Abstract
Concentrated protic ionic liquid (PIL) without dilution has increased saccharification efficiency due to the solubilization of hemicellulose and lignin compounds. However, concentrated PILs are not cost-effective, highly viscous, and toxic to microorganisms. The present study evaluates the effect of PILs on the solubilization of hemicellulose and lignin present in coconut coir and pith. PILs including pyridinium hydrogen sulfate [PyH][HSO4] and triethylammonium hydrogen sulfate [TEA][HSO4] were considered in this study. The sugars and lignin were partially hydrolyzed during the PIL pretreatment of coir and pith. However, the degree of biomass solubilization varied for different types of biomass and PIL. The changes in the biomass after pretreatment were studied through FTIR and XRD analysis. The yield of glucose released from [PyH][HSO4] and [TEA][HSO4] pretreated coir and pith increased 4.12, 4.73 and 7.36, and 6.44, respectively. The results showed that diluted PIL could be used in a biorefinery to increase the glucose yield with almost similar efficiency obtained from concentrated PIL. The recovery and reusability studies showed the recycled PIL could be utilized 3 times. Further application of low concentrations of PIL suggests the possibility of a new process design for a biorefinery to achieve low operating costs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic biomass is an excellent potential source of alternative fuels and different biochemical compounds. It is mainly composed of cellulose, hemicellulose, lignin, and a small fraction of extractives and ash. [1]. Fractionation of biomass improves the overall biomass conversion process, making each component more efficient for commercial and industrial use [2]. The cellulose layer is bound to the lignin matrix through inter- and intra-hydrogen molecular bonds of hemicellulose. This layer can be released by hydrolysis of the lignin and hemicellulose layers using different pretreatment methods under different conditions. Pretreatment methods include physical (milling and grinding), chemical (acid, alkali, and other organic solvents), thermal (hot water, ammonia fiber explosion, and steam explosion), and biological/biochemical (fungi, bacteria, and microalgae) methods [3]. After pretreatment, the exposed cellulose polymer layer is further broken down into glucose monomers during the enzymatic saccharification process. [4, 5]. Glucose monomers are then fermented to ethanol using wild or recombinant yeast species, such as Saccharomyces cerevisiae. [6]. However, under harsh conditions, some pretreatment methods can degrade sugar to furan compounds (furfural and 5-hydroxymethylfurfural) [7]. These compounds act as inhibitors to yeast metabolism, growth, and fermentation [8]. Furan compounds inhibit the glycolysis pathways of many organisms, protein, and RNA synthesis [9]. Acid and alkali are the most widely used conventional pretreatment methods. Acid pretreatment significantly affects cellulose and hemicellulose, whereas alkali pretreatment has a significant effect on lignin solubilization [10]. However, the drawback of this acid and alkali method includes generation of furan compounds, corrosion of equipment [10], generation of wastewater during pH neutralization, and formation of salts [11]. These drawbacks during pretreatment are overcome through ionic liquid (IL) pretreatment.
In recent years, pretreatment of lignocellulosic biomass using ionic liquids has gained momentum. Ionic liquids are composed of anions and cations with physical properties, such as low vapor pressure and high melting point. [12]. Pretreatment using ionic liquids leads to the following: (a) dissolution of cellulose, (b) reduction in cellulose crystallinity, and (c) increase in the surface porosity of lignocellulosic biomass [13]. IL cations are imidazolium, pyrrolidinium, pyridinium, ammonium, sulfonium, and phosphonium. Among these different cations, imidazolium has been studied widely by researchers to dissolve cellulose during pretreatment. However, the main disadvantage of imidazolium-based IL includes (a) toxic to enzymes and fermentative yeast, (b) highly viscous, (c) generation of wastewater with IL residue, (d) high energy input to recover and dry IL, and (e) high cost [14]. Therefore, ammonium- and pyridinium-based ionic liquid with hydrogen sulfate, also referred to as the protic ionic liquid (PIL), has been proposed as alternative options of imidazolium-based IL. Pretreatment of lignocellulosic biomass using ammonium- and pyridinium-based ILs increases the saccharification efficiency [15, 16]. In addition, these PILs are less toxic and lower cost compared to imidazolium ILs [17].
Previously, most IL pretreatment studies were conducted using concentrated ILs with the main goal achieving a high saccharification rate of cellulose to sugars, with the range of 70–90% [18, 19]. However, few attempts using diluted ILs (25–50%) [20, 21] have been made to reduce consumption costs of ILs and reduce the operational difficulty, e.g., high viscosity mixture, although the sugar recovery rate was reduced to 50–80%. On the other hand, pretreatment with a high concentration of ionic liquid has led to an increase in the dissolution of cellulose. However, it has also resulted in difficulties, such as separations of (a) soluble sugars (monomers), (b) sugar degradation products, and (c) ionic liquid. The accumulations of sugars and sugar by-products during ionic liquid pretreatment and recycling decreased the pretreatment efficiency. Therefore, an attempt was made to pretreat different biomass with two types of ILs, ammonium IL (triethylammonium hydrogen sulfate [TEA][HSO4]) and pyridinium IL (pyridinium hydrogen sulfate [PyH][HSO4]), at low concentrations. In addition, it has been reported that [TEA][HSO4] [22] and [PyH][HSO4] [23] are low cost, eco-friendly ionic liquid solvents that can be recovered and recycled. George et al. reported an increase in saccharification efficiency due to delignification rather than decrystallization of cellulose when using [TEA][HSO4] with 20% water [24]. Similarly, Asim et al. reported 79% delignification after pretreatment of wheat straw using [PyH][HSO4] [25]. Low PIL concentration could benefit the biorefineries (e.g., for bioethanol production) and the environment through (a) reduced washing steps during pH neutralization, low toxicity, and viscosity compared to PIL of high concentration. Furthermore, PILs at low concentrations could result in lower residual IL in biomass after washing steps, therefore lowering the inhibition on enzymes/microorganism during the saccharification/fermentation process.
The present study focuses on determining the delignification efficiency after ionic liquid pretreatment. Two different types of coconut residues including coconut coir and coconut pith were considered for this study due to their different cellulose (24–50%), hemicellulose (12–25%), and lignin (40–50%) compositions [26]. In addition, due to their high lignin contents, few reported studies have been associated with the pretreatment of coconut residues with ionic liquids [27, 28]. However, the effect of an ionic liquid at a low concentration on biomass with different lignin and cellulose content has not yet been evaluated and explored. This study evaluated the glucose yield from the pretreated biomass to determine the saccharification efficiency.
2 Materials and methods
2.1 Procurement and preparation of raw materials
Coconut coir and pith were procured from a local coconut coir processing industry in Hyderabad, India. The coir and pith were manually subjected to size reduction, followed by soaking in liquid nitrogen for 5 min. The sample was ground in pestle-mortar until the coir was converted to powder form. The powdered coir sample was sieved using a 1.0-mm pore size steel mesh. The collected sample was dried in a hot air oven at 105 °C for 24 h. The processed coir sample was stored in an airtight container until further use [26].
2.2 Compositional characterization of coir and pith
The sample’s composition (cellulose, hemicellulose, acid-soluble lignin, and acid-insoluble lignin, ash) was determined by the Laboratory Analytical Procedure (LAPs) for biomass developed by NREL, USA [29]. The extractive free dried sample (0.3 g) was hydrolyzed with 3 mL of sulfuric acid (72% w/w) in a hot water bath, set at 30 °C, for 60 min. Mixing was provided at every 15-min interval. The sample was then diluted with 84 mL of distilled water to a total of 87 mL, thereby obtaining a final concentration of 4% w/w sulfuric acid. The mixture was autoclaved at 121 °C for 60 min using a laboratory-scale autoclave to hydrolyze oligomers, such as cellobiose formed during the acid hydrolysis process. The autoclaved mixture was later filtered using a vacuum filtration unit (make: Millipore) with 0.45-µm filter paper (make: Whatman) to separate the solid residue from the liquid. The obtained filtrate was later analyzed for monosaccharides and disaccharides using LC–MS (model: 1260 infinity II, make: Agilent technologies, Germany) [30]. Also, the acid-soluble lignin was determined from the filtrate using LC-DAD/LC–MS method [31]. The obtained solid residue was dried in a hot air oven for 12 h. The sample was put in the muffle furnace and heated at 575 °C for 4 h. The residue from the muffle furnace was weighed to determine the ash content. Acid insoluble lignin was determined from the difference in the solid residue’s weight and the weight of the ash content. The sample’s moisture content was determined, as described in the protocol [32]. The extractives in the sample were determined by using the protocol described by NREL [33]. The chemical composition of coir and pith is summarized in Table 1.
2.3 Synthesis of ionic liquid
The PILs, [PyH][HSO4] and [TEA][HSO4], were prepared according to the published method [34]. The sulfuric acid (H2SO4) was used as the Brønsted acid for the synthesis of [Py][HSO4] and [TEA][HSO4]. Pyridine and trimethylamine were used as Brønsted bases for the synthesis of [PyH][HSO4] and [TEA][HSO4], respectively (Fig. 1). The H-NMR for the synthesized [PyH][HSO4] and [TEA][HSO4] are shown in Fig. 1.
2.3.1 Pyridinium hydrogen sulfate [PyH][HSO4] synthesis
Pyridine (4.9 g, 62 mmol) was cooled in a round bottom flask and placed in an ice bath. With stirring, 12 mL of 5 M H2SO4 (62 mmol) was added dropwise. After the addition, the mixture was stirred at room temperature for 1 h to ensure the completion of the reaction. Water was removed using a rotary evaporator, operated at 70 °C under a high vacuum for 4 h. The product was washed with dichloromethane and dried again using the rotary evaporator. The recovered ionic liquid was a white hygroscopic solid. The reaction for the synthesis of [PyH][HSO4] is shown in Fig. 1a.
2.3.2 Triethylammonium hydrogen sulfate [TEA][HSO4] synthesis
Trimethylamine (3.63 g, 35.8 mmol) was cooled in a round bottom flask and placed in an ice bath. With stirring, 7.2 mL of 5 M H2SO4 (35.8 mmol) was added dropwise. After the addition, the mixture was stirred at room temperature for 1 h to ensure completion of the reaction. Water was removed using a rotary evaporator, operated at 70 °C under high vacuum for 4 h or until the residue’s weight became constant. The product was dissolved in dichloromethane, and the remaining water was absorbed by sodium sulfate (Na2SO4). Then the mixture was filtered, and the dichloromethane was removed using the rotary evaporator. The reaction for the synthesis of [TEA][HSO4] is shown in Fig. 1b.
2.4 Experimental setup — stirred high-pressure batch reactor
The pretreatment process was carried out in a stirred high-pressure batch reactor. The optimum conditions for IL pretreatment were determined using response surface methodology (RSM) (unreported study). The operational parameters, such as biomass loading, reaction time, and PIL concentration, were optimized because they are simply adjustable process parameters (Table 2). The pretreatment of coir and pith was carried out in a 2000-mL-volume stirred high-pressure batch reactor with a working volume of 500 mL. The stirred high-pressure batch reactor vessel was flushed thrice with N2 gas to remove all air in the reactor. The temperature was maintained at 121 °C, and the agitation of the mixture was maintained at 250 rpm. After pretreatment of the biomass under the desired condition, the mixture was collected in a 250-mL Erlenmeyer conical flask. To the mixture, 50 mL of ethanol was added twice to regenerate the dissolved solids (if any). The solids were separated from the hydrolysate by vacuum filtration. The collected solids were washed thrice with 50 mL distilled water to remove any IL residue in the solid. The pH of the filtrate was adjusted to 7.0 using 1 N H2SO4 and 1 N NaOH. The liquid hydrolysate was analyzed for sugar monomers and soluble lignin using LC–MS [30, 31]. Also, the hydrolysate was analyzed to determine the total reducing sugar [35]. The solids were subjected to drying (90 °C for 8 h) followed by enzymatic saccharification to study the effectiveness of PIL pretreatment. The pretreatments were carried out thrice to reduce the variance between the experimental data. The yield of byproducts was calculated from the expression as shown in Eq. (1), and the biomass conversion was determined as shown in Eq. (2).
where P is the product in mg/mL (monosaccharide, 5-hydroxymethylfurfural, furfural, and levulinic acid) obtained after the pretreatment; S.C is the stoichiometric coefficient. The stoichiometric coefficients used were (a) xylan: 1.136, (b) glucan: 0.9, (c) furfural: 0.727, (d) 5-HMF: 0.77, and (e) levulinic acid: 0.716; V is the volume of the sample in mL; S is the substrate (sugar) in mg present in the biomass; \({W}_{1}\) is the initial weight of the dried biomass; \({W}_{2}\) is the final weight of dried biomass after IL pretreatment.
2.4.1 Recovery and recycling of ionic liquid
The ionic liquid in the filtrate and the solids’ washings were recovered by distillation under vacuum using a rotary evaporator (Adithya Scientific, India). Initially, the ethanol solvent was recovered by maintaining the temperature at 40 °C. The ionic liquid was recovered by removing the water under vacuum conditions at 70 °C. The ionic liquid was evaporated until less than 1% of the water content. The distillate was collected in a round bottom flask connected to the evaporator. The residue in the round bottom flask was a mixture of PIL, reducing sugar, furan, and soluble lignin. The ionic liquid recovered from the first cycle of the pretreatment process was subjected to the second and third cycles to study the recycled ionic liquid’s effectiveness in pretreatment.
2.5 Enzymatic saccharification
The saccharification of pretreated coconut coir and pith was carried out using commercial enzymes, such as cellulase (C1794, make: Sigma Aldrich, USA) and β-glucosidase (G0395. Make: Sigma-Aldrich, USA) with enzyme activity of 97 FPU/mL and 5.1 CBU/g, respectively. The concentrations of cellulase and β-glucosidase enzymes considered in this study were 20 FPU/g and 10 CBU/g, respectively [30]. The enzymes were suspended in a medium composed of 0.05 M citrate buffer (pH 4.8), sodium azide (0.3% w/v), and Tween 80 (0.1% v/v). The suspension was made in a 150-mL Erlenmeyer flask. Ten percent w/v of treated solids was added to this mixture, and the flask was placed in an orbital incubator shaker (150 rpm). The treated solids’ enzymatic hydrolysis was carried out in time intervals (24–120 h) and at 50 °C. The sample was collected every 24 h, and the liquid hydrolysate was analyzed for glucose yield using LC–MS. After collecting the sample, the enzymes were denatured at 90 °C for 5 min. The sample was allowed to cool down and then centrifuged at 1792 × g for 10 min to separate the solid residue and liquid hydrolysate. The saccharification process was carried out in triplicates to reduce the error for the obtained experimental data. The yield of glucose after saccharification was determined using Eq. (3).
2.6 Quantification and analysis of reducing sugar using UV–visible spectrometer
A monosaccharide with an exposed carbonyl group (aldoses or keto group) is called as reducing sugar [36]. The estimation of reducing sugar in the liquid hydrolysate was done by following the Miller protocol [35]. In 3 mL of liquid hydrolysate, 3 mL of dinitrosalicylic acid reagent (DNSA) was added. The mixture was heated for 10 min at 90 °C using a dry bath (DRB 200, make Hach). After a reddish color appeared, 1 mL of 40% Rochelle salt (potassium sodium tartrate) was added. The sample was cooled before analysis using a UV–visible spectrometer. The absorbance for the sample was recorded at wavelength 575 nm.
2.7 Quantification of sugar, furan, and soluble lignin using LC–MS
2.7.1 Sugar analysis
The analysis and quantification of sugar were performed using LC–MS (Infinity 1260, make: Agilent, Germany). The equipment was equipped with electron spray ionization (ESI) source, and the analysis was carried out in the negative ionization mode. The sample’s elution was carried out using a 3.0 μm particle size Poroshell HILIC Z column (2.7 mm × 150 mm) column (make: Agilent) kept at 35 °C. The mobile phase was a mixture of — (A) 0.3% v/v ammonium hydroxide (NH4OH) and (B) acetonitrile (ACN) with 0.3% v/v NH4OH ([30]. The standard curve for individual sugars determined concentrations of monosaccharides in each sample, such as xylose, arabinose, fructose, mannose, glucose, galactose, and cellobiose.
2.7.2 Furan analysis
In addition, the hydrolysate was analyzed for the presence of furan (furfural and 5-hydroxymethylfurfural) compounds and quantified using LC-DAD. The compounds were eluted using a Zorbax SB − C18 (2.1 × 50 mm, 1.8 microns) column. The volume of the sample injected into the system was 2 µL. The column oven temperature was kept at 35 °C. The mobile phases for eluting the compounds from the column were — (A) 80% — formic acid (98%, HPLC grade) and (B) 20% — methanol (99.99%, HPLC grade). The isocratic condition was maintained. The standard curve was prepared using different concentrations of furfural and 5-hydroxymethylfurfural. The concentrations of the furan compounds in the hydrolysate were determined using the prepared standard curve [31].
2.7.3 Soluble lignin analysis
The lignin monomer liquid hydrolysate was quantified using the LC–MS. The lignin monomer mixtures were injected into the Poroshell 120 EC-C18 (2.1 × 100 mm, 2.7-micron particle size) column. Two mobile phases used for the elution of the compounds from the column during the run were — (A) 1% (v/v) formic acid (98%, HPLC grade) in MS grade water and (B) methanol (99.99%, HPLC grade). The flow of mobile phases developed for separation of lignin monomer were (i) 0–2 min (A-80%, B-20%), (ii) 2–11 min (A-10%, B-90%), and (iii) 11–16 min (A-80%, B-20%). The column oven temperature was maintained at 35 °C, and the mobile phase flow rate was 0.3 mL/min. The gas temperature, drying gas flow, nebulizer pressure, quadrupole temperature, and capillary voltage (Vcap) were held at 300 °C, 12 L/min, 45 psig, 100 °C, 4500 V, respectively. Calibration curves for mixed lignin monomers were generated using Chemlab workstation software. The low and high detection limits were 0.5 mg/L and 50 mg/L, respectively [31].
2.8 Fourier transform infrared spectroscopy (FTIR) analysis
Fourier transform infrared spectroscopy (FTIR) was used to identify the changes in the treated solid residue samples’ chemical structure and functional groups (Munajad et al., 2018). This study used Fourier transform infrared spectrometer (Alpha II, Bruker) with a spectral range from 500 to 4000 cm−1 at a resolution of 4 cm−1 with 32 scans. The sample was placed evenly over the attenuated total reflection (ATR) crystal. The crystal was made up of zinc (Zn) and selenium (Se).
2.9 X-ray diffraction (XRD) analysis
Xpert Pro (PW3040/60, PANalytical, Germany) was used to obtain X-ray diffraction patterns of the untreated and treated samples. The instrument was equipped with a sealed tube Cu-Kα source, a diffracted beam PreFIX carrier, and a line detector. Samples were cast on microscope slides using double-sided tape. Scans were taken at 45 kV and 40 mA with a Bragg angle (2θ) of 5–80° in steps of 0.0167°. The crystallinity index CrI for the untreated and treated sample was determined from the Bragg angle (2θ) of the amorphous and crystalline cellulose region. The CrI for the samples was calculated (Eq. (3)).
where, \({I}_{cryz}\) is the intensity of peak at Bragg angle 22.4° (crystalline cellulose), \({I}_{am}\) is the intensity of the background scatters at Bragg angle 18.0° (amorphous cellulose) [37].
2.10 Test for significance
The statistical significance for the change of cellulose, hemicellulose, and lignin composition was evaluated by performing Tukey’s test with a p-value of 0.05.
3 Results and discussion
3.1 Protic ionic liquid (PIL) pretreatment
3.1.1 Efficiency of pretreatment
Protic ionic liquid (PIL) pretreatment of coir and pith was carried out by following the experimental conditions shown in Table 2. After pretreatment, the biomass conversion was evaluated according to Eq. (2) and shown in Fig. 2. A difference in biomass conversion was observed between coir and pith after pretreatment with [PyH][HSO4] and [TEA][HSO4], respectively. A biomass conversion of 34.1%, 34.9% and 28.13%, and 31.1% was observed for pith and coir after pretreatment with [PyH][HSO4] and [TEA][HSO4], respectively. While for pith, a slight increase in biomass conversion was observed. The biomass conversion increased 1.1-fold times for coir when pretreated with [TEA][HSO4]. In comparison, biomass conversion was higher when pretreated using [TEA][HSO4]. A solid recovery of 78.15%, 77.825% and 71.87%, and 68.9% were obtained for coir and pith pretreated with [PyH][HSO4] and [TEA][HSO4], respectively.
Semerci and Ersan reported a solid recovery of 57.2% after pretreatment of hornbeam using [TEA][HSO4]. The decrease in the solid was attributed to the solubilization of lignin and the particle size (0.15–1.18 mm) during pretreatment [38]. According to Brandt and others, 30–40% of biomass conversion was observed when pretreating Miscanthus x giganteus with 80% w/w of [TEA][HSO4] at different time intervals (2–4 h). However, the higher biomass conversion may be due to the low lignin content of Miscanthus x giganteus (21–26%). Meanwhile, the lignin content in coconut coir and pith varies between 40 and 45%. Furthermore, in the reported study, the concentration of [TEA][HSO4] was high (80% w/w) [39]. Another study reported fractionation of Miscanthus through combined pretreatment (ethanol-[TEA][HSO4]) with the 60% wt PIL concentration and 40% wt ethanol. The concentration of the PIL was lowered using organic solvents, and the pretreatment efficiency was studied through glucose yield during saccharification. A glucose yield of 87% and 71% was reported for 10% and 50% biomass loading, respectively [40]. However, in the present study, the [TEA][HSO4] concentration for pretreatment of coir and pith was 5.7% and 6.8% with 94.3% and 93.2% water, respectively. Furthermore, another study evaluated the effect of PIL and water as co-solvent on fractionation of Miscanthus and pine softwood [41]. These previous observations were in agreement with the outcome of the present study.
The compositional analysis of the samples before and after treatment was performed to determine the efficiency of ionic liquid pretreatment (Table 3). It was noted that the solid recovery after ionic liquid pretreatment was almost similar in both cases. The removal of xylose after pretreatment was higher in samples pretreated with [PyH][HSO4]. Meanwhile, the delignification of the coir and pith sample was higher when pretreated with [TEA][HSO4]. Furthermore, the changes in the glucose contents in treated coir and pith are not significant. The glucose content in the treated coir and pith increased by 1.11 and 1.21-fold times after pretreating it with [TEA][HSO4], respectively. Therefore, it was implied that glucose could be retained within the biomass if a diluted ionic liquid is utilized. After regeneration of cellulose using anti-solvent, residual sugars are found in the ionic liquid mixture [42]. Therefore, the ionic liquid performance during recycling was reduced. Henceforth, this study provides insights towards the retention of sugars within the biomass after pretreatment under dilute conditions of ionic liquid.
3.1.2 Solubilization of sugars, lignin, and sugar degradation compounds
To evaluate the effect of PIL pretreatment on the biomass fractionations, the solubilized solids (monosaccharides, furan compounds, and lignin derivative compounds) in the collected hydrolysate were analyzed. The monosaccharides and disaccharides identified in the liquid hydrolysate after pretreatment of coir and pith using [PyH][HSO4] and [TEA][HSO4] are shown in Fig. 3. The reducing sugar yields of 25.7% and 24% in liquid coir hydrolysate were collected after pretreatment by using [PyH][HSO4] and [TEA][HSO4], respectively. On the other hand, a reducing sugar yield of 44.21% and 42.67% was observed in pith hydrolysate collected after pretreatment by using [PyH][HSO4] and [TEA][HSO4], respectively. The solubilized sugars’ percentages in the hydrolysate were determined based on the obtained total reducing sugar (Fig. 3a and b). It was noted that the hydrolysate samples in both cases of coir pretreatments were abundant with xylose and followed by glucose. On the other hand, the hydrolysate of pith was abundant with glucose and followed by xylose. This observation provides an insight into the mechanism and effect of PIL on the compositions of coir and pith. Coconut coir is composed of hemicellulose in higher proportions compared to coconut pith. Therefore, it is implied that ionic liquid cleaves the glycosidic bonds (β-1,4-glycosidic bonds) between the xylose units in hemicellulose chains of coir. However, pith with lower hemicellulose has cellulose in higher proportion resulting in the attack of the cations and anions to cellulose chains and followed by hemicellulose chains.
The solubilization of xylose in coir hydrolysate obtained from [PyH][HSO4] pretreatment increased 1.55-fold times and followed by glucose (1.4-fold times) when compared to [TEA][HSO4]-pretreated sample. Similarly, the solubilization of glucose in pith hydrolysate increased 1.08-fold times after pretreated using [PyH][HSO4]. In contrast to coir studies, the solubilization of xylose increased 1.3-fold time for pretreated pith by using [TEA][HSO4]. Therefore, evaluating the results, the correlations between the [PyH][HSO4] pretreatment and sugar contents can be clearly understood. Though [PyH][HSO4] pretreatment resulted in higher solubilization of sugars present in biomass, it is necessary to determine the possibilities of PIL to solubilize the lignin component as well. The dissolution of lignin was studied based on the lignin monomers and their derivative compounds in the hydrolysate (Fig. 3c and d). The analysis of soluble lignin in the hydrolysate of coir and pith showed that ionic liquid could alter or modify the structure of lignin during pretreatment. In the case of coir and pith pretreated using PIL, p-hydroxybenzoic acid, p-hydroxybenzaldehyde, vanillic acid, vanillin, syringaldehyde, and catechol were identified. On the other hand, syringaldehyde and vanillin were not detected in pith hydrolysate after pretreatment. The lignin structure is composed of different compounds bonded by different chemical linkages [43].
These compounds are derivatives of monomer units (syringyl (S), guaiacyl (G), and hydroxyl (H)) [44]. The compounds, such as p-hydroxybenzoic acid and p-hydroxybenzaldehyde, are lignin derivatives of H units. Meanwhile, vanillic acid and vanillin are derivative compounds of G units, and syringaldehyde is a derivative compound of S units. A total soluble lignin of 0.96% and 1.5% was determined for pretreated coir by using [PyH][HSO4] and [TEA][HSO4], respectively. Meanwhile, 0.46% and 4.98% of soluble lignin were determined for pretreated pith by using [PyH][HSO4] and [TEA][HSO4], respectively. In comparison to [PyH][HSO4], maximum solubilization of lignin was achieved when samples were pretreated using [TEA][HSO4]. During pretreatment, the β-O-4 aryl ether bond is cleaved, causing the production of lignin monomers and their derivative compounds. Dutta et al. reported that pretreatment with 90% w/w of [TEA][HSO4] decreased the β-aryl ether bond present in the kraft lignin. Furthermore, maximum recondensation and dehydration pathways were also observed [45]. In another study, 84% delignification was achieved after pretreatment of sugarcane bagasse by using 80% w/w of [TEA][HSO4] [46]. Although the concentrations of [TEA][HSO4] in the present study are low compared to literature, solubilization of lignin in a lower amount was achieved during the pretreatment.
The formation of furan compounds (furfural and 5-hydroxymethylfurfural) during ionic liquid pretreatment was also evaluated to select effective PIL (Fig. 3e and f). The formations of furans were higher in [TEA][HSO4]-pretreated sample compared to [PyH][HSO4]-pretreated sample in both pith and coir, although the difference was small. The degradation of sugar (C6 and C5) increased 1.8 and 1.6-fold times after pretreatment of coir and pith using [TEA][HSO4]. Meanwhile, lower contents of furan compounds were observed in [PyH][HSO4]-pretreated biomass. Though PIL pretreatment resulted in the production of furan compounds, the amounts are lower than the other pretreatment techniques. These furan compounds inhibit the activity of enzymes and yeast during the saccharification and fermentation process. Furfural concentrations above 1000 and 2000 ppm can partially and completely inhibit the fermentation process, respectively [47]. In the present study, the concentrations of furans in coir and pith hydrolysates were less than 50 ppm. Since the concentration of inhibitory compounds is low, the detoxification of the hydrolysate might not be required. However, the solids need to be washed to remove furan, soluble lignin, and IL residues because these compounds can inhibit enzyme activity and lead to lower glucose yield [48]. Overall, this study highlights the importance of different PIL on solubilizing lignin and sugars. In addition, this study also provides insight into the formation of inhibitory compounds.
3.2 XRD analysis
To decipher the effect of PIL pretreatment on cellulose crystallinity (especially amorphous and crystalline cellulose), the XRD analysis was conducted on pretreated samples (Fig. 4). The degree of crystallinity and crystallinity index (CrI) are important parameters to determine the susceptibility of biomass to enzymatic saccharification. The crystalline and amorphous cellulose was studied at Bragg’s angle of 22.4° and 18.0°, respectively. The initial CrI values of the untreated coir and pith were observed to be 80% and 65%, respectively. The increases in CrI values of 1.037 and 1.34 fold were observed for [PyH][HSO4]-pretreated coir and pith, respectively. However, a 1.04-fold decrease in the CrI value of [TEA][HSO4]-pretreated coir was observed. The decrease in the CrI is due to the partial removal of hemicellulose and lignin during pretreatment. Partial solubilization of lignin was observed during pretreatment of coir using [TEA][HSO4] (Fig. 3). On the other hand, a 1.32-fold increase of CrI was observed for coconut pith pretreated with [TEA][HSO4], respectively.
The increase in the CrI value is attributed to the partial removal of lignin or hemicellulose from coconut coir and pith. The lignin layer was modified and removed during the pretreatment, thus exposing the cellulose layer. Hanny and Arief (2017) reported the changes in the CrI value during pretreatment of coconut coir dust using ionic liquid (IL), NaOH and IL + NaOH. The changes in the CrI values were attributed to the removal of hemicellulose/lignin and the transformation of crystalline cellulose to an amorphous state [49]. Another study reported similar findings for increasing the CrI value due to lignin solubilization and xylan removal [50]. The XRD results from this study showed that pretreatments with [PyH][HSO4] and [TEA][HSO4] had more effect on xylan and lignin in coconut pith compared to coconut coir. The increase in CrI value for pith compared to coir also correlated with the soluble lignin contents (Fig. 3). As observed, the amount of soluble lignin detected in IL-treated pith was higher compared to IL-treated coir. Furthermore, [TEA][HSO4]-treated pith displayed a higher phenol derivative compounds in hydrolysate compared to [PyH][HSO4] treated coir. Therefore, it was understood that pith treated with [TEA][HSO4] displayed a higher CrI value compared to treated coir.
3.3 FTIR analysis
The FTIR analysis was carried out to determine the changes in functional groups before and after pretreatment of coir and pith (Fig. 5). The transmittance peak at 847 cm−1 was ascribed to C-H bending vibration (out of plane) in lignin present in the untreated pith. However, this peak was not observed for pith pretreated with [PyH][HSO4]-treated sample. On the other hand, a shift in the transmittance peaks such as 847 cm−1 for [TEA][HSO4]-pretreated solid signified the change in the molecular weight of lignin. The peak at 1040 cm−1 and 1050 cm−1 is attributed to the C-O, C–C, and C–OH strong stretching vibration observed in cellulose, hemicellulose, and lignin. This peak was observed for both untreated and treated solids. However, the intensity of the transmittance peak differed for ionic liquid–treated solids. The transmittance band observed for hemicellulose in untreated pith attributed to the strong C = O stretching vibration in a carboxylic acid. However, the disappearance of this peak in treated solids signifies the solubilization of hemicellulose during pretreatment. On the other hand, the transmittance band 1530 cm−1 and 1525 cm−1 identified in the coir and pith sample signified the C = C stretching vibration in the lignin. This peak was observed for treated solids with different intensities. The reduction in the wavelength for treated solids reflected the change in the molecular weight of lignin or the change in lignin structure.
The peak at 1695 cm−1 for the coir sample was attributed to the stretching vibration of the C = O group in the aromatic structure of the lignin and carboxyl group (COO-) present in the hemicellulose. However, the shift in the peak for treated solids towards a lower wavelength signified the reducing the molecular weight of lignin and hemicellulose for treated solids. Another transmittance peak at 1745 cm−1 in pith ascribed to the stretching vibration of C = O present in the ester group of hemicellulose. The ester functional group observed for the hemicellulose is responsible for the linkages between hemicellulose and lignin. However, this peak was observed for [PyH][HSO4]-treated solids. This result signified that [PyH][HSO4] solubilizes the hemicellulose resulting in higher recovery of reducing sugar during pretreatment. However, this peak shifted to a lower wavelength for [TEA][HSO4]-treated coir caused by the breakage of the ester bonds. This released hemicellulose into the hydrolysate during pretreatment and also modified the structure of lignin. The peak at 3281 cm−1 and 3321 cm−1 for coir and pith samples corresponds to the stretching vibration of the hydroxyl group (OH) present in the cellulose chains.
3.4 Enzymatic saccharification of coconut coir and pith
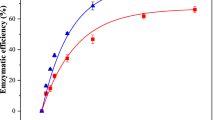
The glucose yield determined the efficiency of the pretreatment with a lower PIL concentration during the saccharification process (Fig. 6). The glucose yields obtained from pretreated coir and pith were higher than 70%. The increased accessibility of the enzyme to the internal structure of the cellulose chain enhanced the conversion of cellulose to glucose. On the other hand, lower yields in untreated samples could be due to the interference of the aromatic lignin and hemicellulose layers. This causes lower exposure of the enzyme to the internal structure of the cellulose fibrils [51]. The reducing sugar yields of [PyH][HSO4]-, and [TEA][HSO4]-treated coir increased 4.12 and 4.73-fold times, respectively. On the other hand, glucose yields of [PyH][HSO4]- and [TEA][HSO4]-treated pith samples increased 7.36 and 6.44-fold times, respectively. It can be observed that the yield of reducing sugar was higher for [TEA][HSO4]-treated coconut coir. On the other hand, the yield of reducing sugar was higher in [PyH][HSO4]-treated pith. Therefore, saccharification of PIL-treated coir and pith at lower concentrations of PILs resulted in reducing sugar yield of 60–85% wt. Furthermore, the glucose yield was correlated with the analysis of the CrI value obtained from the XRD analysis (Fig. 6c). A small effect of PIL pretreatment on the crystalline property of cellulose was observed. However, an increase in the yield during saccharification is attributed to the partial solubilization of hemicellulose and lignin during PIL pretreatment. The findings were compared with different literatures and are summarized in Table 4.
Brandt and Talbot reported the sugar yield of 77% obtained from pretreated Miscanthus x giganteus. The biomass was treated with 80% w/w [TEA][HSO4] at 120 °C for 480 min [39]. On the other hand, a glucose yield of 75% was achieved from treated softwood pine using 80% w/w [TEA][HSO4]. The reaction temperature and pretreatment time were maintained at 170 °C and 30 min [52]. The ionic liquid [TEA][HSO4] has proved to be efficient in the dissolution of cellulose during pretreatment at higher concentrations. However, a lower concentration of [TEA][HSO4] was employed in the present study to achieve maximum glucose yield during pretreatment with mild temperature and longer retention time. Recent developments in studies related to ionic liquid have focused on lowering the concentration of ionic liquid and maximizing the glucose yield during the saccharification process. Shi et al. reported a glucose yield of 81% during saccharification of pretreated switchgrass by using 10–20% w/w of [Emim][OAc] with reaction time and temperature maintained at 180 min and 160 °C (Shi et al., 2013). Similarly, Fu and Mazza demonstrated the significance of using ionic liquid at lower concentration during pretreatment. A glucose yield of 35%, 70%, and 90% was reported for pretreated triticale straw at various conditions, as displayed in Table 4 [53]. Similarly, another study reported a glucose yield of 83% from wheat bran after pretreatment with [Emim][OAc] (10% w/w, 40 min, 150 °C) [54]. Different insights provided by literature and comparing the same with the present study suggested that lower ionic liquid concentration can increase the sugar yield during saccharification. However, an increase in the reaction time is necessary to modify lignin structure and solubilization of hemicellulose.
3.5 Recovery and reusability studies
Recovery and reusability of the ionic liquids are required to improve the economics of the treatment process because ionic liquids are expensive. After pretreatment, the PILs were recycled and investigated for its efficiencies by considering the reducing sugar, furan, and soluble lignin yield (Fig. 7). In this work, the evaporation process was conducted to recover the used ionic liquids and reutilize the same for subsequent pretreatments. It was noted that the ionic liquid mixture was composed of reducing sugar, furan, and soluble lignin. However, the concentration of the by-products increased as the recycled ionic liquid was utilized for subsequent pretreatment of coir and pith. Therefore, it resulted in a decrease in ionic liquid efficiency (during the gradual increase of pretreatment cycles).
During the ionic liquid recycle process, the change in pH for each cycle was observed, and this represented the change in H+ ions during pretreatment. The pH of the first cycle was observed to be around 1.5 ± 0.2, and it increased to 3 ± 0.4 during the fourth cycle. This implied that in the accumulation of byproducts in the recycled IL, the pH was affected, leading to a decrease in the recovery of reducing sugar and soluble lignin. This observation was in agreement with other studies performed to evaluate the performance of ionic liquid during recycle and reusable studies. The effectiveness of [Emim][OAc] was reduced due to increased cycles of pretreatment after recycling and reuse of the ionic liquid [55]. Since the ionic liquid pretreatment efficiency depends on cations and anions, some cations or anions are affected due to the reuse and recycling procedure [56]. An overall higher yield of reducing sugar was observed for coir and pith pretreated with [TEA][HSO4]. After the third recycle, the ionic liquid mixture study gave promising results with maximum reducing sugar and soluble lignin recovery. On the other hand, higher soluble content and reducing sugar was observed for both coir and pith pretreated with [PyH][HSO4]. Therefore, it can be inferred from the results that [PyH][HSO4] and [TEA][HSO4] can be recovered, recycled, and reused up to the third cycle. The sugar in the hydrolysate during the ionic liquid pretreatment was difficult to be separated from the ionic liquid solution. Therefore, additional techniques are needed to recover sugars from the ionic liquid solution. Therefore, the sugars dissolved in the hydrolysate were considered a loss because the mixture of hydrolysate and ionic liquid is rich in furans and lignin compounds that inhibit the fermentation process [57].
Pretreatment of lignocellulosic biomass using low-concentrated PIL paves the way towards the cost-effective production of biofuel and biochemical products from sugars in biorefineries [58, 59]. The recovery and recycling of PIL could reduce the cost of the PIL to overcome the drawback related to the cost of ionic liquid. Low-concentrated PIL has the potential in pretreatment similar to the concentrated PIL. Further studies are required to understand the effect of low-concentrated PIL on the biomass with low and high lignin content. In addition, the effect of PIL (low and high concentration) on enzyme and yeast activity for sugar and ethanol yield, respectively, is yet to be investigated.
4 Conclusion
A low concentration of protic ionic liquid (PILs) was designed for fractionation of coir and pith by using [PyH][HSO4] and [TEA][HSO4], respectively. [PyH][HSO4] and [TEA][HSO4] showed the greater potential for partial lignin solubilization and hemicellulose recovery, respectively. During PIL pretreatment at low concentration, maximum cellulose was retained with the biomass. The glucose yield increased 4.12, 4.73, and 7.36, 6.44-fold during saccharification (72 h) of coir and pith pretreated with [PyH][HSO4] and [TEA][HSO4], respectively. The increase in glucose yield is attributed to the structure modification during PIL pretreatment. The findings were supported by the XRD diffractogram and FTIR spectrum obtained for the pretreated coir and pith. The recovery and reusability studies showed that PIL could be reused for three cycles. Using a low concentration of PILs during pretreatment provides a cost-effective way to produce biofuels and other biochemical products from coconut residues (coir and pith). The present study shows the possibilities for a new process design for IL-mediated biorefinery to achieve a low operational cost.
References
Kim D (2018) Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 23:309. https://doi.org/10.3390/molecules23020309
Ning P, Yang G, Hu L et al (2021) Recent advances in the valorization of plant biomass. Biotechnol Biofuels 14. https://doi.org/10.1186/s13068-021-01949-3
Sankaran R, Parra Cruz RA, Pakalapati H et al (2020) Recent advances in the pretreatment of microalgal and lignocellulosic biomass: a comprehensive review. Bioresour Technol 298:122476. https://doi.org/10.1016/j.biortech.2019.122476
Binod P, Gnansounou E, Sindhu R, Pandey A (2019) Enzymes for second generation biofuels: Recent developments and future perspectives. Bioresour Technol Reports 5:317–325. https://doi.org/10.1016/j.biteb.2018.06.005
Sun C, Ren H, Sun F et al (2022) Glycerol organosolv pretreatment can unlock lignocellulosic biomass for production of fermentable sugars: present situation and challenges. Bioresour Technol 344, https://doi.org/10.1016/j.biortech.2021.126264
Manfredi AP, Ballesteros I, Sáez F et al (2018) Integral process assessment of sugarcane agricultural crop residues conversion to ethanol. Bioresour Technol 260:241–247. https://doi.org/10.1016/j.biortech.2018.03.114
Arenas-Cárdenas P, López-López A, Moeller-Chávez GE, León-Becerril E (2017) Current pretreatments of lignocellulosic residues in the production of bioethanol. Waste and Biomass Valorization 8:161–181. https://doi.org/10.1007/s12649-016-9559-4
Chaturvedi S, Bhattacharya A, Khare SK (2018) Trends in oil production from oleaginous yeast using biomass: biotechnological potential and constraints. Appl Biochem Microbiol. https://doi.org/10.1134/S000368381804004X
Luo C, Brink DL, Blanch HW (2002) Identification of potential fermentation inhibitors in conversion of hybrid poplar hydrolyzate to ethanol. Biomass Bioenergy 22:125–138. https://doi.org/10.1016/S0961-9534(01)00061-7
Cheah WY, Sankaran R, Show PL et al (2020) Pretreatment methods for lignocellulosic biofuels production: current advances, challenges and future prospects. Biofuel Res J 7:1115–1127. https://doi.org/10.18331/BRJ2020.7.1.4
Chuetor S, Ruiz T, Barakat A et al (2021) Evaluation of rice straw biopowder from alkaline-mechanical pretreatment by hydro-textural approach. Bioresour Technol 323:124619. https://doi.org/10.1016/j.biortech.2020.124619
Tan HT, Lee KT, Mohamed AR (2011) Pretreatment of lignocellulosic palm biomass using a solvent-ionic liquid [BMIM]Cl for glucose recovery: an optimisation study using response surface methodology. Carbohydr Polym 83:1862–1868. https://doi.org/10.1016/j.carbpol.2010.10.052
Acharya S, Liyanage S, Abidi N et al (2021) Utilization of cellulose to its full potential: a review on cellulose dissolution, regeneration, and applications. Polymers (Basel) 13.https://doi.org/10.3390/polym13244344
Gundupalli MP, Tantayotai P, Anne Sahithi ST et al (2022) Ionic liquid assisted pretreatment to improve cellulose fractionation of lignocellulosic biomass. Ion Liq Technol Environ Sustain:75–99.https://doi.org/10.1016/b978-0-12-824545-3.00006-4
Nurdin M, Abimanyu H, Putriani H et al (2021) Optimization of OPEFB lignocellulose transformation process through ionic liquid [TEA][HSO4] based pretreatment. Sci Rep 11. https://doi.org/10.1038/s41598-021-90891-3
Saher S, Saleem H, Asim AM et al (2018) Pyridinium based ionic liquid: a pretreatment solvent and reaction medium for catalytic conversion of cellulose to total reducing sugars (TRS). J Mol Liq 272:330–336. https://doi.org/10.1016/j.molliq.2018.09.099
Rocha EGA, Pin TC, Rabelo SC, Costa AC (2017) Evaluation of the use of protic ionic liquids on biomass fractionation. Fuel. https://doi.org/10.1016/j.fuel.2017.06.014
Han SY, Park CW, Endo T et al (2020) Extrusion process to enhance the pretreatment effect of ionic liquid for improving enzymatic hydrolysis of lignocellulosic biomass. Wood Sci Technol 54:599–613. https://doi.org/10.1007/s00226-020-01170-9
Li C, Tanjore D, He W et al (2013) Scale-up and evaluation of high solid ionic liquid pretreatment and enzymatic hydrolysis of switchgrass. Biotechnol Biofuels 6. https://doi.org/10.1186/1754-6834-6-154
N, Liu H, Sathitsuksanoh N et al (2013) Production and extraction of sugars from switchgrass hydrolyzed in ionic liquids. Biotechnol Biofuels 6. https://doi.org/10.1186/1754-6834-6-39
Lynam JG, Chow GI, Hyland PL, Coronella CJ (2016) Corn stover pretreatment by ionic liquid and glycerol mixtures with their density, viscosity, and thermogravimetric properties. ACS Sustain Chem Eng 4:3786–3793. https://doi.org/10.1021/acssuschemeng.6b00480
Gschwend FJV, Malaret F, Shinde S et al (2018) Rapid pretreatment of: miscanthus using the low-cost ionic liquid triethylammonium hydrogen sulfate at elevated temperatures. Green Chem 20:3486–3498. https://doi.org/10.1039/c8gc00837j
Mohammadi S, Abbasi M (2015) Design of ionic liquid sulfonic acid pyridinium hydrogen sulfate as an efficient, eco-friendly, and reusable catalyst for one-pot synthesis of highly functionalized tetrahydropyridines. Res Chem Intermed 41:8877–8890. https://doi.org/10.1007/s11164-015-1934-4
George A, Brandt A, Tran K et al (2015) Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. https://doi.org/10.1039/c4gc01208a
Asim AM, Uroos M, Naz S, Muhammad N (2021) Pyridinium protic ionic liquids: effective solvents for delignification of wheat straw. J Mol Liq 325:115013. https://doi.org/10.1016/j.molliq.2020.115013
Gundupalli MP, Bhattacharyya D (2021) Hydrothermal liquefaction of residues of Cocos nucifera (Coir and Pith) using subcritical water: process optimization and product characterization. Energy:121466. https://doi.org/10.1016/j.energy.2021.121466
Sangian HF, Kristian J, Rahma S et al (2015) Preparation of reducing sugar hydrolyzed from high-lignin coconut coir dust pretreated by the recycled ionic liquid [mmim][dmp] and combination with alkaline. Bull Chem React Eng Catal. https://doi.org/10.9767/bcrec.10.1.7058.8-22
Katinonkul W, Phuriragpitikhon J (2013) Pretreatment of corn husk and coconut husk using ionic liquid to enhance glucose recovery. Bulletin of Applied Sciences 2:26–34
Sluiter A, Hames B, Ruiz RO et al (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure 1617:1–16
Gundupalli MP, Bhattacharyya D (2019) Sequential acid hydrolysis and enzymatic saccharification of coconut coir for recovering reducing sugar: process evaluation and optimization. Bioresour Technol Reports 6:70–80. https://doi.org/10.1016/j.biteb.2019.01.015
Gundupalli MP, Kajiura H, Ishimizu T, Bhattacharyya D (2020) Alkaline hydrolysis of coconut pith: process optimization, enzymatic saccharification, and nitrobenzene oxidation of Kraft lignin. Biomass Convers Biorefinery 1–19. https://doi.org/10.1007/s13399-020-00890-z
Nielsen SS (2010) Determination of moisture content. Food Anal Lab Man:17–27.https://doi.org/10.1007/978-1-4419-1463-7_3
Sluiter A, Ruiz RO, Scarlata C et al (2004) Determination of extractives in biomass. Laboratory analytical procedure (LAP) 1617:1–9
Bano K, Jain A, Sarkar R, Panda TK (2020) Economically viable and efficient catalysts for esterification and cross aldol condensation reactions under mild conditions. ChemistrySelect 5:4470–4477. https://doi.org/10.1002/slct.202000252
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Maillard LC, Maillard LC, Maillard L, Maillard L (1912) Action des acides aminés sur les sucres: formation des mélanoïdines par voie méthodique. Comptes R Acad Sci (Paris) 154:66–68
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Semerci I, Ersan G (2021) Hornbeam pretreatment with protic ionic liquids: cation, particle size, biomass loading and recycling effects. Ind Crops Prod 159. https://doi.org/10.1016/j.indcrop.2020.113021
Brandt-Talbot A, Gschwend FJV, Fennell PS et al (2017) An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem 19:3078–3102. https://doi.org/10.1039/c7gc00705a
Chen M, Malaret F, Firth AEJ et al (2020) Design of a combined ionosolv-organosolv biomass fractionation process for biofuel production and high value-added lignin valorisation. Green Chem 22:5161–5178. https://doi.org/10.1039/d0gc01143f
Abouelela AR, Al Ghatta A, Verdía P et al (2021) Evaluating the role of water as a cosolvent and an antisolvent in [HSO4]-based protic ionic liquid pretreatment. ACS Sustain Chem Eng 9:10524–10536. https://doi.org/10.1021/acssuschemeng.1c02299
Feng D, Li L, Yang F et al (2011) Separation of ionic liquid [Mmim][DMP] and glucose from enzymatic hydrolysis mixture of cellulose using alumina column chromatography. Appl Microbiol Biotechnol 91:399–405. https://doi.org/10.1007/s00253-011-3263-x
Guadix-Montero S, Sankar M (2018) Review on catalytic cleavage of C-C inter-unit linkages in lignin model compounds: towards lignin depolymerisation. Top Catal 61:183–198. https://doi.org/10.1007/s11244-018-0909-2
Lu Y, Lu Y-C, Hu H-Q et al (2017) Structural characterization of lignin and its degradation products with spectroscopic methods. J Spectrosc 2017:1–15. https://doi.org/10.1155/2017/8951658
Dutta T, Isern NG, Sun J et al (2017) Survey of lignin-structure changes and depolymerization during ionic liquid pretreatment. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.7b02123
Nakasu PYS, Clarke CJ, Rabelo SC et al (2020) Interplay of acid–base ratio and recycling on the pretreatment performance of the protic ionic liquid monoethanolammonium acetate. ACS Sustain Chem Eng 8:7952–7961. https://doi.org/10.1021/acssuschemeng.0c01311
Banerjee N, Bhatnagar R, Viswanathan L (1981) Inhibition of glycolysis by furfural in Saccharomyces cerevisiae. Eur J Appl Microbiol Biotechnol 11:226–228. https://doi.org/10.1007/BF00505872
Ladeira Ázar RIS, Bordignon-Junior SE, Laufer C et al (2020) Effect of lignin content on cellulolytic saccharification of liquid hot water pretreated sugarcane bagasse. Molecules 25:623. https://doi.org/10.3390/molecules25030623
Sangian HF, Widjaja A (2017) Effect of pretreatment method on structural changes of coconut coir dust. BioResources 12:8030–8046. https://doi.org/10.15376/biores.12.4.8030-8046
Kundu C, Samudrala SP, Kibria MA, Bhattacharya S (2021) One-step peracetic acid pretreatment of hardwood and softwood biomass for platform chemicals production. Sci Rep 11. https://doi.org/10.1038/s41598-021-90667-9
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26. https://doi.org/10.1007/s00253-004-1642-2
Gschwend FJV, Chambon CL, Biedka M et al (2019) Quantitative glucose release from softwood after pretreatment with low-cost ionic liquids. Green Chem 21:692–703. https://doi.org/10.1039/C8GC02155D
Fu D, Mazza G (2011) Aqueous ionic liquid pretreatment of straw. Bioresour Technol 102:7008–7011. https://doi.org/10.1016/j.biortech.2011.04.049
Araya-Farias M, Husson E, Saavedra-Torrico J et al (2019) Wheat bran pretreatment by room temperature ionic liquid-water mixture: optimization of process conditions by pls-surface response design. Front Chem 7. https://doi.org/10.3389/fchem.2019.00585
Auxenfans T, Buchoux S, Larcher D et al (2014) Enzymatic saccharification and structural properties of industrial wood sawdust: recycled ionic liquids pretreatments. Energy Convers Manag 88:1094–1103. https://doi.org/10.1016/j.enconman.2014.04.027
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38:449–467. https://doi.org/10.1016/j.pecs.2012.03.002
Amezcua-Allieri MA, Sánchez Durán T, Aburto J (2017) Study of chemical and enzymatic hydrolysis of cellulosic material to obtain fermentable sugars. J Chem 2017. https://doi.org/10.1155/2017/5680105
Smuga-Kogut M, Kogut T, Markiewicz R, Słowik A (2021) Use of machine learning methods for predicting amount of bioethanol obtained from lignocellulosic biomass with the use of ionic liquids for pretreatment. Energies 14. https://doi.org/10.3390/en14010243
Gundupalli MP, Chuetor S, Cheenkachorn K et al (2021) Interferences of waxes on enzymatic saccharification and ethanol production from lignocellulose biomass. Bioengineering 8. https://doi.org/10.3390/bioengineering8110171
Funding
The Ministry of Human Resource Development (India) and the Department of Science and Technology (India) funded and supported this work under FAST and FIST programs, respectively. Malinee Sriariyanun was supported by King Mongkut’s University of Technology North Bangkok (Contract No. KMUTNB-FF-65–37).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gundupalli, M.P., Bano, K., Panda, T.K. et al. Understanding the effect of low-concentrated protic ionic liquids (PILs) on coconut (Cocos nucifera) residues. Biomass Conv. Bioref. 14, 3275–3291 (2024). https://doi.org/10.1007/s13399-022-02572-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02572-4