Abstract
The present study demonstrates the maximum production of lipase via solid-state fermentation (SSF) by a novel strain of Chaetomium globosum, through lignocellulosic biomass valorization. After lignocellulosic biomass screening, Vachellia nilotica (babul) yielded the maximum lipase (53.1 ± 0.2 U/mL) activity after 72 h. Different bioprocess parameters, including pH, temperature, moisture content, inoculum size, and time periods, were optimized by the central composite design of response surface methodology (RSM). The maximum lipase yield (52.48 ± 5.3 U/mL) was achieved at pH 9.0, moisture 70%, incubation time 24 h, inoculum mass 1 mL, and temperature 30 °C. F-value 26.21 and p-value 0.00 by the analysis of variance indicate the significance of the proposed model. The coefficient of determination (R2) 95.60% was used to check the goodness of fit of the model, having a value of which indicates the model accuracy. Lipase was precipitated and purified with 80% ammonium sulfate followed by Sephadex G-100 column with 305.8 U/mg specific activity. The molecular weight of purified lipase was 60 kDa, estimated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The optimally purified lipase was immobilized on chitosan (natural biopolymer) using different lipase concentrations (0.5, 1, and 2 mL) to check the beads constancy. It was observed that lipase (0.5 mL) adsorbed on chitosan result in the highest activity compared to the free lipase. Chitosan-immobilized lipase showed high thermal stability at 40 °C for 5 h. It also exhibited stability for up to 7 reuse cycles. Furthermore, lipase showed its good effect in the detergent industry by demonstrating compatibility with the Sufi detergent brand. In conclusion, the recently produced lipase from the lignocellulosic biomass has a great potential to be used as an additive in the detergent industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lipases (E.C.3.1.1.3) belong to the structural superfamily of α/β hydrolases that mostly retain a conserved pentapeptide Gly-X-Ser-X-Gly motif having three amino acids in a common active site: an acidic glutamic or aspartic acid residue, a serine nucleophilic residue, and a histidine basic residue [1, 2]. They catalyze the ester bond hydrolysis of mono-, di-, and triglycerides into their fatty acids and glycerol. Due to their triglyceride hydrolysis capability at a lipid-water interface, they are characterized as triacylglycerol acyl hydrolases and differ from the closely related esterases. This phenomenon is known as the “interfacial activation” and was first recommended to discriminate lipases from esterases. Nowadays, lipase demands are increasing due to unique catalytic properties like alcoholysis, hydrolysis or synthesis of ester bonds, epoxidations, aminolysis, inter-esterifications, and peroxidations. Considering the rising demand for an array of applications, we selected lipase for the current study [3]. Being eco-friendly and nontoxic, it shows vast application prospects in different industries like food, cosmetics, flavor enhancement, biofuels, dairy, detergent, drugs, and biofuels. However, lower stability, high production and recovery cost, reuse, and recycling hinder its broad-spectrum implementation [4]. Growing trends in catalyst immobilization is a key tool to enhance the prospect of a massive and commercial enzyme [5].
Agricultural biomass is deliberated as one of the abundantly available renewable possessions on earth. Every year, the accumulation of biomass in large quantities affects the environment and destroys potentially valuable materials that could be refined and converted into various high-value products. Recently, increasing research interest has been directed towards the utilizing of agro-wastes, i.e., Vachellia nilotica (babul), Pisum sativum (peels), and Millettia pinata (sukhchain): Vachellia nilotica (babul) ratios (1:1) for the production of highly demanded detergent industry enzyme. These agricultural wastes contain huge amounts of nutrients, like nitrogen, carbon, minerals, and biomass residues, which act as a very suitable substrate to produce enzymes in an economically feasible way and solve the problem caused by their disposal in the environment [6, 7].
Lipases can be originated from many species, such as plants, animals, and microorganisms [1]. Microorganisms for lipase production have been found in different habitats, such as industrial wastes, contaminated soil with oil, vegetable oil processing factories, rotten food, coal tips, oilseeds, compost heaps, and hot springs [8]. Microbial sources, such as bacteria, fungi, yeast, and archaea, produce lipases with distinctive features capable of biotechnological applications [9]. Among all these microbes, filamentous fungi are considered an excellent choice/source for lipase production, because they are involved in the production of extracellular enzymes. Additionally, the fungal growth of hyphal mode and their low water activity tolerance make fungi enormously efficient in the bioconversion of solid substrates [10]. The solid-state fermentation (SSF) is an economically viable and practically acceptable technology because of the fermenter’s small size, reduced stirring, lower cost of sterilization, and reduced downstream processing. The SSF methods are considered suitable for enzyme production by filamentous fungi because they reproduce the existing natural environment of such fungi as compared to submerged fermentation having low productivity, high production cost, and complexity of the medium than SSF [3].
Pakistan is an agricultural country, and 50 to 60 million tons of agro-waste generated annually. The present study aimed to utilize agricultural waste biomass for isolation of lipase and optimize different parameters through the central composite design of RSM and to check the interaction between several variables (temperature, incubation days, pH, inoculum size, and moisture content). To the best of our knowledge, the current work is the first that reported valorization of Vachellia nilotica (babul) through SSF using novel strain Chaetomium globosum to produce lipase. Furthermore, the immobilization of lipase was done by chitosan to enhance their thermostability. Then, lipase was used with different detergent brands due to its compatibility in the detergent industry. This project will be beneficial to plan a cost-effective method for lipase production by the easily available substrate. Hence, the resulting lipase will be helpful for the detergent industry to be used as an additive.
2 Materials and methods
2.1 Agricultural waste and proximate analysis
All the analytical grade chemicals were used to conduct the present study. Agricultural wastes, including Vachellia nilotica (babul), Pisum sativum (peels), and Millettia pinata (sukhchain): Vachellia nilotica (babul) ratios (1:1), were collected from the indigenous zone of Gujrat, District of Pakistan. After sun drying, all these substrates were dried in an oven at 60 °C, pulverized into powder form (0.1 mm) and preserved. The proximate analysis was done by the AOAC method to check the nutritional contents of agricultural wastes [11].
2.2 Microorganism
The microbial strain Chaetomium globosum was obtained from the Department of Biochemistry and Biotechnology, University of Gujrat, Pakistan. The fungal cultures were grown and maintained on nutrient agar slants and kept at 4 °C for subsequent use [12].
2.3 Qualitative assay for lipase activity
The strain was grown on freshly prepared Potato Dextrose Agar (PDA) media along with the inducers (mustard oil, sesame oil, linseed oil, Tween 80) in Petri plates (100 × 15 mm) to explore its capability for lipase production.
2.4 Inoculum media
An inoculum medium was designed for the vegetative development of Chaetomium globosum. The medium comprises the following ingredients in a volume of 100 mL: glucose 2 g; (NH4)2SO4 0.05 g; CaCl2 0.05 g; KH2PO4·7H2O 0.02 g, and MgSO4·7H2O 0.05 g in an Erlenmeyer flask (250 mL) covered with a cotton plug. Inoculum media was sterilized at 121 °C, and 15 psi for 30 min. After the inoculation of fungus, all flasks were placed in a water bath at 37 °C for 72 h at 120 rpm [13].
2.5 Agro-waste screening and enzyme extraction
The 5 g of each agro-wastes (Vachellia nilotica (babul), Pisum sativum (peels), and Millettia pinata (sukhchain): Vachellia nilotica (babul) ratios (1:1)) were added in triplicate Erlenmeyer flasks (250 mL), moisturized with 5 mL distilled H2O followed by autoclaving. Three milliliters of freshly prepared inoculum was then added under the sterilized environment. The inoculated flasks were placed in the static incubator (Memmert, Germany) at 37 °C for 72 h. After a speculated time, the crude lipase extraction was done by adding 50 mL distilled H2O in all flasks by shaking for 60 min at 175 rpm and filtered through Whatman No.1 filter paper. All experimental crude was strained and centrifuged at 4000 rpm, 4 °C, for 10 min to obtain clear supernatant, which was used for lipase activity [13].
2.6 Lipase activity assay
Lipase activity was determined through 4-Nitrophenyl palmitate 4-NPP as a substrate. Precisely, 0.03 g of 4-NPP was added into 10 mL of 2-propanol, mixed with 90 mL of 5 mM phosphate buffer (NaH2PO4 0.047 g, Na2HPO4 1.249 g both dissolved in 500 mL distilled water at pH 8) containing 0.20 g of deoxycholic acid and 0.1 g of Arabic gum. Afterwards, 100 μL of the crude enzyme was added in 2 mL of the reaction mixture followed by incubation at 35 °C for 40 min. After the incubation process, the reaction was stopped by the addition of 3 mL of 2 M sodium carbonate (26.5 g of Na2CO3 in 250 mL distilled water), and absorbance was measured at 410 nm. One unit of lipase activity is defined as the amount of enzyme releasing 1 μmol of free p-nitrophenol per minute [14].
2.7 Optimization of various parameters by RSM
The enzyme displays diverse optimal activity on different physical, chemical, and nutritional parameters. After selection of strain and best substrate, we further optimized different parameters such as moisture content (40–80%), pH (4–10), incubation time (12–96 h), inoculum size (0.5–3 mL), and temperature (25–50 °C) with α = 0.5 by RSM through central composite design [15]. The Minitab (17 version) was used for the generation of 32 trials containing five factors.
2.8 Purification and SDS-PAGE
Crude lipase extract was purified up to a homogeneous level by (NH4)2SO4 precipitation and gel filtration chromatography (Sephadex G-100). The lipase was precipitated in pellet from the supernatant through the constant addition of (NH4)2SO4 to achieve 80% saturation with adequate stirring for 30 min at 4 °C. Then, centrifugation was done at 4 °C for 15 min at 4000 rpm [15]. The collected pellet was dissolved in phosphate buffer (pH 7, 10 mM) and placed in a dialysis bag (12,000 Da), dialyzed overnight with intermittent changes against the same buffer. Further purification was carried out by loading the concentrated sample in the Sephadex G-100 column. To check the protein contents of crude and purified lipase, bovine serum albumin (BSA) was used as a standard using the spectrophotometric method. SDS-PAGE was carried out with a discontinuous gel system (5% stacking or 12% resolving) gel to estimate lipase molecular weight by method of Laemmli [16]. Molecular markers (Thermo Scientific protein ladder) having known molecular weight were used for comparison of unknown molecular weight of lipase.
2.9 Lipase immobilization on chitosan beads
For immobilization, acetic acid solution 1% (v/v) was dissolved and melts the chitosan in powder form by orbital shaker with shaking continuously at 120 rpm and 60 °C. The beads formation was checked via varying chitosan concentrations (3, 4, and 5%). Different lipase concentrations (0.5 mL, 1 mL, and 2 mL) were mixed with 2 mL of chitosan solution, and the suspension was drop-wise added into 100 mL of 1 M potassium hydroxide (KOH) buffer. Then, beads were leftover for 240 min at room temperature to become mature. The beads filtration and collection process was done by washing with a potassium-phosphate buffer (pH 7.0) and dried at normal lab temperature. The control of chitosan beads was prepared without adding the free lipase [17].
2.10 Effect of temperature on free and chitosan-immobilized enzyme and reusability
The thermostability was checked by determining the lipase activity after incubating at a different range of temperatures (25–45 °C) for 5 h without the addition of substrate. After testing the preliminary activity of immobilized lipase, it was subjected to multiple cycles to assess recycling efficiency.
2.11 Detergent compatibility
Different indigenous detergent trademarks like Sufi (Sufi group’s detergent and soup Pakistan), Ariel (Procter and Gamble), Surf excel (Unilever, Pakistan), Brite (Colgate Palmolive, Pakistan), and Bonus (Colgate Palmolive, Pakistan) were used to check the lipase compatibility. A solution was prepared by dissolving 7 g of detergent into 100 mL of distilled water laterally boiled at 95 °C for 10 min. A reaction mixture containing 2.5 mL of the substrate, 1 mL of lipase, and 2.5 mL of every detergent solution was incubated for 10 min at 30 °C followed by standard lipase assay. A control was prepared in which 1 mL distilled water was added in the abovementioned protocol of reaction mixture in the place of lipase.
2.12 Statistical analysis
All the experiments were performed in triplicate. The means and standard errors of means (mean ± S.E) were calculated for each treatment and used to draw the figures using MS excel and Minitab version 17.
3 Results and discussion
3.1 Identification of strain
The strain was grown on sterile Petri plates, which contains Potato Dextrose Agar (PDA) media. After their growth, the strain was identified based on their morphology and macroscopic or microscopic appearance. Their growth rate is very fast, and the colony pattern is cottony. Initially, the color of the surface colony was white and became brown when colonies are matured. Their hyphae are hyaline to light brown. There are appearances of green color around the colonies that showed its resemblance with Chaetomium globosum as shown in Fig. 1.
3.2 Qualitative assay for lipase activity
A fungus was tested for lipase production by the hydrolysis of inducers. It is a qualitative type agar plate assay to check the capability of strain for lipase production. After the incubation time, a lipolysis zone (clear zone) was observed around fungal colonies. Fungus showed a clear zone around the growth to release the lipase that causes cleavage of inducers present in oil and Tween 80 in the medium. Results showed that Tween 80 (10 ± 0.3 mm) yielded the maximum hydrolysis zone followed by linseed oil, mustard oil, and sesame oil in Table 1. Our findings show similarity with Lanka and abd [18], who reported Tween 80 as the best inducer for lipase production. Various inducers like Tween 80, Olive oil, Tween 20, and Tributyrin have been used by many researchers like Bharathi and Rajalakshmi [19].
3.3 Screening of substrates
Different substrates, like Vachellia nilotica (babul), Pisum sativum (peels), and Millettia pinata (sukhchain): Vachellia nilotica (babul) ratios (1:1), were cultured with Chaetomium globosum in fermented media for 72 h for lipase production. After the incubation time, all samples were analyzed individually for the activity of lipase. The maximum lipase activity (53.1 ± 0.2 U/mL) was observed by Vachellia nilotica (babul) as a substrate followed by Millettia pinata (sukhchain): Vachellia nilotica (babul) (1:1) and Pisum sativum (peels) as presented in Fig. 2. Our results show similarity with Nadeem et al. [17], who reported that after the screening of different substrates, Vachellia nilotica, Pisum sativum, Millettia pinata: Vachellia nilotica (1:1), Vachellia nilotica furnished the maximum activity. It has also been reported that substrates like apple pomace, beans, corn steep dry, coffee pulp, cane bagasse, lemon peel, rice husk, and wheat bran were also used for lipase production [20]. Table 2 shows the results of the proximate analysis of different agro-wastes based on dry weight. The crude fat of babul was 18.9 ± 0.3%, indicating it as an appropriate substrate for lipase production.
3.4 Optimization of parameters by RSM
The composition of media largely influences the enzymatic production by microbes. The enzyme production in optimized and low-cost media on a large scale is very significant from the commercial perspective. Agricultural waste is the rich source of lignocellulosic biomass and can be used as the cheapest raw source to produce industrially relevant chemicals and enzymes. Different parameters optimized by the central composite design of RSM showed the maximum lipase activity (52.48 ± 5.3 U/mL) at pH 9, time period 24 h, temperature 30 °C, moisture 70%, and inoculum size of 1 mL as shown in Table 3. The variance analysis showed in Table 4 described that the suggested model having F-value of 11.95 and p-value 0.00 shows its significance. The model goodness was determined by the coefficient of determination (R2), displaying the value 95.60% was used, which indicated the model accuracy. It designated that the planned model for lipase optimization did not predict 4.4% variation. From the ANOVA table, it is determined that the main effects of factor A (moisture content p value = 0.010), factor B (incubation time p-value 0.000), and factor D (temperature p-value = 0.021) are significant. The effects of factor C (inoculum size p value = 0.782) and factor E (pH p value = 0.813) were considered insignificant. The interaction effects of all possible combinations are shown in Table 4. The regression equation is presented as:
Figure 3 shows that the predicted and observed values were almost similar, and lipase production increased up to the optimum level, but after optimum conditions, their production started to decline. The contour plots of RSM show the relationships among different variables like incubation time, moisture, inoculum size, temperature, and pH for lipase production, as shown in Fig. 4. These contour plots were constructed by selected two independent variables, and the values of other variables were remained fixed to attain optimum conditions for the maximum lipase yield. The different colors in plots identified different levels of lipase production. Figure 5 presents the desirability chart for lipase production by Chaetomium globosum through solid-state fermentation. These charts showed that if the value of factor A is 73.53, factor B is 12.0, factor C is 0.50, factor D is 25.0, and factor E is 9.33, then the maximum predicted yield would be 59.74 U/mL, which was very close to the experimental values ensuring the validation of model prediction.
Different pH values, i.e., 4, 5, 6, 7, 8, 9, and 10, were designed by RSM and supplied in the SSF medium for the lipase production. Values of different pH were adjusted in distilled water and then provided moisture to the fermented medium. The medium fermented with the Vachellia nilotica gives the highest activity at pH 9.0 for lipase production. Results clarify that this lipase was alkaline in nature and enzyme activity increased continually with a rise in pH of the fermented medium. The maximum enzyme activity in the alkaline pH can be recognized as the enhanced binding of the enzyme to its substrate. Meanwhile, the pH greatly decides the enzyme binding to its substrate [21]. Our result shows similarity with Mesophilic lipase from Pseudomonas fluorescens JCM5963 and the cold-adapted lipase from Psychrobacter sp. 7195, whose maximum activity is also reported at pH 9.0 [22]. Devi et al. [23] reported that lipase produced from Pseudomonas guariconesis registered the maximum activity at pH 9.0 after optimization by different statistical design experiments.
Optimum temperature is significant for the maximum lipase production. Results indicate the highest lipase activity at 30 °C. If the temperature rises beyond its optimal value, it will impede the growth and development of microorganisms and reduce the final product yield, so maintaining temperature should be necessary to attain the maximum results [17]. Azevedo et al. [24] reported that a temperature range from 28 to 32 °C is ideal for fungal lipase production. Lipase produced from Pseudomonas guariconesis has shown the maximum growth at 30 °C after optimization by different statistical designs (Plackett–Burman followed by central composite design) [23]. Behera et al. [25] reported that lipase produced from Staphylococcus hominis followed by optimization with RSM results in the maximum activity at 30 °C.
The incubation period is an imperative factor for microbial growth in the fermented medium to express its maximum activity and enzymes titer. The maximum lipase activity (52.48 ± 5.3 U/mL) was obtained from agro-waste in the fermented medium after 24 h. Beyond the optimum time, the fungus growth and lipase activity start declining due to lessened accessibility of available nutrients and generation of harmful metabolites [26]. Results showed that after the 1-day incubation, the activity of the enzyme goes towards decline. Further increase in the incubation period causes decreased production of enzymes. It also depends upon the microorganisms, nature of the fermented medium, availability of nutrients, and physiological situations. Studies have also revealed that the production of enzymes is influenced by incubation time. Mostly lipase activity was initiated within 24 h, but it was possible that quantitatively lipase activity was enhanced until 72 h [27].
Different levels of moisture content (40, 50, 60, 70, 80) were designed by RSM. Results showed that the maximum yield (52.48 ± 5.3 U/mL) was observed at 70% moisture content. But trials carried out above this level decreased the lipase activity. Our results are in accordance with Gutarra et al. [28], who reported that the lipase produced from P. simplicissimum provided the highest yield at 70% moisture content. According to Gutarra et al. [28], a rise in moisture level beyond its optimal level results in a reduction in the release of microbial metabolites in fermented media because high levels of moisture cause accumulation of media components and inefficient transfer of oxygen.
Different inoculum sizes for lipase, i.e., 0.5, 1, 3, 5, and 7 mL (1 × 107 spores in each mL), were designed by RSM. The maximum lipase activity (52.48 ± 5.3 U/mL) was obtained by adding 1 mL of inoculum. A reduction in the lipase activity at large inoculum size is due to the quick depletion of the available nutrients. A higher density of inoculum increases the H2O content, thus decreasing the aeration process in solid substrate medium and inhibiting fungus and enzyme production. Optimal inoculum size has a significant role in the fermentation process; meanwhile, the aggregation of spores can retard the progression and development of the microorganism [29]. Our findings show similarity with Suyanto et al. [30], who reported that the optimum lipase activity for Aspergillus niger was obtained at a spore concentration of 1 × 106 spore mL−1. Previous studies also demonstrated that 83.33% lipase activity was obtained by 1% (v/v) inoculum in addition to the production medium. The maximum lipase activity and biomass were observed at 1% (v/v) added bacterial seed culture, giving 55 U/mg of lipase activity and 8.5 Log cells/mL at 78 h, preceded by a small decline in cell number and activity [31].
3.5 Purification and SDS
The extract of cell free crude enzyme has an initial lipase activity of 118,357/180 mL along with the specific activity of 98.30 U/mg. This crude lipase was partially purified by 80% NH4SO4 precipitation followed by dialysis against the phosphate buffer for 24 h with periodic changes of buffer. After the dialysis, a rise in specific activity from 131.7 U/mg to 155.0 U/mg, along with an increase in purification fold from 1.34 to 1.57, was observed. The active lipase fraction was subsequently purified up to the homogeneity level using the Sephadex G-100 column along with the specific activity of 305.8 U/mg with a rise in 3.11 purification fold (Table 5). Our results show similarity with Sethi et al. [32] who reported that lipase produced from Aspergillus terreus was purified with 80% NH4SO4 precipitation followed by Sephadex G-100 with a purification fold of 2.56%. Okunwaye et al. [33] reported that lipase produced from Raphia mesocarp had purified with 80% NH4SO4 precipitation followed by gel filtration chromatography with a purification fold of 5.79%. The estimated molecular weight of lipase isolated from Chaetomium globosum was 60 kDa as shown in Fig. 6. Our results show similarity with Shu et al. [34] who reported that molecular weight of lipase 60 kDa isolated from Antrodia cinnamomea BCRC 35,396.
3.6 Lipase immobilization on chitosan biopolymer
Chitosan (3%, 4%, and 5%) in different amounts was tested to check the beads constancy. The result indicates that beads formation with supporting material was stabilized at 4%. Various lipase concentrations (0.5 mL, 1 mL, and 2 mL) were used to form beads with chitosan. It can be shown in the graph that lipase shows the maximum activity at 0.5 mL concentration, as presented in Fig. 7. When enzyme concentration increases, its activity goes towards decline. In the chitosan, immobilization enzyme was stuck to the chitosan (supporting material) by hydrogen and ionic bonding, van der Waals forces, and hydrophobic connections. Chitosan provides numerous benefits, including biodegradability, a greater affinity for proteins, biocompatibility, and harmlessness [35]. It has also been studied that the preparation and application of highly hydrophobic epoxy-alginate/chitosan acted as a support system for the immobilization of lipases from Thermomyces lanuginosus that is commercially available as Lipolase ® (TLL1). The covalent attachment of Lipolase ® was responsible for developing highly active biocatalysts and sixfold more stable in contrast to the free lipase [36].
3.7 Effect of temperature on free and CHI-immobilized lipase
Thermostability is one of the main issues in the usage of enzymes at an industrial scale. Results showed in Fig. 8a that free lipase shows stability at 30 °C but in temperatures above this range, enzyme activity tends to decline. Figure 8b displays that immobilization increase the thermostability of lipase, and lipase shows stability at 40 °C for 5 h. Immobilization altered the flexibility of the enzyme and changed into a more rigid form responsible for enhancing stability against thermal denaturation [17].
3.8 Immobilized lipase reusability in chitosan beads
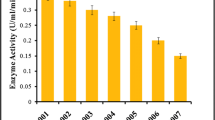
Enzyme stability is one of the important issues when usage is at an industrial level, and immobilization ensures their stability at high temperatures. The stability of immobilized enzymes devoid of their activity loss is significant from an economic perspective. Figure 9 displays the consequence of repetitive rounds on the chitosan-immobilized lipase activity; it has progressively declined after the 7 repeated cycles and later drops more sharply. It has been reported that retained activity of lipase immobilized on beads was 38% of chitosan after 10 times reuse. A significant reason for the loss of specific activity was due to protein leakage from chitosan beads because of poor adsorption of an enzyme that improved by the glutaraldehyde; thus, 65% of enzyme-specific activity was retained [37].
3.9 Detergent compatibility
Lipase was utilized to determine its compatibility with different detergent brands (locally offered) for their commercial use in the detergent industry. The detergent solution was placed in an incubator at 95 °C to eliminate any native enzymes in detergents. Results showed in Fig. 10 that lipase displays the maximum compatibility with Sufi 97% at 30 °C followed by Surf excel 80%, Ariel 70%, Bonus 64%, and Brite 50%. Lipases have good suitability with Sufi brand recommendations as an appropriate additive enzyme for detergents. Our results show similarity with Liu et al. [38], who reported that lipase produced from Fusarium solani N4-2 at alkaline pH 9 and the lowest temperature 30 °C are suitable additives for commercial detergents. Liu et al. [38] reported the highest activity with Ariel, followed by a White cat (commercial detergents).
4 Conclusion
Valorization of lignocellulosic biomass (renewable resources), which is responsible for landfilling and emission of lethal gases, is the best way to produce valuable products like enzymes. In the current research work, Chaetomium globosum revealed good potential for utilizing renewable resources as an inexpensive supportive material to produce lipase. Lipase was immobilized by natural supporting material (chitosan). Characterization of chitosan-immobilized lipase showed thermostability at 40 °C for 5 h. Presently produced lipase from Chaetomium globosum showed good compatibility with the Sufi brand exhibiting a practical approach in the detergent industry. Furthermore, immobilization increases the thermostability and recycling of enzymes. Thermostability is one of the emerging needs of industry, so immobilization fulfills this demand and improves economic revenue by decreasing the amounts of imported enzymes.
References
Mehta A, Bodh U, Gupta R (2018) Isolation of a novel lipase producing fungal isolate Aspergillus fumigatus and production optimization of enzyme. Biocat and Biotrans 36:450–457
Bilal M, Fernandes CD, Mehmood T, Nadeem F, Tabassam Q, Ferreira LFR (2021) Immobilized lipases-based nano-biocatalytic systems—a versatile platform with incredible biotechnological potential. Inter J Bio Macromole 175:108–122
Ramos-Sanchez LB, Cujilema-Quitio MC, Julian-Ricardo MC, Cordova J, Fickers P (2015) Fungal lipase production by solid-state fermentation. J Bioprocess Biotech 5:1
Javed S, Azeem F, Hussain S, Rasul I, Siddique MH, Riaz M, Afzal M, Kouser A, Nadeem H (2018) Bacterial lipases: a review on purification and characterization. Prog Biophy Molecu Bio 132:23–34
Anwar MZ, Kim DJ, Kumar A, Patel SK, Otari S, Mardina P, Jeong JH, Sohn JH, Kim JH, Park JT, Lee JK (2017) SnO 2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Scienti Rep 7:1–11
Amin M, Bhatti HN, Zuber M, Bhatti IA, Asgher M (2014) Potential use of agricultural wastes for the production of lipase by Aspergillus melleus under solid state fermentation. J Anim Plant Sci 24:1430–1437
Mehmood T, Nadeem F, Qamar SA, Bilal M, Iqbal HM (2021) Bioconversion of agro-industrial waste into value-added compounds. Sustainable Bioconversion of Waste to Value Added Products. Springer Nature Switzerland, pp 349–368
Chaturvedi S, Khare A (2016) Isolation and optimization for extracellular lipase using ground nut shell under submerged fermentation. Indo Ame J Pharma Res 6:4727–4732
Faiz S, Nasreen Z, Sha A, Naz S (2020) Isolation, screening and characterization of lipase from bacterial isolates and its application in detergents and oily waste water degradation. Pure App Bio 10:209–224
Duarte AWF, Bonugli-Santos RC, Duarte ALF, Gomes E, Sette LD (2021) Statistical experimental design applied to extracellular lipase production by the marine Antarctic yeast Leucosporidium scottii CRM 728. Biocatal Agri Biotech 32:101954
Saeed S, Tayyab M, Mehmood T, Awan AR, Firyal S, Nadeem F, Irfan M (2021) Valorization of potato peel for production of alginate and optimization of the process through response surface methodology (RSM) by using Azotobacter nigricans. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01357-5
Irshad M, Anwar Z, Mahmood Z, Aqil T, Mehmmod S, Nawaz H (2014) Bio-processing of agro-industrial waste orange peel for induced production of pectinase by Trichoderma viridi; its purification and characterization. Turk J Biochem 39:9–18
Mehmood T, Saman T, Asgher M, Irfan M, Anwar Z, Nadeem F, Siddiqa A (2019) Optimization of cultural parameters for pectin methylestrase and polygalacturonase production from Schizophyllum commune in solid state fermentation. Bangla J Bot 48:65–74
Boonmahome P, Mongkolthanarul W (2013) Lipase-producing bacterium and its enzyme characterization. J Life Sci Technol 1:196–200
Amin M, Bhatti HN, Sadaf S, Bilal M (2021) Enhancing lipase biosynthesis by Aspergillu Melleus and its biocatalytic potential for degradation of polyester Vylon-200. Cataly Letters 1-15. https://doi.org/10.1007/s10562-021-03603-x
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Nadeem F, Mehmood T, Naveed M, Shamas S, Saman T, Anwar Z (2019) Protease production from Cheotomium globusum through central composite design using agricultural wastes and its immobilization for industrial exploitation. Waste Biomass Valor 11:6529–6539
Lanka S, Trinkle BT (2017) Screening and isolation of lipase producing fungi from marine water obtained from Machilipatnam costal region. Inter J Pharmacog Phytochem Res 9:928–932
Bharathi D, Rajalakshmi G (2019) Microbial lipases: an overview of screening, production and purification. Biocatal Agricul Biotech 22:101368
Kumar DS, Ray S (2014) Fungal lipase production by solid state fermentation-an overview. J Anal Bioanal Tech 6:1
Munawar TM, Aruna K, Swamy A (2014) Production, purification and characterization of alkaline protease from agro industrial wastes by using Aspergillus terreus (AB661667) under solid state fermentation. Inter J Adv Res in Eng App Sci 3:12–23
Latip W, AbdRahman RNZR, Leow ATC, Shariff FM, Ali MSM (2016) Expression and characterization of thermotolerant lipase with broad pH profiles isolated from an Antarctic Pseudomonas sp strain AMS3. Peer J 4:e2420
Devi R, Nampoothiri KM, Sukumaran RK, Sindhu R, Arumugam M (2020) Lipase of Pseudomonas guariconesis as an additive in laundry detergents and transesterification biocatalysts. J Basic Microbio 60:112–125
de Azevedo WM, de Oliveira LFR, Alcantara MA, Cordeiro AMTDM, Damasceno KSFDSC, Assis CFD, Sousa Junior FCD (2020) Turning cacay butter and wheat bran into substrate for lipase production by Aspergillus terreus NRRL-255. Prepar Biochem Biotech 50:689–696
Behera AR, Veluppal A, Dutta K (2019) Optimization of physical parameters for enhanced production of lipase from Staphylococcus hominis using response surface methodology. Environ Sci Poll Res 26:34277–34284
Muthulakshmi C, Gomathi D, Kumar DG, Ravikumar G, Kalaiselvi M, Uma C (2011) Production, purification and characterization of protease by Aspergillus flavus under solid state fermentation. Jordan J Biol Sci 4:137–148
Vermelho AB, Supuran CT, Guisan JM (2012) Microbial enzyme: applications in industry and in bioremediation. Enz Res 2012:1–2
Gutarra ML, Cavalcanti ED, Castilho LR, Freire DM, Sant’Anna GL, (2005) Lipase production by solid-state fermentation. App Biochem Biotech 121:105–116
Koser S, Anwar Z, Iqbal Z, Anjum A, Aqil T, Mehmood S, Irshad M (2014) Utilization of Aspergillus oryzae to produce pectin lyase from various agro-industrial residues. J Radia Res App Sci 7:327–332
Suyanto E, Soetarto E, Cahyanto M (2019) Production and optimization of lipase by Aspergillus niger using coconut pulp waste in solid state fermentation. J Phy Conf Ser 1374:012005
Thakur V, Tewari R, Sharma R (2014) Evaluation of production parameters for maximum lipase production by P. stutzeri MTCC 5618 and scale-up in bioreactor. Chi J Bio 2014:1–14. https://doi.org/10.1155/2014/208462
Sethi BK, Nanda PK, Sahoo S (2016) Characterization of biotechnologically relevant extracellular lipase produced by Aspergillus terreus NCFT 4269.10. Braz J Microbio 47:143–149
Okunwaye T, Obibuzor J, Okogbenin E (2015) Purification and biochemical properties of lipase from raphia palm fruit mesocarp. Afri J Biochem Res 9:73–80
Shu CH, Xu CJ, Lin GC (2006) Purification and partial characterization of a lipase from Antrodia cinnamomea. Process Biochem 41:734–738
Dwevedi A (2016) Basics of enzyme immobilization, in Enzyme immobilization. Springer, Cham, pp 21–44
Mendes AA, de Castro HF, Andrade GS, Tardioli PW, de LC Giordano R, (2013) Preparation and application of epoxy–chitosan/alginate support in the immobilization of microbial lipases by covalent attachment. Rea Functio Poly 73:160–167
Gilani SL, Najafpour GD, Moghadamnia A, Kamaruddin AH (2016) Stability of immobilized porcine pancreas lipase on mesoporous chitosan beads: a comparative study. J Mole Catal B: Enzy 133:144–153
Liu R, Jiang X, Mou H, Guan H, Hwang H, Li X (2009) A novel low-temperature resistant alkaline lipase from a soda lake fungus strain Fusarium solani N4–2 for detergent formulation. Biochem Eng J 46:265–270
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nadeem, F., Mehmood, T., Anwar, Z. et al. Optimization of bioprocess steps through response surface methodology for the production of immobilized lipase using Chaetomium globosum via solid-state fermentation. Biomass Conv. Bioref. 13, 10539–10550 (2023). https://doi.org/10.1007/s13399-021-01752-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01752-y