideal inhibitor in rhizosphere soil to reduce the mobility and bioavailability and consequently mitigate the toxicity of Cd, while ZNC is used as a promoter to protect the normal nutrient uptake, net photosynthesis activity, and biomass accumulation of maize under Cd-stressed conditions. Hence, the combination of ZNC and biochar amendment could be a promising measure for the remediation of Cd-contaminated soil. However, for safety and cost-effectiveness, more studies considering the potential physiological and soil biogeochemical mechanisms of ZNC and biochar performance for the steady remediation of Cd-contaminated soils are still needed.
4 Conclusions
The application of ZNC to Cd-contaminated soil alleviated the inhibition of Cd on photosynthesis, thus promoting plant growth of maize. Biochar is an efficient material for tackling Cd pollution in alkaline soil, especially at a higher dosage. The combined application of ZNC and biochar significantly reduced the available Cd content in the 0–10 cm soil, decreasing the extractable Cd fraction, and increasing the stable Cd fraction in the rhizosphere. In summary, the combination of biochar and ZNC to alkaline soils could be an emerging approach for the sustainable remediation of alkaline Cd-polluted soil. Our results provide a theoretical basis for further applying ZNC and biochar in the field to simultaneously increase Cd tolerance and reduce Cd risk in cereal crops.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhao F, Ma Y, Zhu Y, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Techno l 49:750–759
Zama EF, Zhu YG, Reid BJ, Sun GX (2017) The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J Clean Prod 148:127–136
Yu X, Zhou H, Ye X, Wang H (2021) From hazardous agriculture waste to hazardous metal scavenger: tobacco stalk biochar-mediated sequestration of cd leads to enhanced tobacco productivity. J Hazard Mater 413:125303
Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH (2019) Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int 125:365–385
Zhang YC, Xiao X, Zhu XM, Chen BL (2022) Self-assembled fungus-biochar composite pellets (FBPs) for enhanced co-sorption-biodegradation towards phenanthrene. Chemosphere 286:131887
Jing FQ, Sohi SP, Liu YY, Chen JW (2018) Insight into mechanism of aged biochar for adsorption of PAEs: reciprocal effects of ageing and coexisting Cd2+. Environ Pollut 242:1098–1107
Arabia Z, Rinklebe J, El-Naggar A, Hou DY, Sarmah AK, Moreno-Jim’enezd E (2021) Immobilization of arsenic, chromium, and nickel in soils via biochar: a meta-analysis. Environ Pollut 286:117199
Qian GR, Chen W, Lim TT, Chui PC (2019) In-situ stabilization of Pb, Zn, Cu, Cd and Ni in the multi-contaminated sediments with ferrihydrite and apatite composite additives. J Hazard Mater 170:1093–1100
Tang N, Niu CG, Li XT, Liang C, Guo H, Lin LS (2018) Efficient removal of Cd2+ and Pb2+ from aqueous solution with amino and thiol functionalized activated carbon: isotherm and kinetics modeling. Sci Total Environ 635:1331–1344
Chen D, Guo H, Li RY, Li LQ, Pan GX, Chang A, Joseph S (2016) Low uptake affinity cultivars with biochar to tackle Cd-tainted rice-a field study over four rice seasons in Hunan, China. Sci Total Environ 541:1489–1498
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng HY (2015) Physicochemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59
Jiang ZQ, Wang T, Sun YL, Nong Y, Tang LY, Gu TT, Wang SM, Li Z (2020) Application of Pb to probe the physiological responses of fungal intracellular vesicles. Ecotoxicol Environ Saf 194:110441
Awa SH, Hadibarata T (2020) Removal of heavy metals in contaminated soil by phytoremediation mechanism: a review. Water Air Soil Pollut 231(2):47
Etesami H (2018) Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicol Environ Saf 147:175–191
Zare MR, Amin MM, Nikaeen M, Bina B, Rahmani A, Hemmati-Borji S, Rahmani H (2015) Acute toxicity of Hg, Cd, and Pb towards dominant bacterial strains of sequencing batch reactor (SBR). Environ Monit Assess 187:263
Xing YM, Wan YT, Liu YL, Wang PF, Zhao YX, Fan Z, Tian XF (2021) Effects of extracts from paecilomyces variotii on chlorophyll fluorescence transient in leaves of maize under waterlogging stress. J Irrig Drain 40:52–57
Wang XQ, Yao YY, Chen BC, Zhang M, Liu ZL, Wang Q, Ma JZ (2020) Paecilomyces variotii extracts and controlled-release urea synergistically increased nitrogen use efficiency and rice yield. ACS Omega 5:13303–13311
Gasco G, Alvarez ML, Paz-Ferreiro J, Mendez A (2019) Combining phytoextraction by Brassica napus and biochar amendment for the remediation of a mining soil in Rio Tinto (Spain). Chemosphere 231:562–570
Franchi E, Cosmina P, Pedron F, Rosellini I, Barbafieri M, Petruzzelli G, Vocciante M (2019) Improved arsenic phytoextraction by combined use of mobilizing chemicals and autochthonous soil bacteria. Sci Total Environ 655:328–336
Wang XQ, Yao YY, Liu ZG, Chen BC, Zhang M, Wang QB, Ma JZ (2021) Highly active biostimulant Paecilomyces variotii extracts reduced controlled-release urea application while maintaining rice yield. J Sci Food Agr 102:1883–1893
Chen Q, Li ZL, Qu ZM, Zhou HY, Qi YJ, Liu ZG, Zhang M (2020) Maize yield and root morphological characteristics affected by controlled release diammonium phosphate and Paecilomyces variotii extracts. Field Crop Res 255:107862
Hamid Y, Tang L, Hussain B, Usman M, Gurajala HK, Rashid MS, He ZL, Yang XE (2020) Efficiency of lime, biochar, Fe containing biochar and composite amendments for Cd and Pb immobilization in a co-contaminated alluvial soil. Environ Pollut 257:12
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. Int J Environ Anal Chem 51:135–151
Clemente JS, Beauchemin S, MacKinnon T, Martin J, Johnston CT, Joern B (2017) Initial biochar properties related to the removal of As, Se, Pb, Cd, Cu, Ni, and Zn from an acidic suspension. Chemosphere 170:216–224
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal contaminated soils: principles and applicability. Sci Total Environ 633:206–219
Yin GC, Song XW, Tao L, Sarkar B, Sarmah AK, Zhang WX, Lin QT, Xiao RB, Liu QJ, Wang HL (2020) Novel Fe-Mn binary oxide-biochar as an adsorbent for removing Cd (II) from aqueous solutions. Chem Eng J 389:124465
Fan JJ, Cai C, Chi HF, Reid BJ, Coulon F, Zhang YC, Hou YW (2020) Remediation of cadmium and lead polluted soil using thiol-modified biochar. J Hazard Mater 388:122037
Rinklebe J, Shaheen SM (2015) Miscellaneous additives can enhance plant uptake and affect geochemical fractions of copper in a heavily polluted riparian grassland soil. Ecotox Environ Safe 119:58–65
Fellet G, Marmiroli M, Marchiol L (2014) Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci Total Environ 468–469:598–608
Janus A, Waterlot C, Heymans S, Deboffe C, Douay F, Pelfrene A (2018) Do biochars influence the availability and human oral bioaccessibility of Cd, Pb, and Zn in a contaminated slightly alkaline soil? Environ Monit Assess 190(4):218
Wang LW, O’Connor D, Rinklebe J, Ok YS, Tsang DCW, Shen ZT, Hou DY (2020) Biochar aging: mechanisms, physicochemical changes, assessment, and implications for field applications. Environ Sci Technol 54(23):14797–14814
Lu CC, Liu HF, Jiang DP, Wang LL, Jiang YK, Tang SY, Hou XW, Han XY, Liu ZG, Zhang M, Chu ZH, Ding XH (2019) Paecilomyces variotii extracts (ZNC) enhance plant immunity and promote plant growth. Plant Soil 441:383–397
Tu C, Wei J, Guan F, Liu Y, Sun YH, Luo YM (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576
Ren J, Wang FH, Zhai YB, Zhu Y, Peng C, Wang TF, Li CT, Zeng GM (2017) Effect of sewage sludge hydrochar on soil properties and Cd immobilization in a contaminated soil. Chemosphere 189:627–633
Qiu Z, Tang J, Chen J, Zhang Q (2020) Remediation of cadmium-contaminated soil with biochar simultaneously improves biochar’s recalcitrance. Environ Pollut 256:113436
Derakhshan Nejad Z, Rezania S, Jung MC, Al-Ghamdi AA, Mustafa A, Elshikh MS (2021) Effects of fine fractions of soil organic, semi-organic, and inorganic amendments on the mitigation of heavy metal (loid)s leaching and bioavailability in a post-mining area. Chemosphere 271:129538
Houben D, Sonnet P (2015) Impact of biochar and root-induced changes on metal dynamics in the rhizosphere of Agrostis capillaris and Lupinus albus. Chemosphere 139:644–651
Zhu Z, Huang Y, Wu X, Liu Z, Zou J, Chen Y, Su N, Cui J (2019) Increased antioxidative capacity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in Chinese cabbage seedlings. Ecotoxicol Environ Saf 177:47–57
De Maria S, Puschenreiter M, Rivelli AR (2013) Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant Soil Environ 59:254–261
Xiao X, Chen ZM, Chen BL (2020) Proton uptake behaviors of organic and inorganic matters in biochars prepared under different pyrolytic temperatures. Sci Total Environ 746:140853
Kamran M, Malik Z, Parveen A, Zong YT, Abbasi GH, Rafiq MT, Shaaban M, Mustafa A, Bashir S, Rafay M, Mehmood S, Ali M (2019) Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L) cultivated in Cd-polluted soil. J Environ Manag 250:109500
Wang RZ, Wei S, Jia PH, Liu T, Hou DD, Xie RH, Lin Z, Ge J, Qiao YB, Chang XY, Lu LL, Tian SK (2019) Biochar significantly alters rhizobacterial communities and reduces Cd concentration in rice grains grown on Cd-contaminated soils. Environ Sci Technol 676:627–638
Wang RZ, Wei S, Jia PH, Liu T, Tian SK (2019) Biochar significantly alters rhizobacterial communities and reduces Cd concentration in rice grains grown on Cd-contaminated soils. Sci Total Environ 676:627–638
Huang YF, Chen JH, Zhang DR, Fang B, Yangjin T, Zou JW, Chen YH, Su NN, Cui J (2021) Enhanced vacuole compartmentalization of cadmium in root cells contributes to glutathione-induced reduction of cadmium translocation from roots to shoots in pakchoi (Brassica chinensis L). Ecotoxicol Environ Saf 208:111616
Nakamura S, Suzui N, Yin YG, Ishii S, Fujimaki S, Kawachi N, Rai H, Matsumoto T, Sato-Izawa K, Ohkama-Ohtsu N (2020) Effects of enhancing endogenous and exogenous glutathione in roots on cadmium movement in Arabidopsis thaliana. Plant Sci 290:110304
Funding
This study was supported by the Natural Science Foundation of China (grant no. 41807092), the Open Project of Liaocheng University Animal Husbandry Discipline (no. 319312101–18), the Project of Shandong Province Higher Educational Science and Technology Program (KJ2018BAF034), and the Startup Foundation for Ph.D. of Liaocheng University (318051839).
Author information
Authors and Affiliations
Contributions
Xinran Guo took part in data curation, investigation, and original draft writing; Jiyao Xu took part in methodology and material preparation; Dongyu He and Derui Bu involved in data collection and analysis; Yanyan Lu took part in formal analysis; Youxin Zhao and Yurong Chen took part in review and editing; Xiaofei Tian took part in conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, X., Xu, J., He, D. et al. Combining paecilomyces variotii extracts and biochar for the remediation of alkaline Cd-contaminated soil. Biomass Conv. Bioref. 14, 13353–13362 (2024). https://doi.org/10.1007/s13399-022-03308-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03308-0