Abstract
The biofuel yield from lignocellulose biomass depends strongly on the fermentable sugar yield from the hydrolysis step. Enzymatic hydrolysis, the preferable hydrolysis method, gives low sugar yield due to the lignin existence and the cellulose crystallinity. To increase the sugar yield, pretreatment is required to breakdown the recalcitrant nature of lignocellulose biomass. This review paper presents a comprehensive critical review of the lignocellulosic biomass (LCB) pretreatment methods for enhanced fermentable sugar yield. There is a need for an effective and cost-efficient pretreatment method that curbs inhibitory products and reduces the use of chemicals and energy. This paper highlighted recent advances in agricultural-based LCB pretreatment; discussed current challenges, advantages, and disadvantages; and suggested future solutions for agricultural-based biofuel production. Examined methods include pulsed electric energy (PEE), ionic liquid, co-solvent enhanced lignocellulosic fractionation pretreatment, and deep eutectic solvent. Each method was reviewed by its conditions (indicate the use of energy and chemicals), sugar yield, and inhibitory products. The review also researched the synergistic effect of combining more than one pretreatment method as a potential approach to overcome the drawbacks of the individual methods. In addition, the paper suggested improvement for each method and identified the research gaps to be bridged. Also, a comparison, summary, and research perspectives were provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomass-derived fuels are one of the feasible options to reduce carbon emissions while providing energy security [1, 2]. Greenhouse gas emissions can be decreased by 86% using ethanol produced from lignocellulose biomass [3]. In Canada, wheat is being used for ethanol production in Alberta, Manitoba, and Saskatchewan, while corn is being used in Manitoba, Ontario, and Quebec [4]. In 2018, 80% of ethanol production in Canada was from corn and the rest was from wheat. Canada’s 2019 ethanol imports were 1.4 billion liters. Agricultural residues such as straw are low-cost lignocellulose biomass (LCB), and they are readily available. The yearly worldwide lignocellulose biomass residues were estimated to be more than 220 billion tonne [5]. In Canada alone, it was estimated that the average availability of agricultural residues is to be in the range of 24.5–48 million dry tonnes per year [6]. Canadian Prairie Provinces have 72% of lignocellulose biomass with Saskatchewan alone accounting for 36% of the total agricultural residues. Utilizing these amounts could provide ethanol average production of 6.6–13 billion liters per year. Other chemicals such as phenol and furfural can also be produced [7, 8]. Table 1 shows the potential of bioethanol production from Canadian lignocellulose biomass materials [9]. Agricultural residues, forest mill residues, and forest harvest residues together make up to 20.3 billion liters of ethanol per year. Flax straw is difficult to degrade into the soil and it wraps around the equipment during the seeding process. That is why the residual straw is burnt which causes pollution.
The main obstacle for lignocellulose biomass to biofuel commercialization is the recalcitrant nature of LCB [10]. The main constituents of LCB are cellulose, hemicellulose, and lignin. The presence of lignin and the crystalline structure of cellulose affect the conversion of LCB to biofuels [11]. The most common steps for LCB to biofuel process are pretreatment, hydrolysis, fermentation, and separation [12]. The pretreatment step is aimed to increase the exposure of the cellulosic material. The hydrolysis step is meant to produce reducing sugars from the pretreated LCB, mostly using enzymes. Then, the sugars are converted to biofuel via fermentation and finally the biofuel is purified. Among these steps, pretreatment step was identified as the most technical challenging and it affects the yield of biofuel production significantly [10]. In addition, LCB has different sources and therefore different compositions which imply that different pretreatment conditions are needed for each feedstock for optimum biofuel yield [13, 14]. Another challenge of LCB pretreatment is its cost which was estimated to be 19% of the total biofuel production cost [15].
The efficient pretreatment method can be described as the one of low energy requirements, maximum fermentable sugar yield, and low inhibitors [16]. The presence of inhibitors, such as furfural, 5-hydroxymethyl-2-furaldehyd (HMF), acetic acid, and phenolic compounds affects the hydrolysis and the fermentation [17]. The enzymatic digestibility of LCB can be enhanced by lignin removal and decreasing the crystallinity of cellulose. The objective of this paper is to review the pretreatment technologies of lignocellulose biomass for maximum biofuel yield, the opportunities and challenges associated with each method, current status, and the future trends.
2 Lignocellulose biomass characteristics
Lignocellulose biomass is classified into three types: agricultural waste (e.g., wheat straw, bagasse, and corncob), woody residues (e.g., sawdust and bark), and energy crops (e.g., switch grass and miscanthus) [18]. LCB is composed mainly from cellulose, hemicellulose, and lignin. Cellulose accounts for the largest composition of LCB (40–50%) while hemicellulose accounts for 25–35% and lignin for 15–20%. There are also small quantities of other constituents such as pectin, proteins, extractives, and ash [19, 20].
Cellulose is a homopolymer sugar with a polymerization degree of 10,000 or higher, and linear structure consists of D-glucose units connected by β-1,4-glucosidic bonds [20]. Those bonds are hydrogen and van der Waals [21]. Most of cellulose is of crystalline structure which makes the cellulose insoluble in aqueous solution as well as resistant to hydrolysis [21, 22].
Hemicellulose is a heteropolymer sugar with a polymerization degree of less than 200 [23]. It consists of aldopentoses (xylose and arabinose), aldohexoses (mannose, glucose, and galactose), and sugar acids (4-O-methyl-d-glucuronic acid and d-glucuronic acid) [20]. The bonds linking hemicellulose to cellulose and lignin are covalent bonds. Xylans are the major class of hemicellulos and are found mainly in annual plants like straws and grasses [24]. Softwoods are composed mainly of glucomannan. The hydrolysis of hemicellulose is easier than cellulose due to its amorphous structure and the few hydrogen bonds involved. The existence of branches of short lateral chains of different sugars in the hemicellulose differentiates it from cellulose [25].
Lignin is a polymer of amorphous structure that confines cellulose and hemicellulose fractions [23]. The function of lignin is to act as a bonding and water proofing agent for the plant and as a protector from microorganisms and insects attack. It is a non-sugar polymer composed of phenylpropane units [20]. These units are sinapyl alcohol (syringyl propanol), coumaryl alcohol (p-hydroxyphenyl propanol), and coniferyl alcohol (guaiacyl propanol) [26]. The existence of these units depends on the type of LCB, for example, hardwood lignin has guaiacyl propanol as the main building blocks while softwood lignin has coniferyl and sinapyl alcohol as the main building blocks. All of these units are bonded by aryl-ether and C–C linkages. Lignin provides strength and protection to the plant and helps the transport of water in the plant. Therefore, lignin hinders the degradation of the cellulose during the hydrolysis. Lignin itself cannot be hydrolyzed nor fermented.
Straw species are known to be of uniform composition in comparison to wood species [23]. However, straws’ cellulose content is lower than that of wood. Table 2 gives examples of different lignocellulose biomasses and their compositions. As it can be seen from the table, cotton seed hair has the highest cellulose content of 80% or more. Paper is not a raw lignocellulose biomass because it is processed and all or most of hemicellulose and lignin are removed. That is why the cellulose content is very high compared to other LCB. The use of lignocellulose biomass for biorefinery depends on its availability as well as its compositions.
3 Lignocellulose biomass to biofuel
LCB can be converted into intermediates and final products by different processes: thermal, chemical, thermo-chemical, biological, and a combination of them [21]. It can be combusted to provide heat, gasified to provide syngas, or converted to bio-oil. The most common process for biofuel production is through the biological process in which LCB is converted to fermentable sugars through the hydrolysis and then the sugars are converted to biofuel through the fermentation as shown in Fig. 1. The saccharification and the fermentation can be combined in one step which is known as simultaneous saccharification and fermentation (SSF).
Block flow diagram for the conversion of biomass to biofuel without pretreatments (1) and (2) and with pretreatments (3) and (4). Paths (1) and (4) show the flow diagram of separated saccharification and fermentation of biomass. Paths (2) and (3) show the flow diagram of simultaneous saccharification and fermentation of biomass
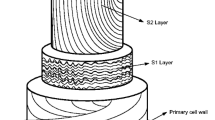
Enzymatic hydrolysis is attractive due to the minimum inhibitory products, and it is environmentally benign [33]. However, the process efficiency is dependent on the LCB characteristics [5, 25]. LCB structure and lignin existence can reduce the yield of fermentable sugars by blocking the enzymes from accessing the cellulosic materials. This resistance is known as biomass recalcitrance. Thus, a pretreatment step is required to expose cellulose by removing lignin and also to decrease cellulose crystallinity (Fig. 2) [13, 34]. Many pretreatment methods have been developed and are classified into physical, physicochemical, chemical, and biological. Each one of these methods has advantages and disadvantages. The pretreatment can result in removal of lignin and/or hemicellulose (totally or partially) and some of the cellulose. The pretreatment conditions need to be adjusted to reduce sugars losses and the production of inhibitory products such as acetic acid, furfural, and HMF [16]. In general, the most important pretreatment factors are the processing time, amount of chemicals and solvents required, and energy consumption.
4 Pretreatment technologies
LCB pretreatment methods are classified to physical, chemical, physicochemical, and biological pretreatments (Fig. 3).
4.1 Physical pretreatment
Physical pretreatment is defined as the pretreatment that does not employ chemicals or microorganisms for LCB pretreatment. The physical pretreatments being used are many such as mechanical comminution, irradiation, and pulsed electric field.
4.1.1 Mechanical pretreatment
Mechanical pretreatment or comminution reduces the material size and makes the cellulosic component more accessible for the enzymes [16, 25]. It does not remove the lignin component from the LCB. Size reduction can be through chipping, milling and grinding. Chipping can reduce the LCB size to 10–30 mm, and grinding or milling can reduce it to 0.2–2 mm [27]. Size below 0.4 mm was found to have no effect on the hydrolysis [35, 36]. Moreover, reducing the particle size excessively results in increasing the production of inhibitory products such as volatile fatty acids [37].
Dahunsi studied the comminution pretreatment effect on different grasses for methane production enhancement [38]. Two stages were used: the first one at a rotation speed of 800 rpm and the second one at 400 rpm. This resulted in methane yield increase of 22%. Also, the study showed that the pretreatment can be scaled up and it is economically feasible. These studies indicate that multiple stages with different machines affect the recalcitrance structure of LCB and could be investigated for bioethanol production too. Another study investigated biogas yield enhancement from meadow grass using two different machines [39]. The rotated plastic sweeping brush against steel roller provided a yield increase of 20% at 600 rpm. In the other machine, where a coarse steel roller was used instead of the brush, the yield was increased by 27% at a speed of 400 rpm. Waste paper pretreatment investigated with Hollander beater enhanced the methane yield by 21% at a beating time of 60 min [40]. Although these machines used for biogas production, the results can be used as a guide to investigate fermentable sugar yield using similar machines.
The advantage of mechanical pretreatment is that it can be performed during the harvesting, which is beneficial to optimize the total energy for harvesting and pretreatment. As an example, the effect of chopping during harvesting on biogas yield was studied [41]. It was found that a methane yield increase of 11–13% is possible with particle size reduction in the range of 33–6 mm. Chopping length below 7–8 mm requires more energy, and it is not recommended to guarantee profitability [42]. Tsapekos et al. carried out the pretreatment of meadow grass using three harvesters, disc-mower, an excoriator, and chopper [43]. The results indicated that using excoriator provided the highest methane yield increase of 20%. Similar investigation can be carried out for bioethanol production.
The energy consumption for size reduction is a function of the material’s type, the final particle size, the machine type, and the moisture content [44]. For example, the energy required to obtain corn stover with a particle size of 9.5 mm is 3.2 Wh/kg while for hardwood size of 1.6 mm, it is 130 Wh/kg [16].
Extrusion pretreatment results in a high shearing which affects the lignocellulose biomass. The extrusion forces are due to compression and transport of the biomass material through the extruder [45]. LCBs with moisture content of more than 15–20% are recommended to be pretreated with colloid mills and extruders, whereas hammer and knife mills are recommended for LCBs with moisture content of 10–15% [44]. The energy required by knife mill to reduce hardwood, straw, and corn stover sizes to 3.2 mm was found to be 50, 6.4, and 20 kWh/tonne, respectively [46]. On the other hand, hammer mill required 115, 21, and 9.6 kWh/tonne for hardwood, straw, and corn stover, respectively. In general, the required energy increases as the moisture content increases and the desired particle size decreases. The required energy for hemp grinding to increase methane yield by 15% was found more than steam pretreatment [47].
4.1.2 Wave-assisted pretreatment
Wave-assisted pretreatment, also known as irradiation pretreatment, can be microwave, ultrasound, electron beam, or gamma ray [48]. Gamma radiation increased the reducing sugars from rice straw by 142.74% [49]. Gamma radiation was found to disrupt the structure of the lignocellulose biomass and affect its thermal stability, and mechanical properties such as tensile strength and elongation are decreased (by 20% and and 14% for Jute fiber, respectively) [50, 51]. These effects are due to chemical reactions such as chain scission and cross-linking induced by radiation. Freed radicals are produced due to the ionization and cause the degradation of the lignocellulose biomass. The studies identified two types of radicals: short-lived and long lived [52, 53]. The short-lived radicals are produced in the amorphous regions, and they decay rapidly causing the primary degradation of the lignocellulos. The secondary degradation of the lignocellulose is caused by the long-lived radicals which are produced in the crystalline regions and their decay continues for days (20 days) [54]. Combined pretreatment of gamma ray and dilute acid increased glucose release from wheat straw by 69.7% [55]. The feasibility of the gamma ray pretreatment can be improved by accelerating the rate of the aftereffect degradation (secondary degradation).
The microwave irradiation was proved to affect the lignocellulose recalcitrant nature, and it could replace the conventional heating method. The advantages of this pretreatment are the easy operation, short processing time, and the disruption of cellulose structure [56]. The performance of microwave pretreatment depends on the electromagnetic energy storage ability of LCB and the conversion of the stored energy into heat [48]. Microwave pretreatment was used for wheat straw and switchgrass at 150 °C for methane production enhancement [57, 58]. The yield of methane from wheat straw was increased by 28% in comparison to the untreated sample. However, no yield improvement was observed for switchgrass and only the time to reach 80% of ultimate methane volume was decreased by 4.5 days. Increasing the microwave temperature was proved in some studies to have an adverse effect on biogas production rate and yield compared to conventional heating as it was tested for grass and agricultural straws [59, 60].
Microwave is usually used as an assistant technique to other pretreatments such as acid or alkaline pretreatments [61,62,63,64,65,66]. For instance, the pretreatment of rice straw using glycerine medium and microwave at a power of 240 W increased the amount of reducing sugars from enzymatic hydrolysis by more than 200% [67]. Microwave-assisted alkaline pretreatment increased the sugar yield from switch grass by 53% in comparison to the conventional heating [68]. Microwave-NaOH pretreatment increased the bioethanol yield from microcrystalline cellulose to a maximum of 58.91% of the theoretical yield [69]. The effect of microwave, microwave–alkali, microwave–acid, and microwave–acid–alkali pretreatments on the hydrolysis of sugarcane bagasse was investigated [70]. The acid used was 1% H2SO4, and the alkali used was 1% NaOH. The fermentable sugar yield was increased by the microwave–acid–alkali pretreatment with a significant effect observed for the alkali. This proves that using of microwave as an assistant technique decreases the amount of chemical needed; however, most of these studies do not provide a relationship or formula between the pretreatment conditions and the performance which could be done through the optimization. Also, providing an overall energy balance will help in comparing the feasibility of microwave to conventional heating. Microwave is also used as an alternative to conventional heating to supply heat required by chemical pretreatment [71].
Ultrasound waves is another type of irradiation pretreatment, and it was found to affect the physical and chemical properties of LCB [72,73,74]. Ultrasound pretreatment breaks the cellulose and hemicellulose by creating cavitation bubbles of small size at first which then grow to unstable size where they collapse resulting in changing of the biomass structure [75]. The main factors of this pretreatment are frequency, duration, reactor type, and solvent type [76]. Among these factors, sonication duration was found to have the maximum effect on the pretreatment performance [77]. Yachmenev et al. performed ultrasound pretreatment on corn stover, and they found that the enzymatic hydrolysis was enhanced significantly [78]. In another study, the released sugar yield from corn stover using sonication at 20 kHz was 10% more than the untreated sample [79]. Ultrasound pretreatment at high power was reported to increase the oxidation extent and improve the degree of nano-fibrillation of the cellulose [80, 81]. Patil et al. found that a power of 50 W is required to obtain a reducing sugar yield of 7.46 mg/ml from sawdust at 4%(w/v) solid loading, 50% duty cycle, 1 h, and 50 °C [82]. Combined microwave and ultrasonic pretreatment was applied for wheat straw, and it showed that the delignification efficiency of the wheat straw was decreased by 50% because of lignin condensation [83].
Irradiation pretreatment is usually tested in ovens equipped with microwave sources. However, there were some successful efforts to make the pretreatment continuous to facilitate its scale up and commercialization [32, 84]. Although irradiation pretreatment has a short time [82], it was reported to be costly and facing problems of treating large volumes and scaling up [74, 85]. In addition, maintaining uniform heat distribution and avoiding hot spots with the LCB are challenging. Selection of an optimum size of the biomass and the stirring/shaking during the irradiation may help in avoiding such problems.
4.1.3 Pulsed electric energy
Pulsed electrical energy (PEE) pretreatment has been used for gene delivery, microbial inactivation, permeation to save energy during drying, and components extraction [86]. The main parameter of PEE is the field strength (E) which is the voltage applied per unit distance between two electrodes. PEE is classified, based on the applied voltage characteristics, into pulsed electric field (PEF) and high-voltage electrical discharge (HVED). PEF method is characterized as a non-destructive and low-temperature technique. The PEF is based on application of a short burst electrical field with specific characteristic on the LCB sample suspended between two electrodes as demonstrated by Fig. 4 [86]. Plant tissues need field strength and time ranges of 500–1000 V/cm and 10−4–10−1 s, respectively, while smaller cells require field strength and time ranges of 20–50 V/cm and 10−5–10−3 s, respectively. The temperature rise during the PEF was found to be less than 10 °C [19]. Square and exponential decay waveforms are the most commonly used pulse geometries. Among the two, square pulses are more efficient in terms of minimizing energy absorption and system heating [87]. On the other hand, HVED is a high voltage technique which makes it more destructive with high-temperature increase.
Applying low-frequency pulses of high strength is energy efficient and minimizes the temperature elevation. Lindmark et al. investigated biogas production from ley crop silage with PEF pretreatment [88]. The pulse energy was kept constant at 67.2 J. The yield of biogas was increased by 16%, and the energy efficiency was 200% at a field strength of 96 kV/cm and number of pulses of 65. The PEF pretreatment of switchgrass and wood chip species was studied at a field strength of 2.5–10 kV/cm, a number of pulses of 1000–10,000, pulse width of 100 µs, and pulsing frequency of 3 Hz [89]. Red dye uptake was used as a permeability measure. The highest dye uptake for switchgrass occurred at a field strength of 8 kV/cm or higher while it was at 10 kV/cm for wood chip. The number of pulses was 2000 pulses for both performances. PEF increased the solute yield from sugar beet tails by 63.05% using a field strength of 450 V/cm for 10 ms [90]. The bioethanol content of the distillate was increased by 3.15%.
There are many PEF studies carried out on biomass other than LCB for production of biogas. Garoma et al. studied the PEF pretreatment of algal biomass as a feedstock for anaerobic digestion process [91]. The methane production was increased by 27.6–110% at an intensity range of 5.4–35 kWh/m3. Carlsson et al. used a field strength of 24 kV/cm with a pulse energy of 67 J for the pretreatment of municipal solid waste and resulted in increasing the biogas yield by 40% [92]. PEF pretreatment of waste activated sludge (WAS) for the enhancement of anaerobic digestion was carried out in a reactor of coaxial electrode and 7-ring electrodes [93]. The biogas production was 2.5 times higher than untreated sludge. This was due to the destruction of sludge cells as shown by SEM analysis.
PEF pretreatment was also employed to increase chemicals extraction from LCB. Loginova et al. investigated the effect of PEF on the extraction kinetics of soluble matter from chicory [94]. According to their results, the activation energy was decreased from 263 kJ/mol with only thermal treatment to 30–40 kJ/mol with addition of PEF pretreatment which enhanced the diffusion process. Bouras et al. studied the impact of PEF on the extraction of polyphenols from Norway spruce bark [95]. The extracted phenol was increased more than eight times using a strength of 20 kV/cm and a pulse width of 10 µs. The temperature elevation was below 7 °C. The results strongly recommended the replacement of the milling pretreatment by the PEF because it requires less energy. Zhao et al. studied the optimization of polysaccharides extraction from corn silk using PEF pretreatment [96]. The optimum yield of polysaccharides was 7.31% ± 0.15% at the strength of 30 kV/cm, pulse width of 6 µs, and liquid-to-solid ratio of 50.
The PEF pretreatment time proved to affect the pretreatment performance significantly. Millisecond and microsecond pulses were applied for the enhancement of the extraction of betanines from red beet, and it was found that the product yield increased by 6.6 times using millisecond pulses (at 0.6 kV/cm and 40 ms) and by 7.2 times using microsecond pulses (at 6 kV/cm and 150 µs) [97]. The results also showed that the microsecond pulses require far less amount of energy than the millisecond pulses to obtain the same yield. For example, the amount of energy required to obtain a yield of 775 µg/g was found to be 6 kJ/kg for microsecond pulses (at 75 µs and 4 kV/cm) and 20 kJ/kg for millisecond pulses (at 40 ms and 0.4 kV/cm).
HVED has been used for electroporation and breaking of cell walls to facilitate the extraction of hemicellulose, lignans, polyphenols, and proteins [98,99,100,101,102]. Brahim et al. studied the effect of HVED pretreatment on the delignification of rapeseed straw [103]. The delignification yield obtained was in the range of 32.2 to 42.3% for treatment time of 10 to 40 min and an energy level range of 204 to 814 kJ/kg. The HVED performance was compared to microwave and ultrasound, and the results showed that in order to obtain the same delignification yield (35%), an energy level of 4.5 times and 9 times more than HVED is required for ultrasound and microwave, respectively. This was attributed to the high strength of the shock waves and bubbles cavitation produced by HVED [104]. El Kantar et al. worked on finding the best pretreatment conditions for the extraction of polyphenol and fermentable sugars from orange peels treated with HVED [105]. The reducing sugar yield was increased by 19% by changing the energy input from 44 to 222 kJ/kg. HVED was also combined with other pretreatments, such HVED-soda, to enhance the enzymatic hydrolysis [106]. An electric field without pulsing was also applied during enzymatic hydrolysis of rice straw [107]. Electric field strength of 12 V/m increased the conversion efficiency by 32.6% compared to without electric field hydrolysis.
In summary, pulsed electric energy has short processing time, low energy requirements, and less waste. However, the application of PEE to lignocellulose biomass to enhance biofuel production in the literature is quietly limited. Although the existed experimental studies indicated a promising enhancement of using PEE for LCB pretreatment, the industrial application is still limited. More studies are needed to develop guidelines for PEF pretreatment considering the LCB type, the desired products, and the integration of PEF in the overall biofuel process. Also, the economic aspects of the process need to be studied.
4.2 Chemical pretreatment
Chemical pretreatments are based on using chemicals to facilitate the structure break down and lignin removal. The following sections discuss the different chemical pretreatment methods.
4.2.1 Ozonolysis pretreatment
Ozone has high reactivity with lignin because of the existence of functional groups of high electron density and double bonds [108]. Therefore, it affects the recalcitrant nature of the LCB and makes cellulose accessible for enzymes [109]. The effect of ozonolysis pretreatment on the LCB main components is in the order lignin, hemicellulose, and cellulose [110]. A linear correlation was found between ozone consumption and lignin removal [111]. The acidity of the pretreated biomass was observed to increase (pH decrease from 6.5 to 3.0) as a result of carboxylic acids formation. The ozonolysis of poplar sawdust for enzymatic hydrolysis resulted in glucose yield equal to the theoretical yield [109]. It was also found that the optimum moisture content of the sawdust was important for the degree of solubilization. The sugar yields from the enzymatic hydrolysis of wheat straw and rye straw were 88.6% and 57% with ozonolysis in comparison to only 29% and 16% without ozonolysis [108]. Travaini et al. used ozonolysis pretreatment for ethanol production from sugarcane bagasse [112]. The enzymatic hydrolysis gave glucose and xylose yields of up to 77.55% and 56.94%, respectively, with a total sugar yield of 2.98 g per g of ozone. Santos et al. investigated the ozonolysis of coffee husks (CH) and the maximum yield of methane (36 NmL CH4/g CH) was obtained at the liquid/solid ratio of 10 ml/g, pH of 11, and ozone loading of 18.5 mg O3/g CH [113]. The yield increased to 49 NmL CH4/g CH by using two-stage ozonolysis.
The advantage of ozonolysis is that no furfural and HMF are produced [114]. However, the formation of carboxylic acids from sugar degradation, which are inhibitory to the fermentation, was reported [115]. There are also inhibitory compounds produced from the degradation of lignin such as vanillin and vanillic acid [116].Washing of the pretreated biomass with water was found to remove the inhibitory products as well as some of the carbohydrates such as xylan [114].Although high sugar yield is obtainable with ozonolysis pretreatment, the ozone requirement is large which increases the pretreatment cost. Ozone production is an energy intensive process (1.65 MJ/100 g ozone) [112]. Finding an effective way to recycle the unused ozone will help in reducing the pretreatment cost. The pretreatment cost can be decreased by considering the recovery of some of the degradation products as a value-added products [116].
4.2.2 Dilute acid pretreatment
Pretreatments with concentrated acids (30% or more) were used for hemicellulose removal and hydrolysis of cellulose [117]. However, using concentrated acid is toxic, is corrosive, and requires acid recovery which makes the pretreatment expensive [118]. Thus, dilute acid pretreatment has been employed instead of concentrated acids. Sulfuric acid is the most used because it is cheap. It was used with different lignocellulose biomass materials include rice hulls, peanut shells, sugarcane bagasse, and cassava stalks [119] as well as saline crops [120], oat hull [121], and oat husks [122]. In a study by Sun and Cheng, dilute sulfuric acid pretreatment for enzymatic hydrolysis of rye straw and Bermuda grass was investigated [123]. The yield of arabinose, galactose, and xylose increased with the increase of acid concentration and the pretreatment time. The glucose yield from rye straw did not change significantly with the pretreatment conditions while it did increase from Bermuda grass as the pretreatment conditions severity increases. Overall, the hydrolysis of Bermuda grass was easier than rye straw which attributed to the different structure and components of both materials. Castro et al. employed sulfuric acid pretreatment for rapeseed straw hydrolysis over a temperature range of 140–200 °C, pretreatment time of 0–20 min, and acid concentration range of 0.5–2% (w/v) [124]. Total conversion of cellulose was achieved at a temperature of 200 °C, time of 27 min, and 0.4% (w/v) H2SO4. However, the concentration of inhibitory products in the hydrolysate was high and the study suggested the use of a detoxification procedure to improve the fermentation process. Dilute sulfuric acid pretreatment has some drawbacks such as strongly catalyzes the formation of furfural, HMF, vanillin, and acetic acid as reported by Agrawal et al. using pilot scale plant for wheat straw hydrolysis [17]. These products inhibit the enzymatic hydrolysis by decreasing the availability of enzymes [125].
Many other dilute acid pretreatments were studied to overcome the sulfuric acid pretreatment problems. Corn stover was pretreated by HNO3, and the result showed that 96% xylose yield is achievable at 0.6% HNO3, 150 °C, and 1 min [126]. Skiba et al. studied dilute HNO3 pretreatment of oat hulls at acid concentration of 4% (w/w) and solid loading of 33.3 g/l [127]. At these conditions and with the assistance of lignin removal with water washing, a reducing sugar yield of 93% was obtained. Dilute phosphoric acid pretreatment of wheat bran and wheat straw was also investigated [128, 129]. Characterization of pretreated cauliflower wastes showed that the H3PO4 helps in hemicellulose and lignin removal which resulted in increasing cellulose accessibility [130]. In addition, according to XRD and SEM analysis, the pretreatment caused significant change in cellulose porosity and crystallinity index which increased the sugar release. Yu et al. found that adding mechanical pulverization step to dilute phosphoric acid resulted in changing the crystallinity index and porosity of corn stover which led to the enhancement of enzymatic hydrolysis and increasing the sugar yield by 106.95% [131]. The pretreatment of corn stover with H2SO4 was better than with H3PO4 due to the high hemicellulose removal achieved by H2SO4 pretreatment [132]. These results agree with what was found by Nair et al. for the pretreatment of wheat bran and whole-stillage fibers at 100 °C [133]. The advantage of using phosphoric acid is that the solid waste can be used as a fertilizer.
Organic acids have also been used for dilute acid pretreatment, and their performances were compared to mineral acids. Among the organic acids investigated, maleic acid showed a superior performance in comparison to sulfuric acid, succinic acid, citric acid, and acetic acid [134, 135]. The maleic acid pretreatment resulted in the highest glucose yield and much less degradation products. These findings were confirmed by Lu and Mosier who investigated the pretreatment of corn stover using maleic acid and sulfuric acid [136]. The pretreated corn stover with maleic acid showed lower xylose degradation by 3–10 times in comparison to sulfuric acid. The xylose yield with maleic acid under the optimum conditions reached 95% with a furfural amount 5 times less than that of sulfuric acid. In other studies, different acids (H2SO4, H3PO4, HCl, HNO3, and trifluoroacetic acid) were used for the pretreatment of loblolly pine (soft wood) [137]. Trifluoroacetic acid pretreatment gave the highest sugar yield of 70% at 150 °C and pH of 1.65, whereas mineral acid (H2SO4, H3PO4, HCl, and HNO3) yield was about 60%. It was observed that increasing the pretreatment temperature leads to the production of degradation products such as furfural and 5-hydroxymethyl-2-furaldehyd (HMF) and the lowest degradation product amount was obtained for trifluoroacetic acid pretreatment. Kootstra et al. compared the performances of fumaric, maleic, and sulfuric acid for the pretreatment of wheat straw using the enzymatic digestibility and degradation products formation as a measurement criteria [138]. The organic acid performance was much better than that of the mineral acid as they gave less amount of furfural at 20% solid loading. The study also found that furfural formation increases with the increase of the solid loading for organic and mineral acids. The good performance of organic acids in terms of high fermentable sugar yields and less degradation is attributed to their weakness in comparison to mineral acids [139]. The dissociation constant of acid (Ka) was found to affect the sugar release and degradation products formation [140]. The higher the Ka value the higher the degradation products level. Therefore, organic acid pretreatment has less degradation products than the mineral acids.
Dilute acid pretreatment is associated with equipment corrosion, formation of degradation products, and waste from acid neutralization. These drawbacks affect the feasibility of dilute acid pretreatment.
4.2.3 Alkali pretreatment
The main advantage of the alkali pretreatment is the delignification of LCB materials [118]. In addition, it can be performed at low temperature (room temperature and lower) [141, 142]. It also eliminates all the acetyl groups as well as reduces the cellulose crystallinity [143]. The chemistry of the alkaline-based pretreatment revealed that the molecules of ester bonds have a saponification reaction which results in the disappearance of those bonds and increasing of pore structure of the lignocellulose material [144].
The alkaline pretreatment depends on the feedstock, the base, and the pretreatment conditions. Sodium hydroxide has gotten the most attention. It was used for the pretreatment of solid residue of olive mill [145]. The saccharification was enhanced 2.5 times at the optimum pretreatment conditions of 100 °C, 20 g solid/g NaOH, and 1 h. The sugar release from sorghum bicolor straw at 2% NaOH was increased by 5.6 times and 4.3 times at 121 °C for 60 min and 60 °C for 90 min, respectively [146]. Low-temperature NaOH pretreatment was investigated for the enhancement of sweet sorghum bagasse enzymatic hydrolysis [147]. The sugar yield from the pretreated bagasse reached 98.7% at 2.5 M NaOH and room temperature for 120 min. The lignin removal was found to be strongly affected by the temperature and reached 90% at 50 °C. A comparison between NaOH, H2SO4, H2O2, and ozone pretreatments showed that NaOH-based pretreatment gave the highest lignin removal and cellulose conversion [148].
Lime is one of the bases studied for the alkaline pretreatment. It was used for the pretreatment of switchgrass [35], poplar wood and newspaper [149], corn stover [150, 151], Jatropha seed cakes [152], and wheat straw [153]. Lime pretreatment is effective at high temperatures and short-time or long pretreatment time and low temperatures [154]. Washing is needed to remove the lime and then the lime is recovered by carbonating the washing water. Yan et al. investigated lime pretreatment of sweet sorghum bagasse and compared the performance to NaOH pretreatment [155]. The results showed that the cellulose conversion upon enzymatic hydrolysis of lime pretreated bagasse was 1.62 times that of NaOH pretreated bagasse which was attributed to the ability of lime to increase the porosity of bagasse as well as the reduction of carbohydrates caused by calcium ions. Combined NaOH and lime pretreatments of switchgrass provided glucose yield of 59.4% and xylose yield of 57.3% at 0.02 g lime/g biomass, 0.10 g NaOH/g biomass, and 6 h [156]. Similar yields were obtained using NaOH only at a loading of 0.2 g NaOH/g biomass; however, the advantage of the combined pretreatment is that lower NaOH loading (50% less) was needed. Other bases were explored for alkaline pretreatment such as potassium hydroxide [72, 143] and ammonia [157,158,159,160].
Alkali pretreatment, in addition to lignin removal, removes hemicellulose and part of cellulose [161]. Therefore, it needs to be applied at low pretreatment severity which results in low delignification. Another solution is to be preceded by another pretreatment such as hot liquid water or dilute acid which degrades the hemicellulose content and the remaining solid can be post-treated with alkali at mild conditions to remove lignin. Rice straw was pretreated with 3% (v/v) H2SO4 and then followed by 4% (v/v) NaOH resulted in 55% removal of lignin and 90% removal of the hemicellulose (Fig. 5) [162]. Ethanol yield from separated xylose and the cellulose obtained after alkali post-pretreatment were 0.468 g/g and 0.40 g/g, respectively. In another study, a biorefinery strategy of 4 stages was developed to produce xylose, ethanol, and adhesive from corncob [163]. Xylose was produced from the hemicellulose fraction using dilute acid pretreatment (H2SO4). The residue was transferred to the second stage where it was converted to lignin-rich liquor and cellulose using dilute alkali pretreatment (NaOH). The cellulose was converted to ethanol in stage 3 via hydrolysis and fermentation while lignin-rich liquor was converted to phenol formaldehyde resin adhesive in stage 4 via resinification. With by-product utilization and process integration, the overall revenue of the biorefinery strategy proposed by this study is 111.3 times the revenue of the process without lignin utilization. Moreover, waste water and greenhouse gas emissions intensity per USD were decreased by 57.8% and 98.9%, respectively. This is promising results and can be applied to other lignocellulose biomass.
The alkaline-based pretreatment helps basically in the delignification of the biomass [148]. However, it has some limitations such as the formation of salts which are not recoverable and can be incorporated into the biomass materials [34, 118]. Therefore, extra treatment is needed, such as neutralization and washing, to remove those salts [141].
4.2.4 Organosolv pretreatment
The organosolv pretreatment or organosolvation pretreatment uses an organic solvent with or without a mineral acid [164,165,166]. Ethanol, methanol, and ethylene glycol are examples of solvents commonly being used. In some studies, pressurized CO2 was used instead of mineral acid [167, 168]. The organosolv pretreatment helps in LCB delignification which increases cellulose conversion to more than 90% [169]. The organosolvation of rice straw was studied for maximization of acetone, butanol, and ethanol yield at 75% (v/v) ethanol and 15% (w/w) sulfuric acid [170]. The highest sugar yield was found for straw pretreated at 150 °C for 60 min. However, the highest yields of acetone, butanol, and ethanol were obtained for straw pretreated at 180 °C for 30 min. Chen et al. compared four different solvents (acetoline, auto-catalyzed ethanol (ACE), formiline, and sulfuric acid–catalyzed ethanol (SACE)) for the pretreatment of wheat straw [171]. The highest delignification and the lowest xylose degradation were obtained for formiline and acetoline. The highest ethanol yield was obtained for formiline while the lowest was for acetoline. Tan et al. investigated the pretreatment of hybrid Pennisetum using four organosolvs (acetone, ethanol tetrahydrofurfuryl alcohol (THFA), γ-valerolactone (GVL)) at 100 °C, 2 h, 0.05 mol/l H2SO4, and liquid-to-solid ratio of 12:1 [172]. The highest enzymatic digestibility (87.5%) was obtained with THFA which was 131.5% higher than the untreated biomass. The TGA results showed that acetone and GVL pretreatment decreased the thermal stability of the pretreated material while THFA and ethanol decreased the thermal stability of only the isolated lignin. A ternary solvent composed of acetone, phenoxyethanol, and water (or APW) was studied for the pretreatment of amorpha [173]. Cellulose recovery of 80.94% and lignin removal of 95.6% were achieved at the optimized conditions (APW volume ratio of 5:11:4, 130 °C, 70 min, 0.15 M H2SO4, and 20 (v/w) liquid-to-solid ratio). SEM results showed that the pretreatment caused tissue separation in the cellulose fraction. The crystallinity index of the treated amorpha was 10.74% lower that the raw amorpha which indicates to the decrystallization effect of the pretreatment. The APW pretreatment was applied to sugarcane bagasse, pine, and corn cob and resulted in over 92% delignification. The pretreatment of sugarcane trash was investigated using glycerol and oxalic acid, and the optimum conditions were found to be 80% v/v glycerol, 170 °C, and 300 mM oxalic acid [174]. At these conditions, cellulose recovery of 71.7% was achieved. In addition, 96.8% and 83.9% of hemicellulose and lignin, respectively, were removed. In another study by Romani et al., the results indicated that the most significant organosolv parameters using ethanol are time and temperature [175]. The maximum ethanol yield was 98.73% obtained at 192.5 °C, 86 min, and 65% ethanol aqueous concentration.
Organosolv has also been used in a combination with other pretreatments such as liquid hot water [176], steam explosion [177], alkaline [178], organic acid and alkaline hydrogen peroxide [179], and microwave [180]. The alkaline–organosolv pretreatment of corn stover using sodium hydroxide–methanol solution resulted in enzymatic digestibility of 97.2% for glucan and 80.3% for xylan [178]..
Using of mineral acids is known to be related to the formation of inhibitory products and equipment corrosion. Therefore, efforts were made to replace those acids with higher efficient solvents. In this regard, organic amine was used with aqueous ethanol for the pretreatment of corn stover [181]. Seven amines were investigated, namely diethylamine, triethylamine, isopropylamine, n-butylamine, isobutylamine, ethylenediamine, and n-propylamine. Among these amines, n-propylamine provided the highest delignification and sugar yield of 81.7% and 83.2%, respectively. Ethanol–hydrazine hydrate pretreatment of corn stover was reported at a solid/liquid ratio of 0.1 g/ml, 60% ethanol concentration, and hydrazine hydrate loading of 10 mmol/g solid [182]. A lignin removal of 77.94% and sugar yield of 90.27% were obtained.
The advantage of the organosolvation is that high delignification rate can be obtained which increases cellulose accessibility. In addition, pure lignin can be separated which could be used for the production of chemicals. However, organosolvation requires the used solvents to be recovered and recycled which may lead to waste generation and increase the cost of the process. Another reason to remove the solvents after an organosolv pretreatment is to prevent any inhibitory action to the enzymatic hydrolysis and the subsequent fermentation.
4.2.5 Co-solvent enhanced lignocellulosic fractionation pretreatment (lignin-first pretreatment)
Co-solvent enhanced lignocellulosic fractionation (CELF) pretreatment or lignin-first pretreatment uses a miscible solution of dilute acid and tetrahydrofuran (THF)–water to efficiently isolate lignin from the biomass [183]. This results in clean lignin product and also facilitates the recovery of cellulosic fraction for sugars production. The mechanism of CELF pretreatment is that THF acts with water as one solvent, and the interactions between it and lignin become equivalent to that between lignin molecules [184]. Therefore, equilibrium shift occurs and lignin dissolves. CELF affects primarily the lignin component of the lignocellulose biomass [185]. The CELF pretreatment of wood chips removed 85% of the lignin and solubilized over 90% of xylan. CELF does not affect the cellulose crystallinity significantly (3% decrease of CrI). The difference between organosolv pretreatment is that organosolv affects, in addition to lignin removal and xylan solubilization, cellulose structure. γ-Valerolactone (GVL) pretreatments resulted in 52% lignin removal and 70% xylan solubilization, respectively. CrI was decreased by 18%, and the cellulose degree of polymerization was decreased by 48%. The performance of CELF was compared with the dilute acid pretreatment at similar conditions [186]. The delignification of corn stover and Populus by CELF was 73.6% and 80.6% higher than dilute acid pretreatment for both materials. Due to the corrosion and formation of undesired products related to the use of dilute acids, some studies investigated CELF in the absence of acids (only THF-water) [187, 188]. The lignin removal from corncob residues was over 71.9%.
The advantage of CELF pretreatment is that simultaneous saccharification and fermentation (SSF) can be performed on the treated biomass and high biofuel yield can be obtained [189]. For instance, application of CELF on corn stover achieved ethanol yield of 89.2% in comparison to 73% using dilute acid pretreatment [190]. More details on the CELF pretreatment are available in the literature [191,192,193,194]. CELF isolates lignin which can be sold as a by-product or converted to value-added products and sold. This can improve the feasibility of the pretreatment. However, an optimization is required between the lignin removal and the yield of total sugars of the pretreated materials.
4.2.6 Ionic liquid pretreatment
Ionic liquids (ILs) are thermally stable and have a minimum environmental impact due to their extremely low volatility [195,196,197]. It was found that ionic liquids help in cellulose dissolution by breaking the intramolecular hydrogen-bonding of the LCB [198, 199]. Amoah et al. compared the pretreatment of sugarcane bagasse using five different ILs: 1-ethyl-3-methylimidazolium diethylphosphate ([Emim][DEP]),1-ethyl-3-methylimidazolium acetate ([Emim][OAc]), 1-butyl-3-methylpyridinium acetate ([Bmim][OAc]), 1-ethyl-3-methylimidazolium chloride ([Emim][Cl]), and 1-butyl-3-methylpyridinium chloride ([Bmpy][Cl]) [200]. The highest sugar yields were obtained with [Bmpy][Cl] which resulted in ethanol yield of 84%.
The main problem with ILs is their high cost; therefore, methods of using aqueous ILs were investigated. Swatloski et al. found that the presence of water decreased cellulose solubilization significantly as cellulose dissolution was not possible at water concentration greater than 1% (w/w) [198]. This is attributed to the competition between water and ILs for hydrogen-bonding with cellulose. The recovery of fermentable sugars from wheat straw was 71.4% at the optimum conditions of 158 °C, 3.6 h, and 49.5% (w/w) ionic liquid concentration [201]. The digestibility of cellulose and xylan was found to change proportionally with time, temperature, and ionic liquid concentration. These results show that a cost-effective aqueous IL pretreatment is possible with optimization of the processing conditions. Hu et al. studied the effect of 50% (w/w) 1-butyl-3-methylimidazolium tetrafluoroborate–water mixture on the corn stack hydrolysis [202]. The enzymatic hydrolysis efficiency was increased by 81.68% at 150 °C for 5 h. Although the crystallinity index was increased, the performance enhancement was explained to be due to the breakdown of lignin–polysaccharide bonding as well as the increase of the specific surface area. The digestibility improvement of IL-treated biomass was explained by FTIR and XRD analysis of energy cane bagasse treated with 1-ethyl-3-methylimidazolium acetate [197]. The pretreatment conditions were 5% (w/w) ILs concentration at 120 °C for 30 min which provided xylan and glucan digestibility (64.3% and 87.0%, respectively) much higher than untreated biomass (2.8% and 5.5%, respectively). FTIR analysis showed that the crystallinity of treated biomass was changed significantly. This was confirmed by XRD which showed that the crystallinity index of treated biomass was decreased by more than 56%. These results explain that ILs help in both delignification and decreasing the cellulose crystallinity.
The high cost of ionic liquids makes the combination with other pretreatments attractive. The combined pretreatment of rice straw using ionic liquids and ammonia was studied to achieve a synergy effect to make the pretreatment feasible [203]. The results showed that the ammonia-ILs (1-ethyl-3-methylimidazolium acetate) provided 97% glucose conversion upon enzymatic hydrolysis. This conversion was higher than individual ammonia pretreatment (52%) and IL pretreatment (76%). In addition, the IL was recycled over 20 times with glucose conversion of 78% of the 20th recycled IL. Also, the enzyme loading could be lowered and still high glucose conversion is achievable (92% and 83% glucose conversion for 50% and 10% enzyme reduction, respectively). Hu et al. studied ultrasound-assisted IL pretreatment of soybean and corn straw [204]. Two ILs were synthesized namely 1-allyl-3-methylimidazolium chloride ([AMIM]Cl) and 1-H-3-methylimidazolium chloride ([HMIM]Cl). It was found that [HMIM]Cl had the highest reducing sugar yield of 26.635% and 25.015% for soybean and corn straw, respectively. Addition of surfactant was reported to enhance the ILs pretreatment [205]. For instance, addition of 3% (w/w) polyethylene glycol 4000 with 1-butyl-3-methyl imidazolium chloride increased the saccharification of sugarcane bagasse by 23% in comparison to IL-treated sample. The optimum conditions for polyethylene glycol 4000 with 1-butyl-3-methyl imidazolium chloride were found in another study to be 154.6 °C, 60 min, and 5% (w/w) of polyethylene glycol concentration [206]. Many ILs combined pretreatments were reported such as IL-alkaline pretreatment [207], oxidative ionic liquid pretreatment [208], aqueous acidified ionic liquid pretreatment [209], microwave-assisted aqueous ionic liquid pretreatment [210, 211], and ultrasound and surfactant-assisted ionic liquid pretreatment [212].
Imidazolium ionic liquids are known to be the most efficient liquid for LCB pretreatment. However, they are expensive and can be inhibitory to the enzymatic hydrolysis [213, 214]. Moreover, they are toxic to the fermentation process as they inhibit the microbial growth even if they present at a very low concentration. Therefore, they must be washed completely which requires a large amount of water or to use ionic liquid-tolerant yeasts [215]. Since cholinium IL is renewable and it has lower cost and toxicity than imidazolium IL, it was studied for the pretreatment of bamboo powder [216]. The cellulose saccharification obtained was 80% at IL/biomass ratio of 3–10 g/g. There was no difference in the composition of the treated biomass and untreated biomass. This explains that the cellulose saccharification improvement was because of structural changes which were confirmed by PXRD as it showed a decrease in the crystallinity index for IL/biomass ratio greater than 3 g/g. An et al. attributed the excellent performance of cholinium to its ability to remove lignin and xylan [217]. They also found that the ILs can be recycled and reused with a saccharification yield of 75% for the eighth cycle. Choline acetate was investigated for sugarcane bagasse saccharification enhancement [31]. The cellulose conversion to glucose was 98.7% at 110 °C for 6 h. This result is close to what was obtained by Ninomiya et al. (95% cellulose conversion for 5 h) [218]. Although the cellulose conversion was slightly lower than other pretreatments (comminution, microwave, and alkaline), the energy profit ratio (energy produced/energy required) was the highest (4.04) which demonstrates that the choline acetate pretreatment is energy efficient.
Morpholinium ILs are less toxic and cheaper than imidazolium ILs [219]. Kahani et al. synthesized two morpholinium ILs: morpholinium acetate ([Morph][Ac]) and N-allyl-N-methylmorpholinium acetate ([AMMorph][Ac]) [220]. Rice straw was used as a feedstock, and dimethyl sulfoxide (DMSO) was used as a co-solvent. [AMMorph][Ac] provided glucose and ethanol yields of 98.1% and 90.8%, respectively, at 120 °C and IL-to-DMSO ratio of 70:30 for 5 h. The ethanol yield obtained with [AMMorph][Ac] was the highest compared to 8% NaOH, 85% H3PO4, and 1-butyl-3-methylimidazolium acetate pretreatments. These results show that the use of dimethyl sulfoxide as a co-solvent decreases the required amount of IL.
4.2.7 Deep eutectic solvent pretreatment
Deep eutectic solvent (DES) is a green solvent and was introduced for the first time in 2003 by Abbott et al. [221]. The first DES was formed by mixing hydroxyethyl trimethylammonium chloride (choline chloride) and urea and the resulted mixture is a liquid at ambient conditions with a freezing point (12 °C) much lower than the individual components (302 °C for choline chloride and 133 °C for urea). This characteristic is described as eutectic which occurs at urea to choline chloride molar ratio of 2. The properties of DES depend greatly on the capability of the mixed components to form hydrogen bonds. Based on these findings, many other DESs were introduced from mixtures of quaternary ammonium salts and amides with unusual solvent properties similar to ionic liquids but sustainable and biodegradable. For example, DES can be synthesized from lignin-derived acids such as p-coumaric acid [222]. The mechanism of DES pretreatment the cleavage of ether bonds which leads to lignin depolymerization and thus its separation [223, 224]. This was confirmed by 13C NMR spectra of the DES-extracted lignin and lignin standard [225].
Francisco et al. investigated the suitability of DESs for the pretreatment of LCB [226]. The results showed that some of the tested DESs such as lactic acid–choline dissolved part of the lignin with low or no dissolution of cellulose. The study did not come out with a single conclusion as the performance was different among different DESs. The study recommended the understanding of hydrogen bonds of DESs and pretreatment optimization. The DESs do not remove only lignin, but also fractions of hemicellulose and cellulose are solubilized [227, 228]. The pretreatment conditions can be optimized to produce lignin of a high purity for commercial use. It was also reported that acetic acid and furfural were detected in the mixture after the pretreatment of wood biomass [225]. This is due to the sugar degradation caused by the pretreatment conditions. Addition of water to DES was proven to enhance the delignification, remove part of the hemicellulose, and improve the enzymatic digestibility [222, 228, 229]. This was attributed to the increase of the solvent thermal stability after addition of a suitable amount of water to the DES (depends on the DES type) [230]. Table 3 provides a summary of some DES pretreatments along with their performances. The main challenge of DES pretreatment is the long processing time as it can be up to 24 h or more. The recovery of DESs and their recyclability need to be investigated to provide an overall evaluation of the pretreatment. The difference between IL and DES pretreatment is that DES selectively removes lignin while IL causes cellulose dissolution [231].
DES efficiency was found to be improved when coupled with alkali post-treatment [241]. p-toluene sulfonic acid-choline chloride (p-TsOH/ChCl) was studied for pretreatment of poplar sawdust (PL) and miscanthus (MC) at 100 °C, 400 rpm, 20 min (MC), and 40 min (PL) and then followed by NaOH post-treatment (1% NaOH, solid loading ratio of 1:80, 2 h, and at room temperature). The coupled pretreatment resulted in a glucose yield (on dry biomass) of 43.2% and 42.51% for poplar sawdust and miscanthus, respectively. The improved enzymatic hydrolysis yield was attributed to the xylan and lignin removal of over 90%. These results were confirmed by XRD and FTIR analyses. The process feasibility can be further enhanced with lignin utilization and recyclability of DES and NaOH.
Combined physical and DES pretreatment was also reported in the literature. Ultrasound pretreatment was combined with three different DESs, specifically choline chloride-lactic acid (ChCl/LA), choline chloride/glycerol (ChCl/G), and choline chloride/urea (ChCl/U) [242]. The reducing sugar yields obtained from oil palm empty fruit bunch were 36.7%, 35.8%, and 35.3% for ChCl/LA, ChCl/U, and ChCl/G, respectively. These results were obtained at 50 °C, sonication power of 210 W, for 15 min. ChCl/LA significantly changed the structure of the biomass. The improved performance of ChCl/LA compared to other DESs was ascribed to its the low surface tension and low viscosity. Another study applied choline chloride-glycerol (ChCl-G) to sugarcane bagasse with assistant of ultrasonic waves [243]. The reducing sugar yield improved by 32.6% in comparison to ChCl alone at optimum conditions of 5.72% (w/w) biomass loading and 60% sonication amplitude for 7.79 min.
The effect of the number of chlorine atoms in the DES was investigated by applying DES containing monochloroacetic acid (MCA) and dichloroacetic acid (DCA) [244]. ChCl-MCA and ChCl-DCA were applied to oil palm fronds and resulted in a delignification yield of 75.96% and 74.89% at 120 °C for 1 h. Enzymatic hydrolysis of biomass pretreated with ChCl-DCA resulted in 89% glucan conversion after 24 h only while biomass pretreated with ChCl-MCA resulted in 82% glucan conversion after 72 h. These results prove that increasing the number of chlorine atoms of DES shortens the time of the enzymatic hydrolysis.
4.3 Physicochemical pretreatment
Physicochemical pretreatments are physical methods with assistance of chemicals or vice versa. These methods include carbon dioxide explosion, liquid hot water, autohydrolysis, and ammonia fiber explosion [56].
4.3.1 Liquid hot water pretreatment
Chemical pretreatments are characterized to be of a high waste generation. Liquid hot water pretreatment (LHW) was studied as alternative technique for expensive chemical-based pretreatments [245]. The pretreated biomass can be transferred to the hydrolysis step without any further processing. Different reactor types were used for LHW including batch autoclave, continuous-flow reactor [246], and semi-continuous fixed bed reactor [247]. The semi-continuous fixed bed reactor has several advantages in comparison to others as it provides better contact between the solid and LHW and no LCB size reduction is needed which saves energy.
Wheat straw pretreated with LHW at 188 °C for 40 min resulted in enzymatic hydrolysis yield of 79.8% [248]. Based on variables analysis, two-step pretreatment was proposed which expected to result in enzymatic hydrolysis yield of 90.6%. Palm-oil residues were treated by LHW for ethanol production and the economic feasibility was used as a criteria for pretreatment conditions selection [249]. The maximum profit was achieved at conditions (185 °C and 15% solid loading for 30 min) different than those for the highest ethanol yield. Pangsang et al. used LHW with palm empty fruit bunch and palm fiber [250]. The sugar yield increased by more than 200% under the optimum conditions (3 MPa and 200 °C for 15 min).
LHW can be used in a combination with solvents such as ethanol [251], with milling [252], and mechanical extrusion [253] to increase the sugar yield. Using of 1,4-butanediol (BDO)-LHW at 200 °C for 40 min with BDO concentration of 20% (v/v) increased the enzymatic digestibility of bamboo by 14% compared to LHW alone [254].
The liquid hot water pretreatment is environmentally friendly as no chemicals are required. Therefore, no waste is generated to be washed and disposed of. LHW also has low degradation product formation at low temperatures. The main disadvantage of LHW is that it is energy intensive (3.5 higher than HCL pretreatment [255]) which increases it is cost (capital and operating).
4.3.2 Steam explosion (autohydrolysis)
In explosion pretreatment, the biomass is treated with high pressure (160–260 °C) steam for short time (few seconds to few minutes) and then the pressure is swiftly changed to atmospheric which results in the explosion of the biomass [27]. Steam explosion helps in decreasing lignin and hemicellulose contents of LCB and increasing cellulose accessibility [256]. Qiu and Chen applied steam explosion to wheat straw and used laccase for the hydrolysis of the pretreated straw [257]. A cellulose conversion of 84.23% was achieved at 1.3 MPa for 5 min. An enzymatic hydrolysis of rice straw treated with steam explosion provided a reducing sugar concentration of 85.4 mg/g which was 129.6% higher than the untreated rice straw [49]. Zhao et al. found that using steam explosion pretreatment decreased the concentration of enzymes required for the hydrolysis by 80% (from 100 to 20 U/g) [258].
Steam explosion requires high energy. Therefore, it is necessary to use it with other pretreatments to make it feasible. Employing of acid pretreatment followed by steam explosion was found highly effective for enzymatic saccharification [259, 260]. The enzymatic digestibility reached 73% for rice straw and 90.1% for corn straw. Grass silage treated with steam explosion at 1% acid loading and 190 °C for 10 min provided 98% sugar yield [261]. Steam explosion was also combined with hydrogen peroxide [262] and alkaline [263]. Combined steam explosion and NaOH for the pretreatment of sugarcane trash and aspen wood was found to prevent the formation of furfural [264].
The pretreatment severity does not affect only the cellulose; it also affects the inhibitors formation. Martin et al. studied the inhibitors formed during steam explosion of Norway spruce in the presence of sulfuric acid or SO2 [265]. Most of inhibitor formation was found related to the presence of SO2. Studying the effect of inhibitors concentration in the range of 12–20% TS (total solid) revealed that formaldehyde concentration was very toxic for yeast as it inhibited the yeast growth when its concentration was just 12% TS.
4.3.3 Ammonia fiber explosion
Ammonia fiber explosion (AFEX) was found to affect the biomass crystallinity [266] and enhance the enzymatic hydrolysis even at low enzymes loading [267]. In AFEX, ammonia at high pressure and moderate temperature is released into a biomass material [268]. AFEX-pretreated Bermuda grass at 100 °C for 30 min resulted in a sugar yield of 94.8% upon hydrolysis [268]. Abdul et al. investigated the effect of AFEX on the enzymatic hydrolysis of oil palm empty fruit bunch fiber [269]. The glucan conversion of the treated sample (1:1 ammonia/solid, 135 °C, 45 min, 50% moisture content) was 90%. It was also observed that the crystallinity index changed due to lignin removal and relocation. AFEX was also studied in a combination with other pretreatments such as diluted acid [270] and hydrogen peroxide [271, 272].
AFEX was found to increase the microbial growth. Pablo Rojas-Sossa et al. showed that the AFEX treated corn stover increased the microbial growth three times compared to untreated feedstock [273]. Furthermore, AFEX treated corn stover provided 22% more biogas production compared to untreated corn stover. A comparative techno-economic analysis showed that the cost of AFEX pretreatment was more than steam explosion and dilute sulfuric acid [274]. This is because of the high-pressure equipment required as well as the energy and the ammonia costs.
4.3.4 Supercritical CO 2 pretreatment (CO 2 explosion)
Supercritical carbon dioxide (SC-CO2) has been used as a low cost, environmentally benign, and recoverable extractive solvent pretreatment [275]. SC-CO2 pretreatment increased the glucose yield from Avicel by 50% [276, 277]. It was proved that CO2 penetrates the crystal lattice of the biomass, and then due to the release of high pressure CO2, explosion happened and the structure of the biomass is disrupted [278]. This increases the surface area of the exposed cellulose for enzymatic hydrolysis. The pretreatment can be performed at low temperature (50–80 °C) but at high moisture content (75%) [279]. However, the low-temperature pretreatment takes longer time (24–48 h) than the high-temperature pretreatment (0.5–2 h at 160–170 °C). The pretreatment pressure was found to be a very important parameter. The effect of pretreatment pressure on rice straw was studied in the range 10–30 MPa [280]. The maximum glucose yield (32.4 ± 0.5%) was obtained at 30 MPa, 110 °C, liquid-to-solid ratio of 1, and 30 min. Scanning electron microscopy (SEM) observations revealed that the pretreatment changed the porosity and the structure of the material with fibers being fluffy and soft which increased cellulose accessibility.
The effect of water on SC-CO2 pretreatment was investigated by Kim and Hong for the pretreatment of hardwood and softwood [281]. They concluded that the sugar yield increases as the moisture content of the treated biomass increases. The maximum sugar yields were 84.7 ± 2.6% for hardwood and 27.3 ± 3.8% for softwood at 73% moisture content, 21.374 MPa, 165 °C, and 30 min. This is because the presence of water swells the biomass assisting the CO2 penetration. The pretreatment of dry wheat straw with SC-CO2 at 190 °C and 30 min provided sugar yield of 14.91%, while the wet wheat straw treated with SC-CO2 at 185 °C and 30 min provided sugar yield of 20.84% (g sugar/g solid) [282]. Combined pretreatment of dry wheat straw using steam explosion (at 200 °C for 15 min) and SC-CO2 (at 12 MPa and 190 °C, for 60 min) resulted in the highest sugar yield (23.46%). SC-CO2 was also combined with ionic liquid (1-butyl-3-methylimidazolium acetate) at 90 °C, 9 MPa, no moisture, and 30 min for the pretreatment of sugarcane bagasse [283]. The total reducing sugar from the combined pretreatment was higher than separate pretreatments (SC-CO2 and ionic liquid). This improvement was attributed to the additional disruption of cellulose structure caused by the ionic liquid. In the presence of ionic liquid, SC-CO2 can be performed at low temperature and results in a high sugar yield. The combined pretreatment of ethanol-ionic liquid (organosolv) and SC-CO2 (at 180 °C, 25 MPa, and 2 h) resulted in a better performance than SC-CO2/ionic liquid [284]. The ratio of the sugarcane bagasse to ionic liquid was lower (1:1) in comparison to another study reported in the literature [283]. The economic and environmental analyses are required for the overall evaluation of the pretreatment.
4.4 Biological pretreatment
The biological pretreatment is based on using microorganisms, mainly fungi, to delignify the lignocellulose biomass materials [285]. In addition to lignin removal, white fungi were found efficient for the removal of other chemicals such as ester-linked p-coumaric, ester-linked ferulic acids, ester-linked phenolic acids, and aromatics [286]. The most used fungi types are brown-rot, white-rot, and soft-rot fungi [287, 288]. Among these three, white-rot fungi are the most used for microbial pretreatment. The effect of 19 different white-rot fungi on the wheat straw hydrolysis was investigated [289]. It was found that only 4 of them provided a significant increase in the sugar yield as shown in Table 4. Lee et al. used three white fungi for the pretreatment of softwood biomass (Ceriporia lacerata, Stereum hirsutum, and Polyporus brumalis) [290]. Stereum hirsutum pretreatment resulted in the maximum sugar yield increase (6.41%) and the highest delignification (14.52%) after 8 weeks. Cellulose degradation was observed for all fungi. Pretreatment of rice straw with Dichomitus squalens resulted in 58.1% enzymatic digestibility within 15 days [291]. XRD and SEM analyses of the fermented biomass showed that the pretreatment changed the crystallinity and structure of the biomass.
Microbial pretreatment in the presence of water is known as solid state cultivation. The effect of moisture was investigated using Phanerochaete chrysosporium with cotton stalks [292]. It was found that increasing the moisture content from 65 to 75% increased the lignin removal by 6%. Salts addition as nutrients was also investigated and the improvement was insignificant. Shi et al. compared the solid state cultivation (SSC with 75% moisture content) and submerged state cultivation (SmC) for the pretreatment of cotton stalks using P. chrysosporium [293]. They found that both methods facilitated lignin removal (35.53% for SSC and 19.38% for SmC). The cellulose conversion of the treated biomass (10.98% for SmC and 3.04% for SSC) was lower than untreated biomass (17.93%). Washing and heating of the treated biomass were found to increase cellulose conversion (14.94% for SmC and 17.81% for SSC).
A summary of biological pretreatments of different LCB materials using different fungi is given in Table 4. The biological pretreatment is environmentally friendly with less pretreatment severity than other methods. The main disadvantage of the biological pretreatment is that it requires long time (in weeks). The pretreatment time of wheat straw by Phanerochaete sordida 37 and Pycnoporus cinnabarinus 115 can be reduced by 1 week in an oxygen environment [289]. In a comparative study, Baral et al. found that the sugar production cost with biological pretreatment was the highest compared to steam explosion, dilute sulfuric acid, and AFEX [274].
5 Pretreatment-generated inhibitors and their effect
By-products of lignocellulose pretreatment affect the hydrolysis and the fermentation processes. The effect of dilute acid pretreatment’s by-products (such as lignin, acetic acid, HMF, vanillin, and furfural) on the enzymatic hydrolysis of avicel was investigated for three commercial enzymes, namely AD from Advanced Enzymes, India; CL from Novozymes, USA; and AC from Genencor, Denmark [17]. The presence of lignin decreased the hydrolysis 10% for AD, 36% for AL, and 17% for AC. Acetic acid inhibitor action differs from enzyme to another, and it increased gradually with the increase of acetic acid concentration except with AD enzymes which experienced a sharp decrease. The effect of acetic acid, furfural, HMF, and vanillin at different concentrations after 24 h of hydrolysis is shown in Table 5. The effect of inhibitors after 48 h of hydrolysis showed similar trend. It can be concluded that the inhibition effect depends on the type of enzyme, inhibitor concentration, and hydrolysis time. Studying of the inhibition mechanism will provide more understanding of its action and will help to develop methods to decrease its effect.
Alkali pretreatment and dilute acid pretreatment were applied to different types of the biomass, namely Douglas fir, poplar, switchgrass, sorghum stalk, bmr sorghum stalk, and corn stover [297]. Alkali pretreatment was carried out at 1% (w/v) NaOH, 121 °C, and 10% solid loading for 30 min. Acid pretreatment was performed at 2% (v/v) H2SO4, 121 °C, 10% solid loading for 30 min for poplar and Douglas fir biomass and at 1% (v/v) H2SO4, 140 °C, 10% solid loading for 30 min for the other biomass. The results showed that sugar loss of alkali pretreatment is larger than acid pretreatment for all types of biomass in the study. This was attributed to the hydrolysis of hemicellulose fraction associated with alkali pretreatment. Moreover, the phenolics produced during alkali pretreatment were larger than those from acid pretreatment for all biomass types, except corn stover. Phenolic compounds are products of lignin degradation. Formation of acetic acid and formic acid seemed to be dependent on the type of biomass as no specific trend observed related to the pretreatment type. The highest acetic acid (12.2%, g/g biomass) and formic acid (3.1%, g/g biomass) were produced from alkali pretreatment of poplar. The lowest acetic acid (1.5%, g/g biomass) was produced from acid pretreatment of Douglas fir while the lowest formic acid (0.3%, g/g biomass) was produced from acid pretreatment of Douglas fir, acid pretreatment of sorghum stalk, and alkali pretreatment of corn stover. HMF and furfural formation were very low (< 0.01%, g/g biomass) for alkali pretreatment in all biomass samples and acid pretreatment in Douglas fir and poplar. These results indicate that biomass type affects the formation of inhibitors. Ferulic acid, fermentation inhibitor, was detected as a by-product of NaOH pretreatment of rice straw [298]. Detoxification was used to study the inhibition effect of the ferulic acid on the fermentation of the hydrolysate. It was found that with detoxification using AEPA250, the ferulic acid content decreased by 94.4% which increased the bioethanol yield by 153.31%. AEPA250 was produced from the residue of rice straw hydrolysis. Examples of inhibitors produced by different pretreatments are shown in Table 6.
6 Outlook and future trends
As described in this review, the inhibitors and degradation product levels are strongly dependent on the pretreatment severity. Also, the pretreatment severity affects the capital cost of the treatment as high pressure, temperature, and corrosion resistant equipment are required. To avoid these problems, methods of high treatment severity should be either improved or avoided completely. Examples for these are ammonia fiber explosion, dilute acid, liquid hot water, and steam explosion. Biological pretreatment seems attractive as they remove lignin efficiently and have low energy requirement. However, it is slow which makes it expensive. Supercritical CO2 pretreatment can benefit from the recent deployment of carbon capture facilities. The biofuel unit needs to be built in the vicinity of a capture plant which will reduce the pretreatment cost associated with CO2 production, transportation, and storage. Combined pretreatment methods are the future trends of LCB pretreatment as they seem to help in cancelling the negative impacts of individual methods. For instance, using microwave with dilute acid pretreatment instead of conventional heating, lime with NaOH, and organosolv with liquid hot water gave a promising result.
As reviewed in this paper, the performance of the most pretreatment methods is studied and analyzed. However, there is a lack in the literature regarding the energy efficiency and the overall economic analysis of these methods. Studying these will provide quantitative measures for the comparison and possibility of commercialization of the pretreatment.
There are other external factors related to the downstream processes (hydrolysis and fermentation). These factors include inhibitor tolerance, enzymes, bacteria or yeast efficiencies, use of detoxification, and the hydrolysis and fermentation approaches (combined or separately). Studying the effect of these factors will help in defining the minimum requirements of a pretreatment for maximum biofuel yield.
In summary, the future research focus should be on:
-
The combination of different pretreatment methods: As an example, combination of physicochemical and chemical pretreatments was investigated. A mixture of Na2S and Na2CO3 (green liquor) was applied after steam explosion for bamboo [304]. The optimum combined pretreatment (31.01% total titratable alkali, 28.01 min, 166.41 °C) resulted in ethanol yield of 20.3% dry matter. FTIR analysis showed the absence of most of chemical bonds of lignin in the residue of the combined pretreatment. The cellulose content was shown to be preserved in the residue. The addition of green liquor pretreatment further decreased cellulose degree of polymerization by more than 27.9%. Another example of the synergistic effect of the combined pretreatments is ionic liquid (1-ethyl-3-methylimidazolium acetate, 25% w/w) pretreatment followed by a biological pretreatment (Cupriavidus basilensis B-8) [305]. The reducing sugar yield increased by 41.7% in comparison to the sole ionic liquid pretreatment. The significant increase of sugar yield was attributed to the structural changes and decreased crystallinity of cellulose by ionic liquid and the removal of lignin by bacteria. In another study, ionic liquid (1-butyl-3-methylimidazolium chloride) pretreatment assisted with p-toluenesulfonic acid of herb residue resulted in a saccharification yield of 98.9% and delignification yield of 79.9% [306]. This Saccharification yield of the co-pretreatment was achieved at 1.0% acid and 79% IL at 130 °C for 2 h. The untreated, acid pretreated, and ionic liquid pretreated saccharification were significantly lower than co-pretreatment (21.1% for untreated, 31.8% for ionic liquid pretreatment, and 39.3% for acid pretreatment). Moreover, the lignin produced from the co-pretreatment was of high quality and can be utilized for different application. The improved performance was found due to the synergistic effect of the co-pretreatment in decreasing cellulose crystallinity and destructing the surface structure of the cell wall.
-
Complete characterization of the LCB before and after pretreatment instead of focusing only on one or two components of the products. This includes the exact components and their compositions, crystallinity, and other structural changes.
-
Finding the optimum operating conditions of each method as well as its mechanism will help in developing ways for more enhancements.
-
The potential of easy to separate by-products such as lignin: biomass-derived DES was found to provide high-quality lignin in addition to the improvement of enzymatic hydrolysis [240]. Choline chloride-lactic acid (ChCl-LA) was applied to poplar sawdust at ChCl/LA molar ratio of 1:2 and 130 °C for 1.5 h. The lignin recovery was 48.6% and glucose yield 75.8%. The recovered lignin can be used for the production of phenolic compounds.
-
In another example of by-product utilization, the residue of enzymatic hydrolysis of rice straw was used to produce detoxification adsorbent for the ferulic acid produced from alkali pretreatment [298].
-
Development of lignin utilization technologies: The pretreatment cost can be reduced by utilizing the by-products and residues of the biorefinery process and converting them into valuable products; for instance, porous carbon can be produced from the enzymatic hydrolysis residue of sugarcane bagasse pretreated with sulfuric acid [307]. The glucose yield and activated carbon yield obtained were 23.4% and 7.1%, respectively. The porous carbon can be used for the manufacturing of super-capacitors.
-
Fast pretreatment methods without chemicals such as pulsed electric field are promising. Mechanistic study and techno-economic analysis of such methods will provide overall evaluation of the method.
-
The ability to treat large volumes in a continuous operation mode
-
The integration between the pretreatment, hydrolysis, fermentation, and lignin utilization processes
-
Studying of the lignocellulose biomass at molecular level for further understanding of the pretreatment action
7 Conclusions
Lignocellulose biomass materials are promising feedstock for biofuel production. The conversion of these materials to biofuels is through the enzymatic hydrolysis to produce fermentable sugars and then the subsequent fermentation of those sugars to biofuel. Hydrolysis feasibility is affected by many factors such as enzymatic digestibility, sugar degradation, lignin removal, inhibitors, and energy and chemical requirements. Therefore, it is necessary to use a pretreatment step to increase the sugar yield with no or minimum sugar loss and inhibitors formation. The pretreatment’s objective is to expose the cellulose, thus increasing its enzymatic saccharification. This can be achieved by removing the lignin and decreasing the cellulose crystallinity. The pretreatment methods are classified into physical, chemical, physicochemical, and biological. Physical methods include mechanical comminution, pyrolysis, irradiation, and pulsed electric energy (PEE). These methods have many advantages such as no chemicals used to be disposed of, recovered, or recycled. Also, no inhibitors and degradation products were directly related to the pretreatments. The disadvantages of the physical methods are their energy requirement and the low performance which make it less efficient and expensive. It can be used as assistant method in a combination with chemical or physicochemical methods. Among all of the physical methods reviewed, PEE pretreatment method seems to be promising as it affects the LCB permeability at a very short time (seconds). However, more investigation is needed to study the effect of PEF on the cellulose structure and lignin, fermentable sugar yield, optimum operating conditions, and the economic feasibility. The chemical pretreatments have drawbacks of inhibitors and degradation product formation, waste generation, chemical recovery and recycle, and equipment corrosion. In addition, washing is required to prevent the effect of the used chemical on the downstream processes. All of these disadvantages affect the techno-economic feasibility of the chemical’s methods.
Physicochemical pretreatments are high-temperature and/or high-pressure methods which require special equipment. This increases their capital and operating costs. Moreover, the high-temperature operation increases the formation of inhibitors and degradation products. Biological pretreatments have high lignin removal and low energy requirement. However, they degrade part of the cellulose too. Moreover, they are very slow with pretreatment time up to weeks.
In order to have technically sound and economically feasible pretreatment, combined pretreatments can be used to maximize the sugar yield while minimizing inhibitor production and the cost. Optimization is a key to decide on the efficiency of a pretreatment.
References
Wang H, Yang B, Zhang Q, Zhu W (2020) Catalytic routes for the conversion of lignocellulosic biomass to aviation fuel range hydrocarbons. Renew Sustain Energy Rev 12. https://doi.org/10.1016/j.rser.2019.109612
Sher F, Iqbal SZ, Liu H, et al (2020) Thermal and kinetic analysis of diverse biomass fuels under different reaction environment: a way forward to renewable energy sources. Energy Convers Manag 203: https://doi.org/10.1016/j.enconman.2019.112266
Wang M, Wu M, Huo H (2007) Life-cycle energy and greenhouse gas emission impacts of different corn ethanol plant types. Environ Res Lett 2:024001. https://doi.org/10.1088/1748-9326/2/2/024001
Bradford H (2019) Canada Biofuels Annual. USDA. https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Biofuels Annual_Ottawa_Canada_8-9-2019.pdf. Accessed 07 Feb 2022
Kumar G, Bakonyi P, Periyasamy S et al (2015) Lignocellulose biohydrogen: practical challenges and recent progress. Renew Sustain Energy Rev 44:728–737. https://doi.org/10.1016/J.RSER.2015.01.042
Li X, Mupondwa E, Panigrahi S et al (2012) A review of agricultural crop residue supply in Canada for cellulosic ethanol production. Renew Sustain Energy Rev 16:2954–2965. https://doi.org/10.1016/J.RSER.2012.02.013
Li J, Wang C, Yang Z (2010) Production and separation of phenols from biomass-derived bio-petroleum. J Anal Appl Pyrolysis 89:218–224. https://doi.org/10.1016/J.JAAP.2010.08.004
Luo Y, Li Z, Li X et al (2019) The production of furfural directly from hemicellulose in lignocellulosic biomass: a review. Catal Today 319:14–24. https://doi.org/10.1016/J.CATTOD.2018.06.042
Mabee WE, Saddler JN (2010) Bioethanol from lignocellulosics: status and perspectives in Canada. Bioresour Technol 101:4806–4813. https://doi.org/10.1016/J.BIORTECH.2009.10.098
Mupondwa E, Li X, Tabil L et al (2017) Status of Canada’s lignocellulosic ethanol: part I: pretreatment technologies. Renew Sustain Energy Rev 72:178–190. https://doi.org/10.1016/j.rser.2017.01.039
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. https://doi.org/10.1021/ie801542g
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861. https://doi.org/10.1016/J.BIORTECH.2009.11.093
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18. https://doi.org/10.1016/J.BIORTECH.2008.05.027
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuel Bioprod Biorefin 2:26–40. https://doi.org/10.1002/bbb.49
Aden A, Ruth M, Ibsen K et al (2002) Lignocellulosic biomass to ethanol process design and economics utilizing co-current dilute acid prehydrolysis and enzymatic hydrolysis for corn stover. NREL. https://www.nrel.gov/docs/fy02osti/32438.pdf. Accessed 29 June 2022
Chiaramonti D, Prussi M, Ferrero S et al (2012) Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenerg 46:25–35. https://doi.org/10.1016/J.BIOMBIOE.2012.04.020
Agrawal R, Satlewal A, Gaur R et al (2015) Pilot scale pretreatment of wheat straw and comparative evaluation of commercial enzyme preparations for biomass saccharification and fermentation. Biochem Eng J 102:54–61. https://doi.org/10.1016/J.BEJ.2015.02.018
Dai L, Wang Y, Liu Y et al (2019) Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: a state-of-the-art review. Renew Sustain Energy Rev 107:20–36. https://doi.org/10.1016/J.RSER.2019.02.015
Vorobiev E, Lebovka N (2017) Application of pulsed electric energy for lignocellulosic biorefinery. In: Miklavčič D (ed) Handbook of electroporation. Springer, Cham, pp 2843–2861
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefin 1:119–134. https://doi.org/10.1002/bbb.4
Maity SK (2015) Opportunities, recent trends and challenges of integrated biorefinery: part I. Renew Sustain Energy Rev 43:1427–1445. https://doi.org/10.1016/J.RSER.2014.11.092
Beguin P, Aubert J-P (1994) The biological degradation of cellulose. FEMS Microbiol Rev 13:25–58
Kuhad RC, Singh A, Eriksson K-EL (1997) Microorganisms and enzymes involved in the degradation of plant fiber cell walls. In: Advances in biochemical engineering/biotechnology. Springer, Berlin Heidelberg, pp 45–125
Agbor VB, Cicek N, Sparling R et al (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29:675–685. https://doi.org/10.1016/J.BIOTECHADV.2011.05.005
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. https://doi.org/10.1021/ie801542g
Pérez J, Muñoz-Dorado J, De La Rubia T, Martínez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63. https://doi.org/10.1007/s10123-002-0062-3
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. https://doi.org/10.1016/S0960-8524(01)00212-7
Lama-Muñoz A, del Mar Contreras M, Espínola F, et al (2020) Characterization of the lignocellulosic and sugars composition of different olive leaves cultivars. Food Chem 329. https://doi.org/10.1016/j.foodchem.2020.127153
Morone A, Pandey RA, Chakrabarti T (2018) Comparative evaluation of OrganoCat and selected advanced oxidation processes as pretreatment to enhance cellulose accessibility of rice straw. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2017.03.039
Reshamwala S, Shawky BT, Dale BE (1995) Ethanol production from enzymatic hydrolysates of AFEX-treated coastal bermudagrass and switchgrass. Appl Biochem Biotechnol 51–52: https://doi.org/10.1007/BF02933410
Asakawa A, Kohara M, Sasaki C et al (2015) Comparison of choline acetate ionic liquid pretreatment with various pretreatments for enhancing the enzymatic saccharification of sugarcane bagasse. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2015.03.073
Peng H, Luo H, Jin S et al (2014) Improved bioethanol production from corn stover by alkali pretreatment with a novel pilot-scale continuous microwave irradiation reactor. Biotechnol Bioprocess Eng. https://doi.org/10.1007/s12257-014-0014-8
Wyman CE, Dale BE, Elander RT et al (2005) Coordinated development of leading biomass pretreatment technologies. Bioresour Technol 96:1959–1966. https://doi.org/10.1016/j.biortech.2005.01.010
Mosier N, Wyman C, Dale B et al (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686. https://doi.org/10.1016/J.BIORTECH.2004.06.025
Chang VS, Burr B, Holtzapple MT (1997) Lime pretreatment of switchgrass. Appl Biochem Biotechnol - Part A Enzym Eng Biotechnol 63–65:3–19. https://doi.org/10.1007/BF02920408
Sharma SK, Mishra IM, Sharma MP, Saini JS (1988) Effect of particle size on biogas generation from biomass residues. Biomass 17:251–263. https://doi.org/10.1016/0144-4565(88)90107-2
Izumi K, Okishio Y, Nagao N et al (2010) Effects of particle size on anaerobic digestion of food waste. Int Biodeterior Biodegradation 64:601–608. https://doi.org/10.1016/J.IBIOD.2010.06.013
Dahunsi S (2019) Mechanical pretreatment of lignocelluloses for enhanced biogas production: methane yield prediction from biomass structural components. Bioresour Technol 280:18–26. https://doi.org/10.1016/J.BIORTECH.2019.02.006
Tsapekos P, Kougias PG, Angelidaki I (2018) Mechanical pretreatment for increased biogas production from lignocellulosic biomass; predicting the methane yield from structural plant components. Waste Manag 78:903–910. https://doi.org/10.1016/J.WASMAN.2018.07.017
Rodriguez C, Alaswad A, El-Hassan Z, Olabi AG (2017) Mechanical pretreatment of waste paper for biogas production. Waste Manag 68:157–164. https://doi.org/10.1016/J.WASMAN.2017.06.040
Herrmann C, Heiermann M, Idler C, Prochnow A (2012) Particle size reduction during harvesting of crop feedstock for biogas production I: effects on ensiling process and methane yields. Bioenergy Res 5:926–936. https://doi.org/10.1007/s12155-012-9206-2
Herrmann C, Prochnow A, Heiermann M, Idler C (2012) Particle size reduction during harvesting of crop feedstock for biogas production II: effects on energy balance, greenhouse gas emissions and profitability. Bioenergy Res 5:937–948. https://doi.org/10.1007/s12155-012-9207-1
Tsapekos P, Kougias PG, Egelund H et al (2017) Mechanical pretreatment at harvesting increases the bioenergy output from marginal land grasses. Renew Energy 111:914–921. https://doi.org/10.1016/J.RENENE.2017.04.061
Kratky L, Jirout T (2011) Biomass size reduction machines for enhancing biogas production. Chem Eng Technol 34:391–399. https://doi.org/10.1002/ceat.201000357
de Vrije T, de Haas GG, Tan GB et al (2002) Pretreatment of Miscanthus for hydrogen production by Thermotoga elfii. Int J Hydrogen Energy 27:1381–1390. https://doi.org/10.1016/S0360-3199(02)00124-6
Cadoche L, López GD (1989) Assessment of size reduction as a preliminary step in the production of ethanol from lignocellulosic wastes. Biol Wastes 30:153–157. https://doi.org/10.1016/0269-7483(89)90069-4
Kreuger E, Sipos B, Zacchi G et al (2011) Bioconversion of industrial hemp to ethanol and methane: the benefits of steam pretreatment and co-production. Bioresour Technol 102:3457–3465. https://doi.org/10.1016/J.BIORTECH.2010.10.126
Hassan SS, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 262:310–318. https://doi.org/10.1016/J.BIORTECH.2018.04.099
Wang K, Xiong X, Chen J, et al (2012) Comparison of gamma irradiation and steam explosion pretreatment for ethanol production from agricultural residues. Biomass and Bioenergy. https://doi.org/10.1016/j.biombioe.2012.08.013
Khan F, Ahmad SR, Kronfli E (2006) γ-radiation induced changes in the physical and chemical properties of lignocellulose. Biomacromolecules 7: https://doi.org/10.1021/bm060168y
Wu X, Chen L, He W, et al (2020) Characterize the physicochemical structure and enzymatic efficiency of agricultural residues exposed to γ-irradiation pretreatment. Ind Crops Prod 150: https://doi.org/10.1016/j.indcrop.2020.112228
Glegg RE, Kertesz ZI (1956) Aftereffect in the degradation of cellulose and pectin by gamma rays. Science (80- ) 124: https://doi.org/10.1126/science.124.3227.893
Khan F (2005) Characterization of methyl methacrylate grafting onto preirradiated biodegradable lignocellulose fiber by γ-radiation. Macromol Biosci 5: https://doi.org/10.1002/mabi.200400137
Yang C, Shen Z, Yu G, Wang J (2008) Effect and aftereffect of γ radiation pretreatment on enzymatic hydrolysis of wheat straw. Bioresour Technol 99: https://doi.org/10.1016/j.biortech.2007.12.008
Hyun Hong S, Taek Lee J, Lee S, et al (2014) Improved enzymatic hydrolysis of wheat straw by combined use of gamma ray and dilute acid for bioethanol production. Radiat Phys Chem 94: https://doi.org/10.1016/j.radphyschem.2013.05.056
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4: https://doi.org/10.1186/s40643-017-0137-9
Jackowiak D, Frigon JC, Ribeiro T et al (2011) Enhancing solubilisation and methane production kinetic of switchgrass by microwave pretreatment. Bioresour Technol 102:3535–3540. https://doi.org/10.1016/J.BIORTECH.2010.11.069
Jackowiak D, Bassard D, Pauss A, Ribeiro T (2011) Optimisation of a microwave pretreatment of wheat straw for methane production. Bioresour Technol 102:6750–6756. https://doi.org/10.1016/J.BIORTECH.2011.03.107
Sapci Z (2013) The effect of microwave pretreatment on biogas production from agricultural straws. Bioresour Technol 128:487–494. https://doi.org/10.1016/J.BIORTECH.2012.09.094
Li L, Kong X, Yang F et al (2012) Biogas production potential and kinetics of microwave and conventional thermal pretreatment of grass. Appl Biochem Biotechnol 166:1183–1191. https://doi.org/10.1007/s12010-011-9503-9
Liu CZ, Cheng XY (2009) Microwave-assisted acid pretreatment for enhancing biogas production from herbal-extraction process residue. Energy Fuels. https://doi.org/10.1021/ef900607f
Cheng XY, Liu CZ (2010) Enhanced biogas production from herbal-extraction process residues by microwave-assisted alkaline pretreatment. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.2278
Diaz AB, Moretti MM de S, Bezerra-Bussoli C, et al (2015) Evaluation of microwave-assisted pretreatment of lignocellulosic biomass immersed in alkaline glycerol for fermentable sugars production. Bioresour Technol. https://doi.org/10.1016/j.biortech.2015.02.112
Ravindran R, Jaiswal S, Abu-Ghannam N, Jaiswal AK (2018) A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour Technol 248:272–279. https://doi.org/10.1016/J.BIORTECH.2017.06.039
Liang J, Xu X, Yu Z, et al (2019) Effects of microwave pretreatment on catalytic fast pyrolysis of pine sawdust. Bioresour Technol 122080. https://doi.org/10.1016/J.BIORTECH.2019.122080
Tsegaye B, Balomajumder C, Roy P (2019) Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers Manag 186:82–92. https://doi.org/10.1016/J.ENCONMAN.2019.02.049
Kitchaiya P, Intanakul P, Krairiksh M (2003) Enhancement of enzymatic hydrolysis of lignocellulosic wastes by microwave pretreatment under atmospheric pressure. J Wood Chem Technol. https://doi.org/10.1081/WCT-120021926
Hu Z, Wen Z (2008) Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem Eng J 38:369–378. https://doi.org/10.1016/J.BEJ.2007.08.001
Peng H, Chen H, Qu Y et al (2014) Bioconversion of different sizes of microcrystalline cellulose pretreated by microwave irradiation with/without NaOH. Appl Energy 117:142–148. https://doi.org/10.1016/J.APENERGY.2013.12.002
Binod P, Satyanagalakshmi K, Sindhu R et al (2012) Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew Energy 37:109–116. https://doi.org/10.1016/J.RENENE.2011.06.007
Zhu Z, Liu Y, Gómez LD, et al (2021) Thermochemical pretreatments of maize stem for sugar recovery: comparative evaluation of microwave and conventional heating. Ind Crops Prod 160: https://doi.org/10.1016/j.indcrop.2020.113106
Sun RC, Tomkinson J (2002) Characterization of hemicelluloses obtained by classical and ultrasonically assisted extractions from wheat straw. Carbohydr Polym 50:263–271. https://doi.org/10.1016/S0144-8617(02)00037-1
Zhang YQ, Fu E, Liang J (2008) Effect of ultrasonic waves on the saccharification processes of lignocellulose. Chem Eng Technol. https://doi.org/10.1002/ceat.200700407
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53. https://doi.org/10.1016/J.PECS.2014.01.001
Kunaver M, Jasiukaityte E, Čuk N (2012) Ultrasonically assisted liquefaction of lignocellulosic materials. Bioresour Technol 103: https://doi.org/10.1016/j.biortech.2011.09.051
Bussemaker MJ, Zhang D (2013) Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind Eng Chem Res 52:3563–3580. https://doi.org/10.1021/ie3022785
Ur Rehman MS, Kim I, Chisti Y, Han JI (2013) Use of ultrasound in the production of bioethanol from lignocellulosic biomass. Energy Educ. Sci Technol Part A Energy Sci Res 30:359–378
Yachmenev V, Condon B, Klasson T, Lambert A (2009) Acceleration of the enzymatic hydrolysis of corn stover and sugar cane bagasse celluloses by low intensity uniform ultrasound. J Biobased Mater Bioenergy. https://doi.org/10.1166/jbmb.2009.1002
Montalbo-Lomboy M, Johnson L, Khanal SK et al (2010) Sonication of sugary-2 corn: a potential pretreatment to enhance sugar release. Bioresour Technol 101:351–358. https://doi.org/10.1016/J.BIORTECH.2009.07.075
Aimin T, Hongwei Z, Gang C et al (2005) Influence of ultrasound treatment on accessibility and regioselective oxidation reactivity of cellulose. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2004.07.003
Chen W, Yu H, Liu Y et al (2011) Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2010.10.040
Patil RS, Joshi SM, Gogate PR (2019) Intensification of delignification of sawdust and subsequent enzymatic hydrolysis using ultrasound. Ultrason Sonochem 58:104656. https://doi.org/10.1016/J.ULTSONCH.2019.104656
Bussemaker MJ, Mu X, Zhang D (2013) Ultrasonic pretreatment of wheat straw in oxidative and nonoxidative conditions aided with microwave heating. Ind Eng Chem Res. https://doi.org/10.1021/ie401181f
Li Q, Guo C, Liu C-Z (2014) Dynamic microwave-assisted alkali pretreatment of cornstalk to enhance hydrogen production via co-culture fermentation of Clostridium thermocellum and Clostridium thermosaccharolyticum. Biomass Bioenerg 64:220–229. https://doi.org/10.1016/J.BIOMBIOE.2014.03.053
Li H, Qu Y, Yang Y et al (2016) Microwave irradiation – a green and efficient way to pretreat biomass. Bioresour Technol 199:34–41. https://doi.org/10.1016/J.BIORTECH.2015.08.099
Golberg A, Sack M, Teissie J et al (2016) Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol Biofuels 9:1–22. https://doi.org/10.1186/s13068-016-0508-z
Knorr D, Geulen M, Grahl T, Sitzmann W (1994) Food application of high electric field pulses. Trends Food Sci Technol 5:71–75. https://doi.org/10.1016/0924-2244(94)90240-2
Lindmark J, Lagerkvist A, Nilsson E et al (2014) Evaluating the effects of electroporation pre-treatment on the biogas yield from ley crop silage. Appl Biochem Biotechnol 174:2616–2625. https://doi.org/10.1007/s12010-014-1213-7
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2011) Pulsed electric field pretreatment of switchgrass and wood chip species for biofuel production. Ind Eng Chem Res 50:10996–11001. https://doi.org/10.1021/ie200555u
Almohammed F, Mhemdi H, Vorobiev E (2016) Pulsed electric field treatment of sugar beet tails as a sustainable feedstock for bioethanol production. Appl Energy. https://doi.org/10.1016/j.apenergy.2015.10.050
Garoma T, Shackelford T (2014) Electroporation of Chlorella vulgaris to enhance biomethane production. Bioresour Technol 169:778–783. https://doi.org/10.1016/j.biortech.2014.07.001
Carlsson M, Lagerkvist A, Ecke H (2008) Electroporation for enhanced methane yield from municipal solid waste. ORBIT 2008 Mov Org Waste Recycl Towar Resour Manag Biobased Econ 6:1–8
Choi H, Jeong S-W, Chung Y (2006) Enhanced anaerobic gas production of waste activated sludge pretreated by pulse power technique. Bioresour Technol 97:198–203. https://doi.org/10.1016/J.BIORTECH.2005.02.023
Loginova KV, Shynkaryk MV, Lebovka NI, Vorobiev E (2010) Acceleration of soluble matter extraction from chicory with pulsed electric fields. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2009.08.009
Bouras M, Grimi N, Bals O, Vorobiev E (2016) Impact of pulsed electric fields on polyphenols extraction from Norway spruce bark. Ind Crops Prod 80:50–58. https://doi.org/10.1016/j.indcrop.2015.10.051
Zhao W, Yu Z, Liu J et al (2011) Optimized extraction of polysaccharides from corn silk by pulsed electric field and response surface quadratic design. J Sci Food Agric. https://doi.org/10.1002/jsfa.4440
Luengo E, Martínez JM, Álvarez I, Raso J (2016) Effects of millisecond and microsecond pulsed electric fields on red beet cell disintegration and extraction of betanines. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2016.01.016
Boussetta N, Lanoisellé JL, Bedel-Cloutour C, Vorobiev E (2009) Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: effect of sulphur dioxide and thermal treatments. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2009.04.030
Boussetta N, Vorobiev E, Reess T et al (2012) Scale-up of high voltage electrical discharges for polyphenols extraction from grape pomace: effect of the dynamic shock waves. Innov Food Sci Emerg Technol. https://doi.org/10.1016/j.ifset.2012.05.004
Boussetta N, Turk M, De Taeye C et al (2013) Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2013.06.004
Rajha HN, Boussetta N, Louka N et al (2014) A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res Int. https://doi.org/10.1016/j.foodres.2014.04.024
Chadni M, Grimi N, Ziegler-Devin I et al (2019) High voltage electric discharges treatment for high molecular weight hemicelluloses extraction from spruce. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115019
Brahim M, El Kantar S, Boussetta N et al (2016) Delignification of rapeseed straw using innovative chemo-physical pretreatments. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2016.09.019
Boussetta N, Lesaint O, Vorobiev E (2013) A study of mechanisms involved during the extraction of polyphenols from grape seeds by pulsed electrical discharges. Innov Food Sci Emerg Technol 19:124–132. https://doi.org/10.1016/J.IFSET.2013.03.007
El Kantar S, Boussetta N, Rajha HN et al (2018) High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res Int 107:755–762. https://doi.org/10.1016/j.foodres.2018.01.070
Brahim M, Checa Fernandez BL, Regnier O et al (2017) Impact of ultrasounds and high voltage electrical discharges on physico-chemical properties of rapeseed straw’s lignin and pulps. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.04.003
Wang Y-Z, Zhang L, Xu T, Ding K (2017) Improving lignocellulose enzymatic saccharification in a bioreactor with an applied electric field. Ind Crops Prod 109:404–409. https://doi.org/10.1016/J.INDCROP.2017.08.061
García-Cubero MT, González-Benito G, Indacoechea I et al (2009) Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour Technol 100:1608–1613. https://doi.org/10.1016/J.BIORTECH.2008.09.012
Vidal PF, Molinier J (1988) Ozonolysis of lignin - improvement of in vitro digestibility of poplar sawdust. Biomass. https://doi.org/10.1016/0144-4565(88)90012-1
Quesada J, Rubio M, Gómez D (1999) Ozonation of lignin rich solid fractions from corn stalks. J Wood Chem Technol. https://doi.org/10.1080/02773819909349603
Li C, Wang L, Chen Z, et al (2021) Ozonolysis of wheat bran in subcritical water for enzymatic saccharification and polysaccharide recovery. J Supercrit Fluids 168: https://doi.org/10.1016/j.supflu.2020.105092
Travaini R, Barrado E, Bolado-Rodríguez S (2016) Effect of ozonolysis pretreatment parameters on the sugar release, ozone consumption and ethanol production from sugarcane bagasse. Bioresour Technol 214:150–158. https://doi.org/10.1016/J.BIORTECH.2016.04.102
dos Santos LC, Adarme OFH, Baêta BEL et al (2018) Production of biogas (methane and hydrogen) from anaerobic digestion of hemicellulosic hydrolysate generated in the oxidative pretreatment of coffee husks. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.05.037
Travaini R, Otero MDM, Coca M, et al (2013) Sugarcane bagasse ozonolysis pretreatment: effect on enzymatic digestibility and inhibitory compound formation. Bioresour Technol 133: https://doi.org/10.1016/j.biortech.2013.01.133
Travaini R, Martín-Juárez J, Lorenzo-Hernando A, Bolado-Rodríguez S (2016) Ozonolysis: an advantageous pretreatment for lignocellulosic biomass revisited. Bioresour Technol 199:2–12. https://doi.org/10.1016/j.biortech.2015.08.143
Schultz-Jensen N, Kádár Z, Thomsen AB, et al (2011) Plasma-assisted pretreatment of wheat straw for ethanol production. Appl Biochem Biotechnol 165: https://doi.org/10.1007/s12010-011-9316-x
Jung YH, Kim KH (2015) Acidic pretreatment. In: Pretreatment of biomass. Elsevier, pp 27–50
Zheng Y, Pan Z, Zhang R (2009) Overview of biomass pretreatment for cellulosic ethanol production. Int J Agric Biol Eng. https://doi.org/10.3965/j.issn.1934-6344.2009.03.051-068
Martin C, Alriksson B, Sjöde A et al (2007) Dilute sulfuric acid pretreatment of agricultural and agro-industrial residues for ethanol production. Appl Biochem Biotechnol 137–140:339–352. https://doi.org/10.1007/s12010-007-9063-1
Zheng Y, Pan Z, Zhang R et al (2007) Evaluation of different biomass materials as feedstock for fermentable sugar production. Appl Biochem Biotechnol 137–140:423–435. https://doi.org/10.1007/s12010-007-9069-8
Lawford HG, Rousseau JD, Tolan JS (2001) Comparative ethanol productivities of different zymomonas recombinants fermenting oat hull hydrolysate. Appl Biochem Biotechnol 91–93:133–146. https://doi.org/10.1385/ABAB:91-93:1-9:133
Makarova EI, Budaeva VV, Skiba EA (2014) Enzymatic hydrolysis of cellulose from oat husks at different substrate concentrations. Russ J Bioorganic Chem 40:726–732. https://doi.org/10.1134/S1068162014070103
Sun Y, Cheng JJ (2005) Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresour Technol 96:1599–1606. https://doi.org/10.1016/J.BIORTECH.2004.12.022
Castro E, Díaz MJ, Cara C et al (2011) Dilute acid pretreatment of rapeseed straw for fermentable sugar generation. Bioresour Technol 102:1270–1276. https://doi.org/10.1016/J.BIORTECH.2010.08.057
Cantarella M, Mucciante C, Cantarella L (2014) Inactivating effects of lignin-derived compounds released during lignocellulosic biomass pretreatment on the endo-glucanase catalyzed hydrolysis of carboxymethylcellulose: a study in continuous stirred ultrafiltration-membrane reactor. Bioresour Technol 156:48–56. https://doi.org/10.1016/J.BIORTECH.2013.12.124
Zhang R, Lu X, Sun Y et al (2011) Modeling and optimization of dilute nitric acid hydrolysis on corn stover. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.2529
Skiba EA, Budaeva VV, Baibakova OV et al (2017) Dilute nitric-acid pretreatment of oat hulls for ethanol production. Biochem Eng J 126:118–125. https://doi.org/10.1016/J.BEJ.2016.09.003
Nair RB, Lundin M, Brandberg T et al (2015) Dilute phosphoric acid pretreatment of wheat bran for enzymatic hydrolysis and subsequent ethanol production by edible fungi Neurospora intermedia. Ind Crops Prod 69:314–323. https://doi.org/10.1016/J.INDCROP.2015.02.038
Nair RB, Lundin M, Lennartsson PR, Taherzadeh MJ (2017) Optimizing dilute phosphoric acid pretreatment of wheat straw in the laboratory and in a demonstration plant for ethanol and edible fungal biomass production using Neurospora intermedia. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.5119
Majumdar S, Naha A, Bhattacharyya DK, Bhowal J (2019) Effective delignification and decrystallization of cauliflower wastes by using dilute phosphoric acid for efficient enzymatic digestibility to produce fermentable sugars. Biomass Bioenerg 125:169–179. https://doi.org/10.1016/J.BIOMBIOE.2019.04.017
Yu H, Xiao W, Han L, Huang G (2019) Characterization of mechanical pulverization/phosphoric acid pretreatment of corn stover for enzymatic hydrolysis. Bioresour Technol 282:69–74. https://doi.org/10.1016/J.BIORTECH.2019.02.104
Um B-H, Karim MN, Henk LL (2003) Effect of sulfuric and phosphoric acid pretreatments on enzymatic hydrolysis of corn stover. Appl Biochem Biotechnol 105:115–126. https://doi.org/10.1385/ABAB:105:1-3:115
Nair RB, Kalif M, Ferreira JA et al (2017) Mild-temperature dilute acid pretreatment for integration of first and second generation ethanol processes. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.08.125
Mosier NS, Sarikaya A, Ladisch CM, Ladisch MR (2001) Characterization of dicarboxylic acids for cellulose hydrolysis. Biotechnol Prog. https://doi.org/10.1021/bp010028u
Mosier NS, Ladisch CM, Ladisch MR (2002) Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation. Biotechnol Bioeng. https://doi.org/10.1002/bit.10316
u Y, Mosier NS (2007) Biomimetic catalysis for hemicellulose hydrolysis in corn stover. Biotechnol Prog 23:116–123. https://doi.org/10.1021/bp060223e
Marzialetti T, Valenzuela Olarte MB, Sievers C et al (2008) Dilute acid hydrolysis of loblolly pine: a comprehensive approach. Ind Eng Chem Res. https://doi.org/10.1021/ie800455f
Kootstra AMJ, Beeftink HH, Scott EL, Sanders JPM (2009) Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem Eng J 46:126–131. https://doi.org/10.1016/J.BEJ.2009.04.020
Kootstra AMJ, Mosier NS, Scott EL et al (2009) Differential effects of mineral and organic acids on the kinetics of arabinose degradation under lignocellulose pretreatment conditions. Biochem Eng J 43:92–97. https://doi.org/10.1016/J.BEJ.2008.09.004
Qin L, Liu Z-H, Li B-Z et al (2012) Mass balance and transformation of corn stover by pretreatment with different dilute organic acids. Bioresour Technol 112:319–326. https://doi.org/10.1016/J.BIORTECH.2012.02.134
Li M-F, Fan Y-M, Xu F et al (2010) Cold sodium hydroxide/urea based pretreatment of bamboo for bioethanol production: characterization of the cellulose rich fraction. Ind Crops Prod 32:551–559. https://doi.org/10.1016/J.INDCROP.2010.07.004
Wan C, Zhou Y, Li Y (2011) Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour Technol 102:6254–6259. https://doi.org/10.1016/J.BIORTECH.2011.02.075
Chang VS, Holtzapple MT (2000) Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol 84–86:5–38. https://doi.org/10.1385/ABAB:84-86:1-9
Chen H, Liu J, Chang X et al (2017) A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol 160:196–206. https://doi.org/10.1016/J.FUPROC.2016.12.007
Abdi N, Hamdache F, Belhocine D et al (2000) Enzymatic saccharification of solid residue of olive mill in a batch reactor. Biochem Eng J 6:177–183. https://doi.org/10.1016/S1369-703X(00)00085-1
McIntosh S, Vancov T (2010) Enhanced enzyme saccharification of Sorghum bicolor straw using dilute alkali pretreatment. Bioresour Technol 101:6718–6727. https://doi.org/10.1016/J.BIORTECH.2010.03.116
Wu L, Arakane M, Ike M et al (2011) Low temperature alkali pretreatment for improving enzymatic digestibility of sweet sorghum bagasse for ethanol production. Bioresour Technol 102:4793–4799. https://doi.org/10.1016/J.BIORTECH.2011.01.023
Silverstein RA, Chen Y, Sharma-Shivappa RR et al (2007) A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour Technol 98:3000–3011. https://doi.org/10.1016/J.BIORTECH.2006.10.022
Chang VS, Nagwani M, Kim C-H, Holtzapple MT (2001) Oxidative lime pretreatment of high-lignin biomass: poplar wood and newspaper. Appl Biochem Biotechnol 94:01–28. https://doi.org/10.1385/ABAB:94:1:01
Kaar WE, Holtzapple MT (2000) Using lime pretreatment to facilitate the enzymic hydrolysis of corn stover. Biomass Bioenerg 18:189–199. https://doi.org/10.1016/S0961-9534(99)00091-4
Kim S, Holtzapple MT (2005) Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour Technol 96:1994–2006. https://doi.org/10.1016/J.BIORTECH.2005.01.014
Liang Y, Siddaramu T, Yesuf J, Sarkany N (2010) Fermentable sugar release from Jatropha seed cakes following lime pretreatment and enzymatic hydrolysis. Bioresour Technol 101:6417–6424. https://doi.org/10.1016/J.BIORTECH.2010.03.038
Reilly M, Dinsdale R, Guwy A (2015) Enhanced biomethane potential from wheat straw by low temperature alkaline calcium hydroxide pre-treatment. Bioresour Technol 189:258–265. https://doi.org/10.1016/J.BIORTECH.2015.03.150
Chang VS, Nagwani M, Holtzapple MT (1998) Lime pretreatment of crop residues bagasse and wheat straw. Appl Biochem Biotechnol - Part A Enzym Eng Biotechnol. https://doi.org/10.1007/BF02825962
Yan Z, Li J, Chang S et al (2015) Lignin relocation contributed to the alkaline pretreatment efficiency of sweet sorghum bagasse. Fuel 158:152–158. https://doi.org/10.1016/J.FUEL.2015.05.029
Xu J, Cheng JJ (2011) Pretreatment of switchgrass for sugar production with the combination of sodium hydroxide and lime. Bioresour Technol 102:3861–3868. https://doi.org/10.1016/J.BIORTECH.2010.12.038
Iyer PV, Wu ZW, Kim SB, Lee YY (1996) Ammonia recycled percolation process for pretreatment of herbaceous biomass. Appl Biochem Biotechnol - Part A Enzym Eng Biotechnol. https://doi.org/10.1007/BF02941693
Foster BL, Dale BE, Doran-Peterson JB (2001) Enzymatic hydrolysis of ammonia-treated sugar beet pulp. Appl Biochem Biotechnol 91–93:269–282. https://doi.org/10.1385/ABAB:91-93:1-9:269
Kim TH, Kim JS, Sunwoo C, Lee Y (2003) Pretreatment of corn stover by aqueous ammonia. Bioresour Technol 90:39–47. https://doi.org/10.1016/S0960-8524(03)00097-X
Prior BA, Day DF (2008) Hydrolysis of ammonia-pretreated sugar cane bagasse with cellulase, β-glucosidase, and hemicellulase preparations. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-007-8084-0
Chen BY, Zhao BC, Li MF, et al (2017) Fractionation of rapeseed straw by hydrothermal/dilute acid pretreatment combined with alkali post-treatment for improving its enzymatic hydrolysis. Bioresour Technol 225: https://doi.org/10.1016/j.biortech.2016.11.062
Kaur A, Kuhad RC (2019) Valorization of rice straw for ethanol production and lignin recovery using combined acid-alkali pre-treatment. Bioenergy Res 12: https://doi.org/10.1007/s12155-019-09988-3
Pang B, Sun Z, Wang L, et al (2021) Improved value and carbon footprint by complete utilization of corncob lignocellulose. Chem Eng J 419: https://doi.org/10.1016/j.cej.2021.129565
Thring RW, Chornet E, Overend RP (1990) Recovery of a solvolytic lignin: effects of spent liquor/acid volume ratio, acid concentration and temperature. Biomass 23:289–305. https://doi.org/10.1016/0144-4565(90)90038-L
Botello JI, Gilarranz MA, Rodríguez F, Oliet M (1999) Preliminary study on products distribution in alcohol pulping of Eucalyptus globulus. J Chem Technol Biotechnol. https://doi.org/10.1002/(SICI)1097-4660(199902)74:2%3c141::AID-JCTB1%3e3.0.CO;2-0
Huijgen WJJ, Telysheva G, Arshanitsa A et al (2014) Characteristics of wheat straw lignins from ethanol-based organosolv treatment. Ind Crops Prod 59:85–95. https://doi.org/10.1016/J.INDCROP.2014.05.003
Roque RMN, Baig MN, Leeke GA et al (2012) Study on sub-critical water mediated hydrolysis of Miscanthus a lignocellulosic biomass. Resour Conserv Recycl 59:43–46. https://doi.org/10.1016/J.RESCONREC.2011.06.007
Muniz Kubota A, Kalnins R, Overton TW (2018) A biorefinery approach for fractionation of Miscanthus lignocellulose using subcritical water extraction and a modified organosolv process. Biomass Bioenerg 111:52–59. https://doi.org/10.1016/J.BIOMBIOE.2018.01.019
Pan X, Arato C, Gilkes N et al (2005) Biorefining of softwoods using ethanol organosolv pulping: preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol Bioeng. https://doi.org/10.1002/bit.20453
Amiri H, Karimi K, Zilouei H (2014) Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour Technol 152:450–456. https://doi.org/10.1016/J.BIORTECH.2013.11.038
Chen H, Zhao J, Hu T et al (2015) A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: substrate digestibility, fermentability and structural features. Appl Energy 150:224–232. https://doi.org/10.1016/J.APENERGY.2015.04.030
Tan X, Zhang Q, Wang W et al (2019) Comparison study of organosolv pretreatment on hybrid pennisetum for enzymatic saccharification and lignin isolation. Fuel. https://doi.org/10.1016/j.fuel.2019.03.117
Chen J, Tan X, Miao C, et al (2021) A one-step deconstruction-separation organosolv fractionation of lignocellulosic biomass using acetone/phenoxyethanol/water ternary solvent system. Bioresour Technol 342: https://doi.org/10.1016/j.biortech.2021.125963
Chotirotsukon C, Raita M, Champreda V, Laosiripojana N (2019) Fractionation of sugarcane trash by oxalic-acid catalyzed glycerol-based organosolv followed by mild solvent delignification. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2019.111753
Romaní A, Larramendi A, Yáñez R et al (2019) Valorization of Eucalyptus nitens bark by organosolv pretreatment for the production of advanced biofuels. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2019.02.040
Weinwurm F, Turk T, Denner J et al (2017) Combined liquid hot water and ethanol organosolv treatment of wheat straw for extraction and reaction modeling. J Clean Prod 165:1473–1484. https://doi.org/10.1016/J.JCLEPRO.2017.06.215
Matsakas L, Raghavendran V, Yakimenko O et al (2019) Lignin-first biomass fractionation using a hybrid organosolv – steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.11.055
Yuan W, Gong Z, Wang G et al (2018) Alkaline organosolv pretreatment of corn stover for enhancing the enzymatic digestibility. Bioresour Technol 265:464–470. https://doi.org/10.1016/J.BIORTECH.2018.06.038
Li M-F, Yu P, Li S-X et al (2017) Sequential two-step fractionation of lignocellulose with formic acid organosolv followed by alkaline hydrogen peroxide under mild conditions to prepare easily saccharified cellulose and value-added lignin. Energy Convers Manag 148:1426–1437. https://doi.org/10.1016/J.ENCONMAN.2017.07.008
Alio MA, Tugui OC, Vial C, Pons A (2019) Microwave-assisted organosolv pretreatment of a sawmill mixed feedstock for bioethanol production in a wood biorefinery. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.12.078
Tang C, Shan J, Chen Y et al (2017) Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin. Bioresour Technol 232:222–228. https://doi.org/10.1016/J.BIORTECH.2017.02.041
Zhong L, Zhang X, Tang C et al (2018) Hydrazine hydrate and organosolv synergetic pretreatment of corn stover to enhance enzymatic saccharification and co-production of high-quality antioxidant lignin. Bioresour Technol 268:677–683. https://doi.org/10.1016/J.BIORTECH.2018.08.063
Meng X, Parikh A, Seemala B et al (2019) Characterization of fractional cuts of co-solvent enhanced lignocellulosic fractionation lignin isolated by sequential precipitation. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.09.130
Smith MD, Mostofian B, Cheng X et al (2016) Cosolvent pretreatment in cellulosic biofuel production: effect of tetrahydrofuran-water on lignin structure and dynamics. Green Chem. https://doi.org/10.1039/c5gc01952d
Meng X, Bhagia S, Wang Y, et al (2020) Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind Crops Prod 146: https://doi.org/10.1016/j.indcrop.2020.112144
Thomas VA, Donohoe BS, Li M, et al (2017) Adding tetrahydrofuran to dilute acid pretreatment provides new insights into substrate changes that greatly enhance biomass deconstruction by Clostridium thermocellum and fungal enzymes. Biotechnol Biofuels 10: https://doi.org/10.1186/s13068-017-0937-3
Jiang Z, He T, Li J, Hu C (2014) Selective conversion of lignin in corncob residue to monophenols with high yield and selectivity. Green Chem 16: https://doi.org/10.1039/c4gc00620h
Yao F, Shen F, Wan X, Hu C (2020) High yield and high concentration glucose production from corncob residues after tetrahydrofuran + H2O co-solvent pretreatment and followed by enzymatic hydrolysis. Renew Sustain Energy Rev 132:.https://doi.org/10.1016/j.rser.2020.110107
Nguyen TY, Cai CM, Kumar R, Wyman CE (2017) Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1704652114
Nguyen TY, Cai CM, Osman O et al (2016) CELF pretreatment of corn stover boosts ethanol titers and yields from high solids SSF with low enzyme loadings. Green Chem. https://doi.org/10.1039/c5gc01977j
Smith MD, Cai CM, Cheng X et al (2018) Temperature-dependent phase behaviour of tetrahydrofuran-water alters solubilization of xylan to improve co-production of furfurals from lignocellulosic biomass. Green Chem. https://doi.org/10.1039/c7gc03608f
Meng X, Parikh A, Seemala B et al (2018) Chemical transformations of poplar lignin during cosolvent enhanced lignocellulosic fractionation process. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.8b01028
Cai CM, Zhang T, Kumar R, Wyman CE (2013) THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. https://doi.org/10.1039/c3gc41214h
Cai CM, Nagane N, Kumar R, Wyman CE (2014) Coupling metal halides with a co-solvent to produce furfural and 5-HMF at high yields directly from lignocellulosic biomass as an integrated biofuels strategy. Green Chem. https://doi.org/10.1039/c4gc00747f
Zhang H, Wu J, Zhang J, He J (2005) 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules. https://doi.org/10.1021/ma0505676
Dadi AP, Schall CA, Varanasi S (2007) Mitigation of cellulose recalcitrance to enzymatic hydrolysis by ionic liquid pretreatment. Appl Biochem Biotechnol 137–140:407–421. https://doi.org/10.1007/s12010-007-9068-9
Qiu Z, Aita GM, Walker MS (2012) Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.04.070
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc. https://doi.org/10.1021/ja025790m
Li C, Wang Q, Zhao ZK (2008) Acid in ionic liquid: an efficient system for hydrolysis of lignocellulose. Green Chem. https://doi.org/10.1039/b711512a
Amoah J, Ogura K, Schmetz Q et al (2019) Co-fermentation of xylose and glucose from ionic liquid pretreated sugar cane bagasse for bioethanol production using engineered xylose assimilating yeast. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2019.105283
Fu D, Mazza G (2011) Optimization of processing conditions for the pretreatment of wheat straw using aqueous ionic liquid. Bioresour Technol. https://doi.org/10.1016/j.biortech.2011.06.023
Hu X, Cheng L, Gu Z et al (2018) Effects of ionic liquid/water mixture pretreatment on the composition, the structure and the enzymatic hydrolysis of corn stalk. Ind Crops Prod 122:142–147. https://doi.org/10.1016/J.INDCROP.2018.05.056
Nguyen T-AD, Kim K-R, Han SJ et al (2010) Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Bioresour Technol 101:7432–7438. https://doi.org/10.1016/J.BIORTECH.2010.04.053
Hu X, Xiao Y, Niu K et al (2013) Functional ionic liquids for hydrolysis of lignocellulose. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2013.04.061
Nasirpour N, Mousavi SM, Shojaosadati SA (2014) A novel surfactant-assisted ionic liquid pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Bioresour Technol. https://doi.org/10.1016/j.biortech.2014.06.023
Nasirpour N, Mousavi SM (2018) RSM based optimization of PEG assisted ionic liquid pretreatment of sugarcane bagasse for enhanced bioethanol production: effect of process parameters. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2018.06.008
Xu JK, Sun YC, Sun RC (2015) Synergistic effects of ionic liquid plus alkaline pretreatments on eucalyptus: lignin structure and cellulose hydrolysis. Process Biochem. https://doi.org/10.1016/j.procbio.2015.03.014
Pang Z, Lyu W, Dong C et al (2016) High selective delignification using oxidative ionic liquid pretreatment at mild conditions for efficient enzymatic hydrolysis of lignocellulose. Bioresour Technol. https://doi.org/10.1016/j.biortech.2016.04.095
Trinh LTP, Lee YJ, Park CS, Bae HJ (2019) Aqueous acidified ionic liquid pretreatment for bioethanol production and concentration of produced ethanol by pervaporation. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2018.09.008
Hou X, Wang Z, Sun J et al (2019) A microwave-assisted aqueous ionic liquid pretreatment to enhance enzymatic hydrolysis of Eucalyptus and its mechanism. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.10.003
Rigual V, Domínguez JC, Santos TM et al (2019) Autohydrolysis and microwave ionic liquid pretreatment of Pinus radiata: imaging visualization and analysis to understand enzymatic digestibility. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2019.03.068
Sharma V, Nargotra P, Bajaj BK (2019) Ultrasound and surfactant assisted ionic liquid pretreatment of sugarcane bagasse for enhancing saccharification using enzymes from an ionic liquid tolerant Aspergillus assiutensis VS34. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.121319
Datta S, Holmes B, Park JI et al (2010) Ionic liquid tolerant hyperthermophilic cellulases for biomass pretreatment and hydrolysis. Green Chem. https://doi.org/10.1039/b916564a
Ouellet M, Datta S, Dibble DC et al (2011) Impact of ionic liquid pretreated plant biomass on Saccharomyces cerevisiae growth and biofuel production. Green Chem. https://doi.org/10.1039/c1gc15327g
Sitepu IR, Enriquez LL, Nguyen V et al (2019) Ethanol production in switchgrass hydrolysate by ionic liquid-tolerant yeasts. Bioresour Technol Reports. https://doi.org/10.1016/j.biteb.2019.100275
Ninomiya K, Soda H, Ogino C et al (2013) Effect of ionic liquid weight ratio on pretreatment of bamboo powder prior to enzymatic saccharification. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.10.097
An YX, Zong MH, Wu H, Li N (2015) Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: biomass fractionation, enzymatic digestion and ionic liquid reuse. Bioresour Technol. https://doi.org/10.1016/j.biortech.2015.05.064
Ninomiya K, Utami ARI, Tsuge Y et al (2018) Pretreatment of bagasse with a minimum amount of cholinium ionic liquid for subsequent saccharification at high loading and co-fermentation for ethanol production. Chem Eng J. https://doi.org/10.1016/j.cej.2017.10.113
Mohammadi M, Shafiei M, Abdolmaleki A et al (2019) A morpholinium ionic liquid for rice straw pretreatment to enhance ethanol production. Ind Crops Prod 139:111494. https://doi.org/10.1016/J.INDCROP.2019.111494
Kahani S, Shafiei M, Abdolmaleki A, Karimi K (2017) Enhancement of ethanol production by novel morpholinium ionic liquids. J Clean Prod. https://doi.org/10.1016/j.jclepro.2017.09.008
Abbott AP, Capper G, Davies DL, et al (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun 70–71. https://doi.org/10.1039/b210714g
Chen L, Yu Q, Wang Q et al (2019) A novel deep eutectic solvent from lignin-derived acids for improving the enzymatic digestibility of herbal residues from cellulose. Cellulose. https://doi.org/10.1007/s10570-018-2190-8
Xia Q, Liu Y, Meng J et al (2018) Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem 20:2711–2721. https://doi.org/10.1039/c8gc00900g
Lou R, Ma R, Lin KT et al (2019) Facile extraction of wheat straw by deep eutectic solvent (DES) to produce lignin nanoparticles. ACS Sustain Chem Eng 7:10248–10256. https://doi.org/10.1021/acssuschemeng.8b05816
Alvarez-Vasco C, Ma R, Quintero M, et al (2016) Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): a source of lignin for valorization. Green Chem 18: https://doi.org/10.1039/c6gc01007e
Francisco M, Van Den Bruinhorst A, Kroon MC (2012) New natural and renewable low transition temperature mixtures (LTTMs): screening as solvents for lignocellulosic biomass processing. Green Chem 14:2153–2157. https://doi.org/10.1039/c2gc35660k
Procentese A, Johnson E, Orr V et al (2015) Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour Technol 192:31–36. https://doi.org/10.1016/j.biortech.2015.05.053
Kumar AK, Parikh BS, Pravakar M (2016) Natural deep eutectic solvent mediated pretreatment of rice straw: bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ Sci Pollut Res 23:9265–9275. https://doi.org/10.1007/s11356-015-4780-4
Hou XD, Feng GJ, Ye M et al (2017) Significantly enhanced enzymatic hydrolysis of rice straw via a high-performance two-stage deep eutectic solvents synergistic pretreatment. Bioresour Technol 238:139–146. https://doi.org/10.1016/j.biortech.2017.04.027
Yiin CL, Quitain AT, Yusup S et al (2018) Sustainable green pretreatment approach to biomass-to-energy conversion using natural hydro-low-transition-temperature mixtures. Bioresour Technol 261:361–369. https://doi.org/10.1016/j.biortech.2018.04.039
Degam G (2017) Deep eutectic solvents synthesis, characterization and applications in pretreatment of lignocellulosic biomass. Dissertation, South Dakota State University
Zhang CW, Xia SQ, Ma PS (2016) Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour Technol 219:1–5. https://doi.org/10.1016/j.biortech.2016.07.026
Procentese A, Raganati F, Olivieri G et al (2017) Low-energy biomass pretreatment with deep eutectic solvents for bio-butanol production. Bioresour Technol 243:464–473. https://doi.org/10.1016/j.biortech.2017.06.143
Chen Z, Reznicek WD, Wan C (2018) Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour Technol 263:40–48. https://doi.org/10.1016/j.biortech.2018.04.058
Lynam JG, Kumar N, Wong MJ (2017) Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour Technol 238:684–689. https://doi.org/10.1016/j.biortech.2017.04.079
Zulkefli S, Abdulmalek E, Abdul Rahman MB (2017) Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzymatic hydrolysis of pretreated oil palm trunk. Renew Energy 107:36–41. https://doi.org/10.1016/j.renene.2017.01.037
Jablonský M, Škulcová A, Kamenská L et al (2015) Deep eutectic solvents: fractionation of wheat straw. BioResources 10:8039–8047. https://doi.org/10.15376/biores.10.4.8039-8047
Hong S, Song Y, Yuan Y et al (2020) Production and characterization of lignin containing nanocellulose from luffa through an acidic deep eutectic solvent treatment and systematic fractionation. Ind Crops Prod 143:111913. https://doi.org/10.1016/j.indcrop.2019.111913
Zhao Z, Chen X, Ali MF et al (2018) Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour Technol 263:325–333. https://doi.org/10.1016/j.biortech.2018.05.016
Su Y, Huang C, Lai C, Yong Q (2021) Green solvent pretreatment for enhanced production of sugars and antioxidative lignin from poplar. Bioresour Technol 321: https://doi.org/10.1016/j.biortech.2020.124471
Zhou X, Huang T, Liu J, et al (2021) Recyclable deep eutectic solvent coupling sodium hydroxide post-treatment for boosting woody/herbaceous biomass conversion at mild condition. Bioresour Technol 320: https://doi.org/10.1016/j.biortech.2020.124327
Lee KM, Hong JY, Tey WY (2021) Combination of ultrasonication and deep eutectic solvent in pretreatment of lignocellulosic biomass for enhanced enzymatic saccharification. Cellulose 28: https://doi.org/10.1007/s10570-020-03598-5
Sharma V, Nargotra P, Sharma S, Bajaj BK (2021) Efficacy and functional mechanisms of a novel combinatorial pretreatment approach based on deep eutectic solvent and ultrasonic waves for bioconversion of sugarcane bagasse. Renew Energy 163: https://doi.org/10.1016/j.renene.2020.10.101
Tnah SK, Wu TY, Ting DCC, et al (2022) Effect of chlorine atoms in choline chloride-monocarboxylic acid for the pretreatment of oil palm fronds and enzymatic hydrolysis. Renew Energy 182: https://doi.org/10.1016/j.renene.2021.09.068
Liu S (2015) A synergetic pretreatment technology for woody biomass conversion. Appl Energy 144:114–128. https://doi.org/10.1016/j.apenergy.2015.02.021
Rogalinski T, Ingram T, Brunner G (2008) Hydrolysis of lignocellulosic biomass in water under elevated temperatures and pressures. J Supercrit Fluids 47:54–63. https://doi.org/10.1016/J.SUPFLU.2008.05.003
Ingram T, Rogalinski T, Bockemühl V et al (2009) Semi-continuous liquid hot water pretreatment of rye straw. J Supercrit Fluids. https://doi.org/10.1016/j.supflu.2008.10.023
Pérez JA, Ballesteros I, Ballesteros M et al (2008) Optimizing liquid hot water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel. https://doi.org/10.1016/j.fuel.2008.06.009
Cardona E, Llano B, Peñuela M et al (2018) Liquid-hot-water pretreatment of palm-oil residues for ethanol production: an economic approach to the selection of the processing conditions. Energy. https://doi.org/10.1016/j.energy.2018.07.045
Pangsang N, Rattanapan U, Thanapimmetha A et al (2019) Chemical-free fractionation of palm empty fruit bunch and palm fiber by hot-compressed water technique for ethanol production. Energy Rep. https://doi.org/10.1016/j.egyr.2019.02.008
Goh CS, Tan HT, Lee KT, Mohamed AR (2010) Optimizing ethanolic hot compressed water (EHCW) cooking as a pretreatment to glucose recovery for the production of fuel ethanol from oil palm frond (OPF). Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2010.03.029
Weiqi W, Shubin W, Liguo L (2013) Combination of liquid hot water pretreatment and wet disk milling to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.08.130
Tian D, Shen F, Yang G et al (2019) Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel. https://doi.org/10.1016/j.fuel.2019.04.155
Liu J, Li R, Shuai L et al (2017) Comparison of liquid hot water (LHW) and high boiling alcohol/water (HBAW) pretreatments for improving enzymatic saccharification of cellulose in bamboo. Ind Crops Prod 107:139–148. https://doi.org/10.1016/J.INDCROP.2017.05.035
Yu Q, Zhuang X, Lv S et al (2013) Liquid hot water pretreatment of sugarcane bagasse and its comparison with chemical pretreatment methods for the sugar recovery and structural changes. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.11.099
Hongzhang C, Liying L (2007) Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Bioresour Technol 98:666–676. https://doi.org/10.1016/J.BIORTECH.2006.02.029
Qiu W, Chen H (2012) Enhanced the enzymatic hydrolysis efficiency of wheat straw after combined steam explosion and laccase pretreatment. Bioresour Technol 118:8–12. https://doi.org/10.1016/J.BIORTECH.2012.05.033
Zhao X, Moates GK, Wilson DR et al (2015) Steam explosion pretreatment and enzymatic saccharification of duckweed (Lemna minor) biomass. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2014.11.003
Chen WH, Tsai CC, Lin CF et al (2013) Pilot-scale study on the acid-catalyzed steam explosion of rice straw using a continuous pretreatment system. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.10.111
Wang W, Ling H, Zhao H (2015) Steam explosion pretreatment of corn straw on xylose recovery and xylitol production using hydrolysate without detoxification. Process Biochem. https://doi.org/10.1016/j.procbio.2015.06.001
Niemi P, Pihlajaniemi V, Rinne M, Siika-aho M (2017) Production of sugars from grass silage after steam explosion or soaking in aqueous ammonia. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2017.01.022
Verardi A, Blasi A, Marino T et al (2018) Effect of steam-pretreatment combined with hydrogen peroxide on lignocellulosic agricultural wastes for bioethanol production: analysis of derived sugars and other by-products. J Energy Chem. https://doi.org/10.1016/j.jechem.2017.11.007
He L, Wang C, Shi H et al (2019) Combination of steam explosion pretreatment and anaerobic alkalization treatment to improve enzymatic hydrolysis of Hippophae rhamnoides. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.121693
Mihiretu GT, Chimphango AF, Görgens JF (2019) Steam explosion pretreatment of alkali-impregnated lignocelluloses for hemicelluloses extraction and improved digestibility. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.122121
Martín C, Wu G, Wang Z et al (2018) Formation of microbial inhibitors in steam-explosion pretreatment of softwood impregnated with sulfuric acid and sulfur dioxide. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.04.074
Gollapalli LE, Dale BE, Rivers DM (2002) Predicting digestibility of ammonia fiber explosion (AFEX)-treated rice straw. Appl Biochem Biotechnol - Part A Enzym Eng Biotechnol 98–100:23–35. https://doi.org/10.1385/ABAB:98-100:1-9:23
Teymouri F, Laureano-Perez L, Alizadeh H, Dale BE (2005) Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour Technol. https://doi.org/10.1016/j.biortech.2005.01.016
Lee JM, Jameel H, Venditti RA (2010) A comparison of the autohydrolysis and ammonia fiber explosion (AFEX) pretreatments on the subsequent enzymatic hydrolysis of coastal Bermuda grass. Bioresour Technol 101:5449–5458. https://doi.org/10.1016/J.BIORTECH.2010.02.055
Abdul PM, Jahim JM, Harun S et al (2016) Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour Technol. https://doi.org/10.1016/j.biortech.2016.02.135
Chundawat SPS, Vismeh R, Sharma LN et al (2010) Multifaceted characterization of cell wall decomposition products formed during ammonia fiber expansion (AFEX) and dilute acid based pretreatments. Bioresour Technol. https://doi.org/10.1016/j.biortech.2010.06.027
Zhao C, Shao Q, Ma Z et al (2016) Physical and chemical characterizations of corn stalk resulting from hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2015.12.018
Zhao C, Qiao X, Cao Y, Shao Q (2017) Application of hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment of energy crops. Fuel. https://doi.org/10.1016/j.fuel.2017.05.073
Rojas-Sossa JP, Zhong Y, Valenti F et al (2019) Effects of ammonia fiber expansion (AFEX) treated corn stover on anaerobic microbes and corresponding digestion performance. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2019.105263
Baral NR, Shah A (2017) Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.02.068
Ritter D, Campbell A (1991) Supercritical carbon dioxide extraction of southern pine and ponderosa pine. Wood fiber Sci 23:98–113
Zheng Y, Lin HM, Wen J et al (1995) Supercritical carbon dioxide explosion as a pretreatment for cellulose hydrolysis. Biotechnol Lett. https://doi.org/10.1007/BF00129015
Zheng Y, Lin HM, Tsao GT (1998) Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol Prog. https://doi.org/10.1021/bp980087g
Narayanaswamy N, Faik A, Goetz DJ, Gu T (2011) Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour Technol 102:6995–7000. https://doi.org/10.1016/J.BIORTECH.2011.04.052
Zhao M, Xu Q, Li G, et al (2019) Pretreatment of agricultural residues by supercritical CO 2 at 50–80 °C to enhance enzymatic hydrolysis. J Energy Chem. https://doi.org/10.1016/j.jechem.2018.05.003
Gao M, Xu F, Li S et al (2010) Effect of SC-CO2 pretreatment in increasing rice straw biomass conversion. Biosyst Eng. https://doi.org/10.1016/j.biosystemseng.2010.05.011
Kim KH, Hong J (2001) Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour Technol 77:139–144. https://doi.org/10.1016/S0960-8524(00)00147-4
Alinia R, Zabihi S, Esmaeilzadeh F, Kalajahi JF (2010) Pretreatment of wheat straw by supercritical CO2 and its enzymatic hydrolysis for sugar production. Biosyst Eng. https://doi.org/10.1016/j.biosystemseng.2010.07.002
Ji Q, Jiang H, Yu X, et al (2020) Efficient and environmentally-friendly dehydration of fructose and treatments of bagasse under the supercritical CO2 system. Renew Energy 162: https://doi.org/10.1016/j.renene.2020.07.123
Silveira MHL, Vanelli BA, Corazza ML, Ramos LP (2015) Supercritical carbon dioxide combined with 1-butyl-3-methylimidazolium acetate and ethanol for the pretreatment and enzymatic hydrolysis of sugarcane bagasse. Bioresour Technol 192: https://doi.org/10.1016/j.biortech.2015.05.044
Okano K, Kitagawa M, Sasaki Y, Watanabe T (2005) Conversion of Japanese red cedar (Cryptomeria japonica) into a feed for ruminants by white-rot basidiomycetes. Anim Feed Sci Technol 120:235–243. https://doi.org/10.1016/J.ANIFEEDSCI.2005.02.023
Akin DE, Rigsby LL, Sethuraman A et al (1995) Alterations in structure, chemistry, and biodegradability of grass lignocellulose treated with the white rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus. Appl Environ Microbiol 61:1591–1598. https://doi.org/10.1128/aem.61.4.1591-1598.1995
ten Have R, Teunissen PJM (2001) Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem Rev 101:3397–3414. https://doi.org/10.1021/cr000115l
Rabinovich ML, Bolobova A V., Vasil’chenko LG (2004) Fungal decomposition of natural aromatic structures and xenobiotics: a review. Appl. Biochem. Microbiol.
Hatakka AI (1983) Pretreatment of wheat straw by white-rot fungi for enzymic saccharification of cellulose. Eur J Appl Microbiol Biotechnol. https://doi.org/10.1007/BF00504744
Lee J-W, Gwak K-S, Park J-Y et al (2007) Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J Microbiol 45:485–91
Bak JS, Kim MD, Choi IG, Kim KH (2010) Biological pretreatment of rice straw by fermenting with Dichomitus squalens. N Biotechnol. https://doi.org/10.1016/j.nbt.2010.02.021
Shi J, Chinn MS, Sharma-Shivappa RR (2008) Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour Technol 99:6556–6564. https://doi.org/10.1016/J.BIORTECH.2007.11.069
Shi J, Sharma-Shivappa RR, Chinn M, Howell N (2009) Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2008.04.016
Yu H, Zhang X, Song L et al (2010) Evaluation of white-rot fungi-assisted alkaline/oxidative pretreatment of corn straw undergoing enzymatic hydrolysis by cellulase. J Biosci Bioeng 110:660–664. https://doi.org/10.1016/J.JBIOSC.2010.08.002
Alexandropoulou M, Antonopoulou G, Fragkou E et al (2017) Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J Environ Manage. https://doi.org/10.1016/j.jenvman.2016.04.006
Noor Arbaain EN, Bahrin EK, Noor NM et al (2019) Chemical-free pretreatment of unwashed oil palm empty fruit bunch by using locally isolated fungus (Schizophyllum commune ENN1) for delignification. Food Bioprod Process. https://doi.org/10.1016/j.fbp.2019.09.001
Guragain YN, Wang D, Vadlani P V. (2016) Appropriate biorefining strategies for multiple feedstocks: critical evaluation for pretreatment methods, and hydrolysis with high solids loading. Renew Energy 96: https://doi.org/10.1016/j.renene.2016.04.099
Hou J, Zhang X, Zhang S et al (2022) Improvement of bioethanol production using a new fermentation system: the process analysis and micro-mechanisms study. Process Saf Environ Prot 162:837–845. https://doi.org/10.1016/j.psep.2022.04.061
Qin L, Liu ZH, Jin M, et al (2013) High temperature aqueous ammonia pretreatment and post-washing enhance the high solids enzymatic hydrolysis of corn stover. Bioresour Technol 146: https://doi.org/10.1016/j.biortech.2013.07.099
Zhang D, Ong YL, Li Z, Wu JC (2013) Biological detoxification of furfural and 5-hydroxyl methyl furfural in hydrolysate of oil palm empty fruit bunch by Enterobacter sp. FDS8. Biochem Eng J 72: https://doi.org/10.1016/j.bej.2013.01.003
Liu K, Atiyeh HK, Pardo-Planas O, et al (2015) Butanol production from hydrothermolysis-pretreated switchgrass: quantification of inhibitors and detoxification of hydrolyzate. Bioresour Technol 189: https://doi.org/10.1016/j.biortech.2015.04.018
Travaini R, Barrado E, Bolado S (2016) Effect of ozonolysis parameters on the inhibitory compound generation and on the production of ethanol by Pichia stipitis and acetone-butanol-ethanol by Clostridium from ozonated and water washed sugarcane bagasse. Bioresour Technol 218: https://doi.org/10.1016/j.biortech.2016.07.028
Wang J, Gao Q, Zhang H, Bao J (2016) Inhibitor degradation and lipid accumulation potentials of oleaginous yeast Trichosporon cutaneum using lignocellulose feedstock. Bioresour Technol 218: https://doi.org/10.1016/j.biortech.2016.06.130
Gao H, Wang Y, Yang Q, et al (2021) Combined steam explosion and optimized green-liquor pretreatments are effective for complete saccharification to maximize bioethanol production by reducing lignocellulose recalcitrance in one-year-old bamboo. Renew Energy 175: https://doi.org/10.1016/j.renene.2021.05.016
Liu Y, Yan Z, He Q, et al (2021) Bacterial delignification promotes the pretreatment of rice straw by ionic liquid at high biomass loading. Process Biochem 111: https://doi.org/10.1016/j.procbio.2021.08.026
Wei HL, Bu J, Zhou SS, et al (2021) A facile ionic liquid and p-toluenesulfonic acid pretreatment of herb residues: enzymatic hydrolysis and lignin valorization. Chem Eng J 419: https://doi.org/10.1016/j.cej.2021.129616
Wang X, Cao L, Lewis R, et al (2020) Biorefining of sugarcane bagasse to fermentable sugars and surface oxygen group-rich hierarchical porous carbon for supercapacitors. Renew Energy 162:. https://doi.org/10.1016/j.renene.2020.09.118
Acknowledgements
The authors are thankful for access to the state-of-the-art research infrastructure in the Clean Energy Technologies Research Institute (CETRI). The authors are grateful for their support.
Funding
The financial support was provided by Mitacs Accelerate (IT29592), Natural Sciences and Engineering Research Council of Canada (NSERC DG: RGPIN-2018–03955), Canada Foundation for Innovation (CFI JELF: 37758), and the Vice-President (Research) Discretionary Fund at the University of Regina.
Author information
Authors and Affiliations
Contributions
Conceptualization: Hussameldin Ibrahim; methodology: Ishag Alawad, Hussameldin Ibrahim; software: Ishag Alawad; validation: Ishag Alawad, Hussameldin Ibrahim; formal analysis: Ishag Alawad; investigation: Ishag Alawad; resources: Hussameldin Ibrahim; data curation: Ishag Alawad; writing—original draft preparation: Ishag Alawad; writing—review and editing: Hussameldin Ibrahim; visualization: Ishag Alawad; supervision: Hussameldin Ibrahim; project administration: Hussameldin Ibrahim; funding acquisition: Hussameldin Ibrahim. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclaimer
The views expressed herein are those of the writers and not necessarily those of our research and funding partners.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alawad, I., Ibrahim, H. Pretreatment of agricultural lignocellulosic biomass for fermentable sugar: opportunities, challenges, and future trends. Biomass Conv. Bioref. 14, 6155–6183 (2024). https://doi.org/10.1007/s13399-022-02981-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02981-5