Abstract
Almond peel waste was collected, characterized, and then used as an adsorbent for removing malachite green (MG) dye from aqueous solutions. The environmental conditions of MG dye adsorption were pH, 2.5–10.5; almond peel dose, 0.25–1.5 g/L; initial MG concentration, 10–60 mg/L; and adsorption time, 0–180 min. These were optimized by using an artificial neural network (ANN) tool. At pH = 8.5, 96.1% of the MG dye could be removed using almond peels. According to the coefficient of determination results, the Langmuir isotherm proved to be the equation that best fit the data of the isotherm study. Furthermore, the kinetic study showed that the data on MG adsorption of the almond peel waste was consistent with the pseudo-second-order model. The ANN model was developed by using a three-layer, feed-forward network with an optimum architecture of 4:10:1. Sigmoid functions were employed in both inputs and hidden layers, as also those hidden in the output layers. The results indicated a high correlation value (R = 0.976) to predict the entire experimental dataset, which indicated the applicability of the ANN tool, to describe the MG adsorption data in a highly accurate manner. The important conclusion of this study, after comparison with other similar adsorbents used in the adsorption process of dye wastewater, revealed that almond peel waste is a cheap, recyclable, and effective adsorptive agent, thus a good alternative to remove dyes from aqueous solutions.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The huge quantity of industrial effluents containing organic pollutants, and their being efficiently treated prior to being pumped into the environment, has created immense interest among researchers [1]. One of the organic pollutants most detected in water bodies are dyes. In fact, these compounds are the major pollutants in wastewaters of textile, paper, leather, and cosmetic industries [1, 2]. On account of their non-biodegradable property and high toxicity to humans, wastewater containing dyes must go through efficient purification processes prior to discharge into the environment [3]. In addition, dyes can disturb aquatic life because of their ability to reduce the penetration of sunlight that is essential for the process of photosynthesis [4]. The malachite green (MG) dye is considered one among the synthetic azo dyes and is typically used in the dyeing process of paper, fabrics, clothes, and leather, in addition to being used as a raw material in the manufacture of paints and inks [5]. Moreover, MG dye is one of the antimicrobial medicines that is used in the fish breeding fields. Owing to its direct effect on human health because of its carcinogenic nature, the use of MG dye had been prohibited in many countries for nearly 2 decades [6, 7]. It is noteworthy that studies which discuss removing hazardous dyes from wastewater are among the priorities of scientific research in the field of environmental pollution treatment [8, 9].
Currently, several advanced treatment technologies are used for the purpose of treating dye-laden wastewater; the most efficient are adsorption membranes, flotation, advanced oxidation, and microbial degradation [10, 11]. Adsorption is said to be simple in design and operation, and an efficient, environment friendly, and cost-effective technology, for removal of different organic and inorganic pollutants [12]. Major mechanisms related to the removal of pollutants by adsorption include ion exchange, chemical reaction, electrostatic process, and bonding processes. The success of the adsorption treatment depends greatly on the characteristics of the adsorbent used, in terms of its ability to adsorb the target pollutants [13]. However, different materials have been applied as adsorptive agents to remove dye pollutants from contaminated solutions, such as activated carbon [14], sugarcane [15], alumina [16], ash particles [17], chitin [18], and bentonite [19]. On the other hand, the use of abundant adsorbents is important in large-scale wastewater treatments. In recent times, more attention has been given to adsorbents prepared from agricultural waste [8]. This is due to the exceptional properties that agricultural waste has, such as availability in large quantities, cheap price, ease of handling and use, and its effectiveness in the adsorption process [20, 21]. Almond is a plant that grows over an area of more than 122,000 hectares in northern Iran and has a high economic value in this country. In addition, the estimated amount of almond exported from Iran in 2020 was about 1% [22, 23]. The outer peel makes up about 35–75% of the weight of the almond fruit [24]. During the harvest season, large quantities of almond peel were generated and disposed of as agricultural waste.
To identify the relationship between the variables, the model that is used for the description of the adsorption process has to satisfy some conditions, such as the dependence of the evaluated parameters on the adsorption mechanism. Therefore, one of the effective tools for this approach is the artificial neural network (ANN), which could perform the mathematical functions for linear and nonlinear systems [25]. In general, the ANN tool can handle a large number of datasets and it also has the ability to detect all possible interactions between the dependent and independent variables. This tool has been commonly used in numerous studies for the optimization of experimental parameters that affect the water treatment process [25,26,27]. In reality, the ANN tool is a significant method that can be used to shorten the duration of the operation and reduce the total cost of the treatment.
To date, there are very few studies related to the ability of the almond peel to remove the MG dye. Moreover, there is no study that has examined the optimization of the environmental parameters of the MG dye adsorptions using the ANN tool [28]. Therefore, the objective of this research is to investigate the adsorptive performance of the almond peel waste, as a cheap and efficient adsorbent, and to remove MG molecules from aqueous solutions. The properties regarding the morphology and structure of the used adsorbent have been characterized by using advanced techniques. In addition, the adsorption performance of the almond peel for the removal of MG molecules has been examined at different values of the environmental parameters. The ANN tool has been used to optimize and predict the impact of the important environmental parameters on the MG adsorption efficiency. Ultimately, the reusability potential of the almond peels was tested through five successive cycles of MG adsorption.
2 Materials and methods
2.1 Materials
The required quantity of almond peels was collected from several forests in Iran, from the almond fruit harvest. The collected quantity was first cleaned with tap water to remove the dirt and then sun dried and crushed. Following that, it was ground using a planetary mill and sieved using 60-mesh and 200-mesh sieves, to obtain powdered almond peels with a particle size of: 75–250 µm. Finally, the powdered quantity was washed with tap water, thrice with deionized water, and then dried in a digital oven at 55 °C for 2 days. Eventually, the dried almond peels was placed in a desiccator for dehumidification, prior to its use in the adsorption experiments.
In addition, an analytical grade MG stock solution of properties listed in Table 1 was purchased from Merck (Germany). Deionized water was used to prepare and dilute the MG solution. Furthermore, the pH values of the used solutions were controlled by NaOH and HCl solutions (0.1 N).
2.2 Characterization
Field emission scanning electron microscopy (FESEM, ZEISS Sigma VP-500) was used for characterization of the surface properties. The specifications of the pore size and surface area of the almond peels were determined by the Brunauer–Emmett–Teller (BET) test, using Belsorp Mini II (Bel Japan Co.). In addition, Fourier transform infrared spectroscopy (FTIR) analysis (AVATAR 370 model) was applied, to determine the functional groups of an almond peel sample and to determine their involvement in the MG dye adsorption reaction.
2.3 Experiments of MG adsorption
The MG adsorption experiments were performed at a temperature of 25 ± 1 °C, using 500-mL conical flasks, filled with 200 mL of MG dye solution. The pH value of the MG solutions was controlled and adjusted by a pH meter (pH 765 m manufactured by the Calimatic Company of Germany) using 0.1 N NaOH and HCl. The working MG–almond peel solution was agitated at a speed of 300 rpm with a shaker machine (KS260 model manufactured by IK Germany) for 180 min. Accordingly, the adsorption capacity of the almond peels was determined at different environmental conditions: pH, 2.5–10.5; adsorbent dose, 0.25–1.5 g/L; initial MG concentration, 10–60 mg/L; and adsorption time, 0–180 min. These conditions were chosen based on the previous adsorption studies and the reported condition of most of the liquid waste generated from industries [29,30,31].
After inception of the limited time of the adsorption process, a 4 mL sample was taken from each flask. The samples were subjected to filtration using a Whatman 42, filter paper (15 mm), followed by centrifugation (Select-a-Fuge Bio Dynamics centrifuge) at 6000 rpm for 30 min, for solid–liquid separation. The initial and remaining MG concentrations were measured using a UV/Vis spectrophotometer at a wavelength of 616 nm. To obtain accurate results, all UV/Vis readings were measured in triplicate. The percentage of removal of MG by almond peels (%R) was calculated using Eq. (1).
where C0 and Ct are the MG dye concentrations (mg/L) in the solution at inception and after a specific time after inception of the adsorption process, respectively.
In addition, the adsorption capacity (qt) was measured using Eq. (2).
where M (g) is the weight of the almond peels used in the adsorption experiments and V (0.2 L) is the MG solution volume. It must be noted that at the equilibrium status, the concentration and adsorption capacity are denoted by Ce (mg/L) and qe (mg/g), respectively.
2.4 ANN model
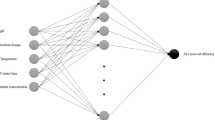
This model is based on the biological neural networks and is used to model and simulate the nonlinear and complex processes. The structure of the neural network includes neurons that are connected as input, hidden, and output layers. To minimize the error and to get accurate results, the neurons and hidden layers have been determined using a trial and error method [32]. As shown in Fig. 1, a three-layer, feed-forward ANN, with 4:10:1, architecture, was used to model the adsorption parameters of the MG dye using almond peels. In addition, the input variables selected in the present study were the initial pH, adsorbent dose, initial dye concentration, and adsorption time. The output variable or process response was the percentage of MG dye removal achieved via adsorption on the almond peels. Various architectures, with 2 to 20 neurons, were tested to compute the optimal number of neurons in the hidden layer of the ANN model. In the ANN analyses, the sum square error (SSE) statistical parameter was used as a loss function for training the ANN. Figure 2 shows the determined results of the SSE values versus the number of neurons in the hidden layer. Notably, the minimum error was obtained by selecting 10 neurons in the middle layer (SSE = 0.196). The hidden layer (fx) of the neural network used the sigmoid function.
The numbers from the 154 data were used for the training phase and 38 for the network evaluation. The data used for the evaluation phase were randomly selected as the initial data. As the Sigmoid function was used as an activation function for the hidden layer neurons, all data had to be in the range of 0.1 to 0.9. Therefore, all inputs (Xi) were converted to the new value (Ai), using Eq. (3).
To compare the experimental data with the one obtained by the ANN model, we converted these last data back to the range (0–100) using Eq. (3).
3 Results and discussion
3.1 Characterization analyses of almond peels
Figure 3a shows the FESEM micrographs (at k × 2000 magnification) of the almond peel sample before and after the MG adsorption. Clearly, the SEM figure before MG adsorption shows that the prepared almond peels are rough and highly porous adsorbents. The conclusion that can be drawn from this characterization is that almond peel is a good material for adsorbing elements. This conclusion must be considered from the BET results, which demonstrate that the specific surface area of almond peels and average diameter of the pores are equal to 49.52 m2/g and 59.283 nm, respectively. After MG adsorption, the MG molecules can be clearly noticed as bright dots on the almond peels.
The FTIR spectra of the almond peel before and after adsorption are illustrated in Fig. 3b. This study found that many functional groups occurring at 577, 701, 988, 1428, 1662, and 3467 cm−1 are responsible for the adsorption of MG molecules on almond peels. The FTIR spectrum of the almond peel sample shows a sharp peak at 3467 cm−1, due to OH group stretching, which in turn is due to the intermolecular hydrogen bonding of polymer compounds, including alcohol or phenol [33, 34]. The weak peak, close to 2918 cm−1, is attributed to the stretching vibrations of the C–H bond of the aliphatic group [33, 35]. The existence of a peak close to 1735 cm−1 is also attributed to the stretching vibration of C = O, which indicates a hemicellulose content. The sharp peak at 820 cm−1 confirms the presence of lignin in the structure of the almond peels [33, 36]. By comparing the FTIR spectra of almond peels before and after the adsorption process of MG molecules, it was found that the peaks of the functional groups present on the almond peels decreased significantly after the adsorbent was used. Such behavior can be attributed to the reaction between the functional groups and MG dye in the solution and illustrates the role of chemical reactions in the adsorption process.
3.2 Effect of initial pH
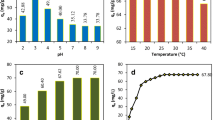
pH is one of the most important parameters that can change the efficiency of pollutant removal in adsorption treatment systems [37]. In the current study, the effect of pH has been tested at values of 2.5–10.5, under the following conditions: MG dye concentration, 30 mg/L; almond peels dose, 0.5 g/L; and adsorption time, 0–180 min. The results are plotted in Fig. 4a. In addition, Fig. 4b depicts the effects of pH on the removal efficiency, at equilibrium condition. The results of the pHpzc analysis are presented in Fig. 4c, according to which the pHpzc of the almond peel used is around 2.88. Thus, at pH below pHpzc (adsorbent zero point), the adsorbent charge is positive; therefore, the adsorbent and pollutant repel each other, decreasing the adsorption efficiency. However, at a pH higher than pHpzc, the dominant charge on the adsorbent surface will be negative, increasing the dye adsorption. Moreover, the pKa of the MG molecule is reported to be 6.9 [5, 6]. The results of this experiment indicate that an increase in the pH value has a positive effect on the efficiency of MG molecule removal. The maximum removal efficiency is determined at alkaline pH values. The findings can be explained as follows: At low pH, the surface charge of the adsorbent is positively charged because of the protonation of the active sites on the almond peel surface, which include polar groups like the hydroxyl, carboxyl, and phenolic groups [37]. Due to the cationic nature of malachite green, the positive surface charge created in the acidic medium reduces the amount of intermolecular interaction between the various polar surface factors of the MG dye. Therefore, there is a reduction in the amount of adsorbed MG molecules. Contrary to that, at pH > 7, due to the presence of the phenoxide and hydroxide groups, the density of the negative charges on the adsorbent surface increases. Thus, the electrostatic attraction increases between the positive ions of malachite green and the negative charges on the adsorbent surface. Consequently, the adsorption efficiency increases. As the dye undergoes restructuring at a pH value above 10, no significant changes in the adsorption rate are found in the pH range from 6.5 to 10.5. Thus, further experiments have been done with pH 6.5 as the optimum pH.
3.3 Effect of the almond peel dose
The adsorbent dosage is one of the significant parameters that strongly affects the adsorption process [38]. Therefore, the effect of the almond peel was examined in the current study by varying its dose from 0.25 to 1.5 g/L (conditions: MG dye concentration, 30 mg/L; pH, 6.5; and adsorption time, 0–180 min). According to Fig. 4d, an increase in almond peels increased the efficacy of MG molecule adsorption. These findings could be related to the increase in the number of sorption sites of almond peels needed for MG molecule adsorption [39]. As noticed, in the initial stages and due to the availability and vacancy of a large number of adsorption sites on almond peels, the adsorption process was fast and high. On reaching saturation, the adsorption speed decelerated gradually and stayed constant until it reached a steady state. This state was reached after filling a majority of the available sites, after about 60 min. A similar behavior was discussed by Lee et al. (2019) [40]. After equilibrium and by varying the amount of almond peel adsorbent used from 0.75 to 15 g/L, the efficiency of MG molecule removal obtained remained constant with a negligible difference of about 4 to 5%. Therefore, all experiments performed used 0.75 g/L of almond peels.
3.4 Effect of initial concentration
To evaluate the effect of the initial concentration on MG removal from an aqueous solution, the concentrations were varied from 10 to 60 mg/L in the following conditions: almond peels dose, 0.5 g/L; pH, 6.5; and adsorption time, 0–180 min) (Fig. 4e). The adsorption efficiency of the MG molecules decreased with the increase in MG concentration. One of the main reasons for this decrease was related to the limited and constant sorption capacity of the almond peels used. Thus, as the MG concentration increased, the capacity uptake of the used almond peels decreased [41]. Indeed, the main reason for the decrease in the adsorption efficiency following the increase in the initial concentration could be attributed to filling the sorption sites with adsorbent particles. According to Othmani et al., after equilibrium, the number of accessible sites of adsorption decreased, such that the MG molecules took a longer period of time to access the most inaccessible sites [37]. Similar findings were also obtained by Sharma et al. (2013), in their specific study on adsorption of MG dye by Timber Waste [42].
3.5 Effect of contact time
According to Fig. 4a, d, and e, it can be seen that the adsorption time has played a positive effect on the adsorption of MG onto the almond peel. An increase in contact time leads to an increase in MG removal efficiency, until it becomes saturated. The equilibrium was reached after 60 min, when most of the MG dye used was fixed onto the available sites of the almond peels.
3.6 Modeling of the isotherm and kinetics data
The isotherm experimental data were modeled based on the Langmuir, Freundlich, and Temkin models. In addition, the experimental data on the MG kinetics process were analyzed by utilizing the pseudo-first- and pseudo-second-order models. Furthermore, the kinetic data were modeled using the intraparticle diffusion kinetic model. For more information about the theoretical background of these four models, one can refer to the following references [29,30,31] [43]. The isotherm and kinetic model parameters are listed in Table 2. The statistical analyses using the coefficient of determination (R2), chi-square test (X2), and root mean square error (RMSE) showed that the isotherm and kinetics data were consistent with the Langmuir and pseudo-second-order models, respectively. Such data indicated that the MG dye molecules were adsorbed via chemical reactions onto the monolayer of the homogeneous active sites of almond peels [44]. From Table 2, the C value was observed to be > 0. This meant that the boundary layer diffusion might have been the rate-limiting step in the adsorption process of MG onto almond peels and not intraparticle diffusion [45].
3.7 Modeling of the adsorption of malachite green by ANN
Figure 5 shows output values (dye removal percentage) of the neural network model and experimental data. The obtained results show that the linear coefficient of determination for the whole data reached 0.976. Based on the modeling data, the ANN model can be successfully applied for the adsorption of MG, because of the almond peels. It may also well describe the dye removal rate for new inputs.
Figure 6 shows a bar graph of the relative effect of each variable of the input layer in the ANN tool. It is noted that all input variables have a significant effect on the response variable or on the percentage of dye removal, but none of them can be ignored. A comparative study of the experimental and theoretical findings has been carried out after studying the effects of pH, the almond peel dose used, and the adsorbent time for the adsorption of MG molecules, after having performed the process using the ANN model. The comparison has been done in compliance with the mean values obtained from the model. The results illustrated in Fig. 7A to C show a good correlation between the ANN model results and those of MG adsorption.
3.8 Comparative study and recyclability test
Table 3 presents the maximum uptake (qm) of almond peels and other previously reported adsorbents used for MG dye adsorption. Based on the values reached, it can be inferred that almond peels are superior adsorbents for the removal of MG molecules from aqueous solutions.
In addition, the recyclability of the used adsorbent was tested in the five adsorption–desorption cycles of MG. The condition of this experiment was fixed at the optimum values determined from the optimization study. The results are depicted in Fig. 8 showing that, compared to the former cycle a decrease in the removal efficiency of MG by the almond peels could be detected. In the first cycle, the removal efficiency was observed to be 85.2%, while it reached 82.00% in the fifth cycle. This small decrease in the removal efficiencies during five cycles of treatment is representative of the high reusability level of almond peels in the adsorption treatment of MG wastewater.
4 Conclusion
In the current study, the adsorption process of the MG dye on almond peel waste was comprehensively examined, using the ANN tool for optimization of the environmental conditions that had an effect on the adsorption mechanism. The characterization properties of the used adsorbent were determined by BET, FTIR, and FESEM analyses, which indicated that almond peels possess excellent morphological properties and functional groups. In addition, these analyses confirmed the adsorption phenomenon of MG dye molecules on used almond peels. The environmental conditions for MG molecule removal by almond peels were optimized by using the ANN tool. The findings showed that the maximum removal efficiency was 96.1% at the optimized conditions (pH, 8.5; almond peels dose, 0.75 g/L; MG concentration, 10 mg/L; and adsorption time, 60 min). A kinetics study showed that the experimental data fitted well with the pseudo-second-order model. Furthermore, the isothermal data were consistent with Langmuir model. The findings of this model revealed that the maximum adsorption capacity of almond peel waste for MG dye was 34.6 mg/g, which was much higher than what was obtained from other adsorbents used for the same purpose. The key finding of this study is that the ANN tool has high applicability to model the MG dye adsorption process, as a high correlation was determined between the determined results of ANN tool analyses and the experimental values of the MG molecule adsorption process.
Data availability
Not applicable.
References
Rasalingam S, Peng R, Koodali RT (2014) Removal of hazardous pollutants from wastewaters: applications of TiO2-SiO2 mixed oxide materials. Journal of Nanomaterials 2014
Zaharia C, Suteu D (2012) Textile organic dyes characteristics, polluting effects and separation/elimination procedures from industrial effluents a critical overview. In. https://doi.org/10.5772/32373
Samchetshabam G, Hussan A, Gon Choudhury T, Gita S, Soholars P, Hussan A (2017) Impact of textile dyes waste on aquatic environments and its treatment.
Ventura-Camargo B, Marin-Morales M (2013) Azo Dyes: characterization and toxicity– a review. Textiles and Light Industrial Science and Technology 2
Raval NP, Shah PU, Shah NK (2017) Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl Water Sci 7(7):3407–3445
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66(3):319–329
Fahmy A, El-Zomrawy A, Saeed AM, Sayed AZ, El-Arab MAE, Shehata H, Friedrich J (2020) Degradation of organic dye using plasma discharge: optimization, pH and energy. Plasma Research Express 2 (1):015009
Ben Arfi R, Karoui S, Mougin K, Ghorbal A (2017) Adsorptive removal of cationic and anionic dyes from aqueous solution by utilizing almond shell as bioadsorbent. Euro-Mediterranean Journal for Environmental Integration 2(1):20. https://doi.org/10.1007/s41207-017-0032-y
Arfi RB, Karoui S, Mougin K, Ghorbal A (2017) Tunisian almond shell for efficient removal of eriochrome black T and malachite green dyes from aqueous solution. Euro-Mediterranean Conference for Environmental Integration. Springer, pp 1383–1385
Rauf M, Ashraf S (2008) Radiation induced degradation of dyes-an overview. J Hazard Mater 166:6–16. https://doi.org/10.1016/j.jhazmat.2008.11.043
Hassaan MA, El Nemr A, Madkour FF (2017) Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. The Egyptian Journal of Aquatic Research 43(1):11–19. https://doi.org/10.1016/j.ejar.2016.09.006
Deng F, Luo X-B, Ding L, Luo S-L (2019) 5 - Application of nanomaterials and nanotechnology in the reutilization of metal ion from wastewater. In: Luo X, Deng F (eds) Nanomaterials for the Removal of Pollutants and Resource Reutilization. Elsevier, pp 149–178. https://doi.org/10.1016/B978-0-12-814837-2.00005-6
Lv, S.H., 7 - High-performance superplasticizer based on chitosan, in Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials, F. Pacheco-Torgal, et al., Editors. 2016, Woodhead Publishing. p. 131–150.
Yu M, Han Y, Li J, Wang L (2017) CO2-activated porous carbon derived from cattail biomass for removal of malachite green dye and application as supercapacitors. Chem Eng J 317:493–502. https://doi.org/10.1016/j.cej.2017.02.105
Mohamad M, Mohammad R, May T, Wei L (2019) Removal of malachite green by sugarcane bagasse biochar using response surface methodology, vol 2068. https://doi.org/10.1063/1.5089328
Kannan C, Sundaram T, Palvannan T (2008) Environmentally stable adsorbent of tetrahedral silica and non-tetrahedral alumina for removal and recovery of malachite green dye from aqueous solution. J Hazard Mater 157(1):137–145
Yin Y, Li C, Song C, Tao P, Sun M, Pan Z, Wang T, Shao M (2016) The design of coal-based carbon membrane coupled with the electric field and its application on the treatment of malachite green (MG) aqueous solution. Colloids Surf, A 506:629–636. https://doi.org/10.1016/j.colsurfa.2016.07.038
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209–210:218–225. https://doi.org/10.1016/j.jhazmat.2012.01.010
Bulut E, Özacar M, Şengil İA (2008) Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microporous Mesoporous Mater 115(3):234–246. https://doi.org/10.1016/j.micromeso.2008.01.039
Ani JU, Akpomie KG, Okoro UC, Aneke LE, Onukwuli OD, Ujam OT (2020) Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl Water Sci 10(2):69. https://doi.org/10.1007/s13201-020-1149-8
Bello OS, Owojuyigbe ES, Babatunde MA, Folaranmi FE (2017) Sustainable conversion of agro-wastes into useful adsorbents. Appl Water Sci 7(7):3561–3571. https://doi.org/10.1007/s13201-016-0494-0
Sorkheh K, Shiran B, Rouhi V, Asadi E, Jahanbazi H, Moradi H, Gradziel T, Martinez-Gomez P (2009) Phenotypic diversity within native Iranian almond (Prunus spp.) species and their breeding potential. Genet Resour Crop Evol 56:947–961. https://doi.org/10.1007/s10722-009-9413-7
Rahemi A The development of almond orchards in Iran. In: III International Symposium on Pistachios and Almonds 591, 2001. pp 177–180
Pirayesh H, Khazaeian A (2012) Using almond ( Prunus amygdalus L.) shell as a bio-waste resource in wood based composite. Composites Part B-engineering - COMPOS PART B-ENG 43. https://doi.org/10.1016/j.compositesb.2011.06.008
Ghaedi M, Zeinali N, Ghaedi AM, Teimuori M, Tashkhourian J (2014) Artificial neural network-genetic algorithm based optimization for the adsorption of methylene blue and brilliant green from aqueous solution by graphite oxide nanoparticle. Spectrochim Acta Part A Mol Biomol Spectrosc 125:264–277. https://doi.org/10.1016/j.saa.2013.12.082
Anupam K, Dutta S, Bhattacharjee C, Datta S (2016) Artificial neural network modelling for removal of chromium (VI) from wastewater using physisorption onto powdered activated carbon 57(8): 3632–3641.
Arghavan FS, Al-Musawi TJ, Allahyari E, Moslehi MH, Nasseh N, Hossein Panahi A (2021) Complete degradation of tamoxifen using FeNi3@SiO2@ZnO as a photocatalyst with UV light irradiation: a study on the degradation process and sensitivity analysis using ANN tool. Mater Sci Semicond Process 128:105725. https://doi.org/10.1016/j.mssp.2021.105725
Ozdes D, Gundogdu A, Duran C, Senturk HB (2010) Evaluation of adsorption characteristics of malachite green onto almond shell (Prunus dulcis). Sep Sci Technol 45(14):2076–2085. https://doi.org/10.1080/01496395.2010.504479
Al-Musawi TJ, Mahvi AH, Khatibi AD, Balarak D (2021) Effective adsorption of ciprofloxacin antibiotic using powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles. Journal of Porous Materialshttps://doi.org/10.1007/s10934-021-01039-7
Alwared AI, Al-Musawi TJ, Muhaisn LF, Mohammed AA (2021) The biosorption of reactive red dye onto orange peel waste: a study on the isotherm and kinetic processes and sensitivity analysis using the artificial neural network approach. Environ Sci Pollut Res 28(3):2848–2859. https://doi.org/10.1007/s11356-020-10613-6
Nasseh N, Al-Musawi TJ, Khosravi R, Hossein Panahi A, Arghavan FS, Barikbin B (2021) FeNi3@SiO2@CuS magnetic nanocomposite: synthesizing, characterization, and application for methylene blue adsorption. Desalin Water Treat 210:402–414
Pauletto P, Gonçalves J, Pinto L, Dotto G, Salau N (2020) Single and competitive dye adsorption onto chitosan–based hybrid hydrogels using artificial neural network modeling. J Colloid Interface Sci 560:722–729
Yahya MD, Abubakar H, Obayomi KS, Iyaka YA, Suleiman B (2020) Simultaneous and continuous biosorption of Cr and Cu (II) ions from industrial tannery effluent using almond shell in a fixed bed column. Results in Engineering 6:100113. https://doi.org/10.1016/j.rineng.2020.100113
Li X, Liu Y, Hao J, Wang W (2018) Study of almond shell characteristics Materials (Basel) 11(9):1782. https://doi.org/10.3390/ma11091782
Li X, Liu Y, Hao J, Wang W (2018) Study of almond shell characteristics Materials 11(9):1782
Trivedi, M., et al., Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. 2015.
Othmani A, Kesraoui A, Boada R, Seffen M, Valiente M (2019) Textile wastewater purification using an elaborated biosorbent hybrid material (luffa–cylindrica–zinc oxide) assisted by alternating current. Water 11 (7). https://doi.org/10.3390/w11071326
Farnane M, Machrouhi A, Elhalil A, Abdennouri M, Qourzal S, Tounsadi H, Barka N (2018) New sustainable biosorbent based on recycled deoiled carob seeds: optimization of heavy metals remediation. J Chem 2018:5748493. https://doi.org/10.1155/2018/5748493
Uddin MT, Rahman MA, Rukanuzzaman M, Islam MA (2017) A potential low cost adsorbent for the removal of cationic dyes from aqueous solutions. Appl Water Sci 7(6):2831–2842. https://doi.org/10.1007/s13201-017-0542-4
Lee S-L, Park J-H, Kim S-H, Kang S-W, Cho J-S, Jeon J-R, Lee Y-B, Seo D-C (2019) Sorption behavior of malachite green onto pristine lignin to evaluate the possibility as a dye adsorbent by lignin. Applied Biological Chemistry 62(1):37. https://doi.org/10.1186/s13765-019-0444-2
Ibrahim SM, Badawy AA, Essawy HA (2019) Improvement of dyes removal from aqueous solution by nanosized cobalt ferrite treated with humic acid during coprecipitation. Journal of Nanostructure in Chemistry 9(4):281–298. https://doi.org/10.1007/s40097-019-00318-9
Uma Y, Sharma U (2013) Removal of malachite green from aqueous solutions by adsorption on to timber waste 4:631–638
Belhachemi M, Addoun F (2011) Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl Water Sci 1(3):111–117. https://doi.org/10.1007/s13201-011-0014-1
Sartape AS, Mandhare AM, Jadhav VV, Raut PD, Anuse MA, Kolekar SS (2017) Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab J Chem 10:S3229–S3238. https://doi.org/10.1016/j.arabjc.2013.12.019
Wang C-J, Li Z, Jiang W-T (2011) Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals. Appl Clay Sci 53(4):723–728. https://doi.org/10.1016/j.clay.2011.06.014
Zhang J, Li Y, Zhang C, Jing Y (2008) Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root. J Hazard Mater 150(3):774–782. https://doi.org/10.1016/j.jhazmat.2007.05.036
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265(1):159–168. https://doi.org/10.1016/j.desal.2010.07.047
Santhi T, Manonmani S, Vasantha VS, Chang YT (2016) A new alternative adsorbent for the removal of cationic dyes from aqueous solution. Arab J Chem 9:S466–S474. https://doi.org/10.1016/j.arabjc.2011.06.004
Acknowledgements
The authors express their sincere gratitude to the Payame Noor University of Tehran, Birjand University of Medical Sciences and Al-Mustaqbal University college for financing and scientific supporting of the current study.
Funding
Birjand University of Medical Sciences has provided financial support for this research.
Author information
Authors and Affiliations
Contributions
N.N., conceptualization, methodology, and project administration; T.A.M., investigation, resources, and data curation; S.F.A., writing—original draft preparation, software, and validation; S.M.A.A and E.A, performed neural network analyzes; T.A.M and A.O., writing—review and editing, resources, and data curation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Musawi, T.J., Arghavan, S.M.A., Allahyari, E. et al. Adsorption of malachite green dye onto almond peel waste: a study focusing on application of the ANN approach for optimization of the effect of environmental parameters. Biomass Conv. Bioref. 13, 12073–12084 (2023). https://doi.org/10.1007/s13399-021-02174-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02174-6