Abstract
With the draining of petroleum derivatives, expanding natural contamination issues, there has been rising enthusiasm for the examination of lignocellulosic biomass for an alternative fsource of energy. Characterization of various biomass, its intermediate, and products is a need for conversion of any biomass to biofuels. Chemical composition of lignocellulosic biomass is an essential point for developing potent pretreatment technologies to break its rigid structure, conversion of sugar by different enzymes mainly cellulose to glucose and even various microorganisms which can ferment sugars into bioethanol and other value-added green chemicals. In this present review work, the main focus is on the proximate and ultimate analysis of different feedstocks, and altered pretreatment techniques such as physical, chemical, physicochemical, and biological methods for bioethanol production have been addressed, which ultimately will help in overcoming the recalcitrance of lignocellulosic biomass by degrading the lignin fraction, breaking down of lignocellulose components, hydrolysis, and fermentation process. Recently, combined pretreatment is gaining popularity as it is more favorable and profitable for improving chemical yield and process of enzymatic hydrolysis of LBs, but it increases the cost of operation. Acid pretreatment, steam explosion, and hydrothermal processes all together show a comparatively high effect on degrading hemicelluloses fraction. Alkali, oxidative, and organosolv pretreatment are more efficient in removing and degrading of lignin portion. This present study will empower a better idea and knowledge of the available process with the upcoming advanced processes which would help to overcome the limitations and establish technology to facilitate the pretreatment methods to make an authentic concept of biorefinery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
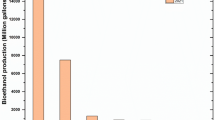
Now a rising shortage of energy is considered a global threat. Bioethanol is treated as the most influential renewable fuel which will compensate for fossil-derived fuels of petrol and diesel. Around the globe, bioethanol production has increased from 50 million m3 in 2007 to over 100 million m3 in 2012 [1]. The global bioethanol demand could very well exceed 125–130 billion liters by 2020, and global annual production of lignocellulosic biomass is about 181.5 billion tons, which is an abundantly available bioresource [2, 3]. Ethanol is mainly applied for engine fuel and fuel additive, with the most common varieties of available fuel mixes. It has the most suitable properties for spark ignition IC engines. Its Motor Octane Number (MON) and Research Octane Number (RON) are 90 and 109, respectively, whereas the regular gasoline bears MON and RON Value 91 and 99, respectively. Ethanol does not burn by compression ignition and not even easily miscible with diesel fuel because of the low octane number. To upgrade the utilization of ethanol in compression ignition (CI) engine vehicles, some initiatives have been taken, such as the addition of an emulsifier to intensify the ethanol-diesel miscibility. Incorporation of ethyl hexyl nitrate or diterbutyl peroxide is moreover used to enhance the octane number. The use of a dual fuel operation in which ethanol and diesel are introduced separately into the cylinder or the modification of diesel engines to adapt their characteristics of auto-ignition [4]. Production of biofuel most commonly as bioethanol is a current issue that begun 10 years ago by the China government to counterbalance the intensifying gap between crude oil consumption and quick economic growth which further decreases the domestic reserves and production. In the northeast of China in Jilin Province, the first ethanol plant was established in August 2003 where corn was studied as the valuable feedstock. Presently utilizing the starch-based feedstocks which include corn, wheat, and cassava, five well-known fuel ethanol producers produce 1.52 million tons of ethanol annually across the country. Due to the enormous population and accelerated urbanization, there is a decrease in cultivable land, and further, the current fuel ethanol production is not viable for the long-term process. Contrarily, China is the dominant and large-scale grain producer and abundant in agricultural residues which assemble about 600–700 million tons annually [1]. After all, there are no reasonable developed technologies for convenient conversion of biomass so most of the beneficial biomass is burnt by the farmers which ultimately causes environmental pollution and further interrupts the air by smoky clouds. [4]. In countries like Brazil and the USA, approximately 80% of bioethanol of the world supply mostly prefers corn or sugarcane. This creates a food vs. fuel all over the world. In Europe, bioethanol is produced from wheat (3.9 million tons), maize (4.1 million tons), sugar beet (12.1 million tons), barley (0.4 million tons), and rye (0.4 million tons). The bioethanol production capacity increased to about 8.5 billion liters per year in 2012, with an actual annual production of about 4.8 billion liters or 57% of the total capacity. Bioethanol in major countries and regions in 2019 is depicted by statistics. In that year, the USA produced about 15.8 billion gallons amount of bioethanol in the world, and Brazil ranked second generating nearly 8.6 billion gallons as shown in Table 1. (Published by M. Garside, Feb 26, 2020) In India, total agricultural lignocellulosic abundance biomass available in 2013 was estimated to be 382.7 million metric tons (MMT) annually which can be converted into 129.6 billion liters of ethanol as shown in Table 2 [5]. It has been recognized globally that agricultural residues and their by-products are most superior to compensate grains for ethanol production without threatening food security despite undergoing many challenges for the conversion of feedstock commercially due to their recalcitrance [6] inclusive of their exclusive chemical composition. Biomass is the most popular carbon-based feedstock that is obtained from plants and microorganisms which is also well known to be lignocellulose-based biomass that has become familiar biomass which significantly increases the bioethanol production and is, therefore, the theme of the present review paper. Primarily, plant cell walls are composed of the cellulosic component which contains structural carbohydrates like cellulose and hemicelluloses and heterogeneous polymer lignin as its primary components, and their contents fluctuate considerably which rely upon the different species, variety, climate, and soil fertility. For agricultural-based biomass collected from corn stover, wheat and rice straw, sugarcane bagasse, cotton stalk, etc., the cell walls constitute about 40% cellulose, 30% hemicellulose, and 15% lignin [7]. Due to cell division, there is a development of primary cell walls with crystalline cellulose microfibrils which are submerged firmly in a matrix of polysaccharides such as hemicelluloses. The adjacent cells are wrapped by a sticky layer known as middle lamellas which are mainly comprised of pectins which organize together into conducting tissue system which is put in order in numerous vascular bundles. A new secondary cell wall is gradually formed between the plasma membrane and the primary cell wall for the growth of mechanical strength and structural reinforcement mainly known as lignin which gives a better explanation of lignocellulosic biomass that can be further transformed into biofuels and beneficial chemicals [8]. The growth of the conducting tissue system with the rigid secondary cell wall plays a versatile role in the growth and development of crops and land plants which promotes the transport of water and nutrients along with wide growth, but it even elevates its recalcitrance to degradation due to cross-linking connection of three main components: cellulose, hemicellulose, and lignin [6]. Industrial ethanol commonly was used for alcoholic beverages and profusely used as biofuel, which is processed petrochemically via acid-catalyzed hydration of ethylene and further fermented where certain species of yeast (e.g., Saccharomyces cerevisiae) or bacteria (e.g., Zymomonas mobilis) metabolize sugars in oxygen-lean conditions to massively produce ethanol and carbon dioxide. Hexose sugars are more productively fermented to ethanol and carbon dioxide by species like Saccharomyces cerevisiae, than pentose sugars that are present in hemicelluloses [9]. However, many efforts are done in biotechnology for suggesting a way to overcome these hindrances leading to a promising harvest [10]. Many challenging perspectives for the commercial production of bioethanol are also highlighted in this review. The chemical composition of lignocellulosic biomass is a fundamental point for developing effective pretreatment to destroy its rigid structure, new enzymes required to release sugars mainly cellulose to glucose, and even different microorganisms to convert sugars into ethanol and other value-added chemicals. Here, bioethanol production from lignocellulosic biomass is considered mainly focusing on the characteristics of the feedstocks, significant pretreatment techniques, which helps in overcoming the recalcitrance of lignocellulosic biomass by degrading the lignin fraction and breaking down of lignocellulose components, enzymatic hydrolysis of the pretreated biomass, and fermentation of the pentose and hexose sugars after hydrolysis as shown in Fig. 1.
2 Different sources of lignocellulosic biomass
The lignocellulosic biomass is mainly available for energy purposes and mainly obtained from three sectors: (a) agriculture residues, (b) forest residues, and (c) industrial residues. It can be divided into several groups with their respective examples such as agricultural waste (leaves, stovers, straws, husk, pods, seeds, bagasse, roots, cobs, seedpods, solid cattle manure, etc.), forest biomass (softwood, hardwoods, cedar, spruce, willow, etc.), forest wastes (slashes, branches from dead trees, forest thinning, burning residues, sawdust, wood chips, etc.), industrial wastes (chemical pulps and primary wastewater solids, etc.), and municipal solid wastes (food waste, newspaper waste, kraft paper, and sorted refuse). Agricultural wastes and forest residues are considered the most favorable biomass feedstocks due to their abundance, availability, and comparatively low cost [11]. The potentiality and different prospects related to the energy of various biomass by-products and organic wastes rely upon the yield, the availability of total land area, and the kind of production. The widespread tertiary source, i.e., municipal solid waste (MSW), is not at least a significant source but a reasonable and economic source of biomass that covers almost all domestic and industrial trash that is gathered in a particular area [9]. Agro-residues are derivatives of agriculture that mainly include cotton stalks, wheat and rice straw, coconut shells, maize cobs, jute sticks, and rice husks [10]. The agricultural residues that are produced have a low density. Forestry residues cover biomass that is not harvested or removed from hardwood and softwood production or removal of dead and dying trees. Forestry waste comprises wood chips, sawdust, and bark which provide 65% of the biomass energy potential [7, 8]. Broadly, the sources can be divided into five categories such as agricultural wastes, industrial waste, forest and woody residues, and municipal solid wastes (MSW) as illustrated in Fig. 2.
3 Lignocellulosic biomass composition
Various lignocellulose biomasses have different physical appearance and strength, but all lignocellulosic biomass has a major composition of homopolymeric cellulose, heteropolymeric hemicellulose, and lignin (Table 3).
3.1 Cellulose
Cellulose is a crucial constituent that supports and makes stiff to the cell wall of the green plants. It is a polysaccharide or complex carbohydrates which are composed of linear glucose unit in the form of a long chain that is connected by β-1, 4-glycosidic bonds with repeated cellobiose residues (glucose-glucose dimer) as shown in Fig. 3. Due to different degrees of polymerization with varying resources, they are stacked into microfibrils and have a strong tendency to form both intra- and intermolecular hydrogen bonds by hydroxyl groups [12]. Most of the biomass contains 40–50% of cellulose molecules that are mostly held together by intermolecular hydrogen bonds, but the molecules even have a strong tendency to form intra- and intermolecular hydrogen bonds, and this specific tendency increases the rigidity of cellulose that make insoluble and resistant towards organic solvents [13].
3.2 Hemicellulose
Hemicellulose is the second most abundant polymer which is mainly composed of several heteropolymers by β-(1,4) linked backbone structure mostly comprised of pentose sugars (C5) such as xylose and arabinose, and hexose sugars (C6) includes repeated units of mannose, galactose, and glucose that have similar equatorial configuration at C1 and C4 as shown in Fig. 4 [14]. Hemicelluloses and celluloses have an almost similar structure which is advantageous from conformational homology which gives rise to a persistent non-covalent connection with cellulose microfibrils [15]. Hemicellulosic content in biomass is approximately 25–35% and with an average molecular weight of < 30,000. Cellulose and hemicellulose bind tightly with non-covalent attractions to the surface of each cellulose microfibril. Hemicelluloses were obtained through the intermediates in the biosynthesis of cellulose [13].
3.3 Lignin
Lignin is another component in lignocellulosic biomass which is mainly a cross-linked polymer, i.e., heterogeneous and hydrophobic. It is composed primarily of aromatic subunits, i.e., monolignol monomers such as p-coumaryl, coniferyl, and sinapyl alcohols as shown in Fig. 5 [5, 12, 16]. Lignin acts like an adhesive by filling the gap between the cellulose and hemicellulose complexion with the polymers. It is present in all types of plant biomass; thus, it is considered a by-product or residue in the bioethanol production process. Lignin is mostly composed of phenylpropane units (3 carbons attached with 6 carbon atom rings linked by ether bonds). This phenyl-propane indicated as 0, I, and II methoxyl groups attached to rings gives special structures I, II, and III. These groups depend on the plant source from which they are obtained. Structure I exists in plants (grasses) and structure II observed in wood (conifers), while structure III is seen in deciduous wood [13].
4 Characterization of different biomass
The thermo-chemical conversion system largely depends on the basic characteristic properties, e.g., moisture content, elemental composition and bulk density, particle size, and porosity. Moisture is the most influencing factor which diminishes the overall energy content of the fuel and hence reduces its thermal conversion efficiency [14]. Moisture content was reported to be in the range of 4.3–9.5 in wheat straw [17] nearly 8.3% in sugarcane stalk [17], and many researchers emphasized the importance of moisture content on the boiler design and operation which states that the higher moisture content reduces overall thermal efficiency and boiler output [14, 18].
The essential characteristic features of the residues that are important for analysis before designing a biomass conversion unit include proximate analysis (ash content, moisture content, volatile matter, and fixed carbon), ultimate analysis or elemental compositions (carbon, hydrogen, oxygen, sulfur, calcium, nitrogen, etc.), and heating value determination. Appropriate review of these properties consequently improves the overall plant efficiency. For characterization, the experimental phase includes proximate analysis, ultimate analysis, and heating value determination, and this analysis is conducted according to the available standard methods [14].
4.1 Proximate analysis
Proximate analysis is used for calculating different parameters that include moisture content, ash content, volatile matter, and fixed carbon. Moisture content is determined by a gravimetric method using a standard hot air oven [19]. Determination of ash content in various biomasses is determined according to the standard procedure of heating in the muffle furnace at 575 °C ± 25 °C. The volatile matter was determined by muffle furnace by heating the sample for 7 min at 925 °C ± 5 °C. Fixed carbon content is determined by the difference between the total compositions. High heating values are calculated using some correlation models based on the proximate and ultimate analysis. Different types of biomass have a different specific amount of moisture which directly affects their heating values [15, 19]. Proximate analysis of different biomass has been reviewed in Table 4. Some convenient standard methods for the determination of proximate analysis reviewed are mentioned below:
-
The standard method for moisture determination involves heating of 1 g biomass sample in a hot air oven to 105 ± 5 °C using the following equation.
Moisture (% M) = (A-B)/C × 100
ACrucible weight and the air-dried sample (g)
BCrucible Weight and oven-dried sample (g)
CSample weight (g)
-
Ash is defined as the weight of residue after complete burning of 1 g of the biomass at 575 ± 25 °C in a muffle furnace.
Ash (% A) = (A-B)/C × 100
ACrucible weight and oven-dried sample (g)
BCrucible weight and residue (g)
COven dried sample weight (g)
-
Volatile matter (% VM): It is termed as the weight loss due to heating of 1 g of biomass at 925 °C ± 5 °C in a furnace for 7 min.
Weight loss due to VM = Total loss of weight-loss due to moisture.
-
Fixed carbon (FC): The content of fixed carbon is determined by subtracting the sum of ash %, volatile matter, and % moisture from the total 100% composition.
FC100-(% A + % VM + % M)
Heating value
The heating or calorific value of any fuel is the amount of heat liberated under specific conditions of combustion which is a function of the fuel’s chemical composition [27]. The higher heating value (HHV) is the total amount of heat energy available in the fuel which also includes the energy contained in the water vapor in the exhaust gases. The lower heating value (LHV) does not include the energy embodied in the water vapor. A bomb calorimeter is used to determine the calorific value of fuel by combusting a known quantity of the fuel under constant volume in a bomb [18].
4.2 Ultimate analysis
This analysis is important for determining the elemental composition (C, N, H, S, O, etc.) of the biomass and is also useful for calculating their heating value. It was carried out by using a CHNS analyzer [15, 28]. The ultimate composition of various lignocellulosic feedstocks has been provided (Table 5).
4.3 Chemical characterization of lignocellulosic biomass
4.3.1 Lignin quantification method
According to Mafei et al. [36], the quantification of insoluble lignin, soluble lignin, and total lignin derived three different equations. For the quantification, 300 mg weight of sample was taken with 72% of 3 ml sulfuric acid at 30 °C for 60 min. After completion of 60 min, the treated sample was mixed with 79 ml of deionized water to makeup the volume, autoclaved for 60 min at 121 °C. After the cooling process, filter them (filtered paper should be dried previously at 105 °C for 1 h). The retained material was washed with 5 ml of deionized water (repeated for 2 times), and dry them up to reach their constant mass. This dried residue is called insoluble lignin [37, 38]. Quantification was done by the given equation:
where LI is the insoluble lignin content (g), mfilt+res is the mass dry sinterized filter containing insoluble lignin (g), and mfilt is the mass dry sinterized filter empty (g).
The amount of soluble lignin was calculated by taking absorbance measurement of filtrate in a UV-vis spectrophotometer. The concentration of soluble lignin was measured by considering the molar extinction coefficient of 105 L g−1 cm−1, which is the average of absorptivities presented by lignin models [37, 38]. The soluble and total lignin mass was calculated by Eqs. (1) and (2), respectively.
where Ls is the soluble lignin content (g), Ahid is the acid hydrolyzates absorbance (205 nm), f is the acid hydrolyzate dilution factor, LT is the total lignin content (%), and Mi is the fractions initial dry mass (g).
4.3.2 Carbohydrates quantification method
According to Mafei et al. [36], acid hydrolyzates were filtered and injected in liquid chromatography (HPLC). The chromatographic analysis should follow this five conditions: (a) BIO-RAD Aminex HPX-87H column (300 × 7.8 mm); (b) temperature at 60 °C; (c) H2SO4 5 mM as eluent (0.6 mL min − 1); (d) 20 μL sample volume; and (e) refractive index detector at 60 °C (Shimadzu, model RID-20A). All experiment conducted in triplicate and the results would give as average percentages with respective standard deviation.
The amount of cellulose, xylan, and arabinose side groups was calculated according to the given formula:
where C is the cellulose, xylan, arabinosyl, or acetyl groups content (%); Mf is the glucose, xylose, arabinose, or acetic acid mass (g); f is the hydrolysis factor for cellulose (0.9), xylan (0.88), arabinosyl groups (0.88), and acetyl groups (0.72); and Mi is the initial fractions dry mass (g).
Mendes et al. [39] determined that total carbohydrates operate with the same procedure as mentioned by Mafei et al., but there is a difference in the temperature value, i.e., 45 °C.
4.4 Determination of extractive methods
Methods for extractive determination were described by one standard procedure by using Soxhlet for 4 h during extraction with toluene-ethanol mixture. The extracted sample again followed 4-h ethanol extraction along with the hot water extraction of the previously extracted and moisture-free sample for 3 h. In second standard method, ethanol extractive has done after 24-h Soxhlet extraction process which is gravimetric determination of extractives. This method was found to be more applicable for agri-residues, soft and hardwood, and waste paper [40].
NREL established two extraction methods, in which the traditional standard method is consuming 24 h for each extraction step, and another upcoming method for extraction operated by using the (ASE) Dionex accelerated solvent extraction system at high temperature and high pressure which ultimately lower both extraction time and solvent use as compared to the traditional method [41].
The variation in the Soxhlet method is automated and accelerated Soxtec procedure lowering the extraction time about 75%. The main deviation compared to the traditional Soxhlet methods is that the thimble contained fewer samples and was absorbed in the boiling solvent [42, 43].
5 Pretreatment
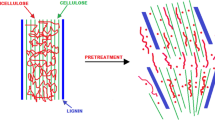
Lignocellulosic biomass is constituted of various types of carbohydrates and polymers which are compactly stuffed by distinct layers of cellulose, hemicelluloses, and lignin as shown in Fig. 6. Lignin layers protect them from degradation and enzymatic hydrolysis. Pretreatment is a crucial step that is necessary for the breakdown of the lignin layer by which affects cellulose and hemicellulose that can easily be extracted by enzymatic action. The pretreatment results decrease the crystallinity of cellulosic moiety and increase biomass surface area that ultimately decomposes hemicellulose [44]. It formulates cellulose more available to enzymes so that carbohydrate polymers are easily converted into fermentable sugars rapidly with high yield. Pretreatment is a combination of different types of treatment for further analysis and is a valuable step in which cellulosic biomass converts into fermentable sugar [45]. The pretreatment methods can be broadly divided into four different categories such as physical, chemical, physicochemical, and biological [46] (Fig. 7).
5.1 Types of pretreatment
5.1.1 Physical pretreatment
Approachable surface area, size, and pores will increase and the crystallinity properties. The physical pretreatment diminishes the degrees of polymerization of cellulose. In common physical treatments, feedstock degrades its lignocellulosic residue by the applications of steaming, grinding, milling, irradiation, temperature, and pressure (Table 5).
Grinding and milling
Grinding and milling is the primary pretreatment method, in which any biomass can be reduced in its particle size and crystallinity properties. For glucose recovery and energy-saving process, grinding of rice straw gives better results in wet disk milling than the ball milling process [47]. Based on the different biomass, improvement in pretreatment methods enables enzymatic saccharification, e.g., ball milling, roll milling, and wet disk milling. In rice straw pretreatment, ball milling is considered the desirable pretreatment, resulting in an ethanol yield of 116.65 and 147.42 mg/g for 2.5% DR (digested residue) and 10% DR, respectively, which may be considered a potential cellulosic feedstock.
Microwave treatment
This pretreatment is effective due to its high heating efficiency and easy operation property. Due to microwave irradiation, the ultrastructure of cellulose [48] breaks lignin and hemicelluloses in lignocellulosic materials and also enhances the enzymatic susceptibility [49]. The enzymatic hydrolysis is enhanced in the presence of water [49, 50] and also in a glycerine medium with a lesser amount of water [51]. As compared to the raw straw, microwave-treated rice straw gives almost an equal level of hydrolysis rate and diminishes sugar yield [52]. It is an efficient method to diminish the recalcitrance of miscanthus and switchgrass lignocellulosic biomass. To accelerate, the destruction is carried out at higher microwave processing temperature, and power also improves the solubilization of biomass [31].
Ultrasound treatment
Sonication is quite an innovative technique and viable for a laboratory that is utilized for the pretreatment process. In ultrasound treatment, cellulose and hemicellulose fractions are accessible to cellulose-degrading enzymes by ultrasound waves that produce both physical and chemical effects by small bubbles that disrupt the morphology for efficient breakdown into simple reducing sugars. The maximum cavitation was formed at the optimum temperature of 50 °C for the degradation of enzymes [53]. Greater is the duration of sonication higher is the effect on pretreatment of biomass. In wheat straw, ultrasonic irradiation in presence of an alkaline medium enhances the purity, yield, and lignin destruction which increases with the sonication time from 5 to 35 min [33]. Optimized ultrasonic treatment increases the production of fermentable sugar from starch in case of corn stover sonication increases the sugar yield when exposed to higher power [34].
Pyrolysis
In the biorefinery process, pyrolysis treatment is utilized for pretreatment of lignocellulosic biomass that is mainly applied for the formation of bio-oil. Regardless of the fact that pyrolysis studies are confined, there are few reviews for reducing sugar production on the utility of pyrolysis in pretreatment of chemically pretreated biomass. Pyrolysis is a thermal degradation process where feedstocks are exposed to elevated temperatures (500 to 800 °C) in the absence of an oxidizing agent. Pyrolysis increases effectiveness during the presence of oxygen at lower temperatures [54, 55]. Agricultural wastes undergo pyrolysis with mild sulfuric acid where 85% of cellulose gets converted into monomeric sugars [35].
Pulsed electric field (PEF)
Pulsed electric field pretreatment is a unique treatment where the biomass is subjected to a blow of high voltage between 5.0 and 20.0 KV/cm for short durations (nano to milliseconds) by revealing the cellulose present in the biomass by building pores in the cell membrane thus penetrating of agents that will further disrupt cellulose into constituent sugars. The main benefits of PEF i.e. it requires low energy as duration (100 μs) is short and even the treatment can be carried out at ambient conditions. Another advantage is a simplification of the PEF instrument design because of the lack of moving parts [54].
5.1.2 Chemical pretreatment
Chemical pretreatment is considered by different acids, alkali, ionic liquids, organic solvent, ammonia, etc. as shown in Table 6. Different chemicals used for the pretreatment of LCB to convert lignocellulosic to fermentable sugar are explained below:
Alkali treatment
In this pretreatment method, alkali like NaOH and KOH solutions are used to eliminate lignin and a few parts of hemicellulose and make the cellulose more approachable to the enzyme. Increase in saccharification production done by this alkali pretreatment method. Usually, pretreatment of alkali occurred at low temperature, but it can be performed with approximately long duration and high concentration of the base. In this pretreatment, breaking of an ester bond between the components such as lignin, hemicellulose, and cellulose and avoiding fragmentation was done effectively as compared with acid or oxidative reagents [73] Diminishing the amount of lignin by 36% can be done by alkali pretreatment of chopped rice straw with 2% sodium hydroxide with 20% solid loading at 85 °C for 1 h [74]. Sodium hydroxide treatment effectively acts on rupturing the ester bond cross-linking lignin and xylan which increases the porosity in the feedstock, by the delignification process [75]. An increase in the porosity and surface area helps in effective enzymatic hydrolysis in rice straw with the help of some separated and exposed microfibrils. Oberoi et al. had observed that pretreatment of native straw with 1% NaOH which decreases lignin by about 47% and increases glucan by about 50%. By increasing the alkali concentration to more than 1% for treatment leads to biomass loss and increases the solubilization of hemicellulose. The pretreatment of rice straw when treated with the combination of alkali, i.e., ammonia and ionic liquid ([Emim]Ac) treatment, demonstrated a synergy effect with 82% of the cellulose recovery and 97% conversion of the enzymatic glucose. This collaborative effect revealed over 90% of the glucose conversion with less enzyme usage and incubation time [61].
Acid treatment
Pretreatment with a different type of acids at normal temperature enhances the anaerobic digestibility. Dilute acid pretreatment largely affects hemicellulose with limited influence on lignin degradation. Acid pretreatment solubilizes the hemicellulose resulting in the cellulose more accessible to enzymes. In this process, mineral acids like HCl and H2SO4 are mostly utilized for the treatment. In dilute acid treatment, for hydrolysis of the carbohydrates, the cellulase enzyme is needed in the treated biomass. Dilute acid pretreatment is a simple single-stage process in which biomass is treated with dilute sulfuric acid temperatures for a period of time and at suitable acid concentrations. To reduce dependence on enzyme requirements, the National Renewable Energy Laboratory (NREL) in Golden, Colorado, has developed a two-stage process. Dilute acid hydrolysis of rice straw has limited reviews because there is no specific condition to remove lignin and has low sugar yield [76]. Rice straw when treated with dilute acid i.e. 1% (w/w) sulfuric acid with 1–5 min reaction time at 160 ̊C to yield 83% of sugar and also increases the efficiency of 70% during enzymatic hydrolysis [56]. Oberoi et al. suggested that when the rice straw is treated with dilute sulfuric acid, i.e., 10% (w/w), it results in diminishing the crystallinity property of the rice straw and ultimately enhances the productivity of enzymatic hydrolysis.
Solvent treatment
Solvent pretreatment is the most important fractionating along with a separating process during which an organic or aqueous organic solvent is employed to deconstruct LCCs in the presence or absence of catalysts [77, 78]. Triethylene glycol, methanol, ethanol, ethylene glycol, tetrahydrofurfuryl alcohol, glycerol, n-butanol, acetone, phenol, etc. have been examined to hydrolyze hemicellulose and even to destruct lignin so that further it can render cellulose for enzymatic hydrolysis. Organic solvents treatment is more profitable on top of other chemical pretreatments due to the fact that those are comparatively low-molecular-weight lignin and pure can be regained as a by-product. At the same time due to heavy expenses of organic solvents and the intensive energy consumption connected with easy recovery of the solvent which builds this approach economically non-competitive.
-
a.
Ionic liquids
Another technique, i.e., pretreatment by ionic liquids (ILs), resides as liquids at room temperature, and mainly they are salts including a large organic cation and a small anion and at less vapor pressure [79]. Based on chemistry, the anion and cation have an extensive classification of ILs [80]. It has aimed for lignin degradation and breaks down the crystalline structure of cellulose for enzymatic hydrolysis. Consequently, IL pretreatment has been broadly studied, and the majority of ILs can be retrieved which not only diminishes their usage but also makes them eco-friendlier [81]. Imidazolium salts are the most common ionic liquids rupturing the hydrogen bonding [82]. Moreover, to produce ethanol, there are still many challenges for ILs, but it can be beneficial in the pretreatment of biomass for the upcoming generation. Rice straw was mixed with ionic liquid at 1:20 solid-liquid ratio and incubated in an oven at 130 °C for 24 h where the recovery rate is higher of glucan and xylan, 74% cellulose recovery, and 78% conversion to glucose [61].
-
b.
Organosolv
In this method, the different feedstocks include the addition of various aqueous organic solvents such as ethanol, methanol, ethylene glycol, and acetone under a particular state of temperature and pressure [83, 84]. In organosolv pretreatment, the important factor is the temperature (rely on sort of feedstock and catalyst) and mainly takes place in presence of an acid, base, or salt catalyst [85]. This method is mainly utilized for the degradation of lignin, and apart from lignin, sugars fractions such as cellulose and hemicellulose are also produced during the process. Pretreatment of rice straw with 75% (v/v) aqueous ethanol containing 1% w/w sulfuric acid at 150 °C for 60 min results in the total sugar concentration of 31 g/L in the enzymatic hydrolysis and increases the production of solvent, i.e., acetone, butanol, and ethanol [60].
-
iii.
Deep eutectic solvents (DES)
Nowadays, the researchers have deviated their emphasis on deep eutectic solvents as a green method for biomass utilization [86]. These mixtures are developed by a combination of hydrogen bond donor (alcohols, amides, and carboxylic acids) and hydrogen bond acceptor (quaternary ammonium salts) at moderate temperature (60–80 °C) which mainly comprises of non-symmetric ions that have a low melting point and lattice energy [86,86,88]. DES is efficient enough for forming hydrogen bonds because of the presence of strong electron donors and acceptors by controlling its solvation property [89]. Without any effect of cellulose, it enhances the solubilization of LCB polymers with more selective solubilization of lignin [90]. Deep eutectic solvents (DESs) based pretreatment considered to a “Green” and economical process due to its clarity in the synthesis process, requisite demand of solvents, and purification steps. DESs are generally less toxic, easily biodegradable, and recyclable [86].
Salt pretreatment
Numerous studies reveal that metal salts can catalyze the deconstruction of LCB [91, 92]. The basic principle is that it forms various complexes of Lewis acids and metal in water and these metal cations serve as Lewis acids which can break the glycosidic bond of hemicelluloses that ultimately result in the formation of xylose [93]. Metal salt solutions are more efficient in increasing the capacity of the breakdown of hemicellulose fraction with minimized xylan degradation [94]. The significant benefits related with metal salts assisted treatments are improving lignin removal, hemicelluloses degradation [91], and complete conversion of biomass, higher reaction rate, improved enzymatic hydrolysis [95], nontoxic, environmentally benign, and no requirement of expensive non-corrosive reactors [90, 96]. Bak et al. [65] observed that when rice straw (RS) pretreated using water soaking-based electron beam irradiation (WEBI), that makes the treatment eco-friendly without any discharge or creation of inhibitory compounds such as hydroxymethylfurfural(HMF) and furfural. It even enhances the yielding of rice straw during the process of enzymatic hydrolysis and fermentation. Yu et al. [67] investigated that structural properties of corn stover such as the reduction in particle size were observed after treatment using mechanical pulverization in the presence or absence of phosphoric acid treatment. This also showed that the compact structure of corn stover gets loosened due to the pulverization effect and even the hemicelluloses content was reduced and increase in accessibility of β-1, 4 glycosidic bonds for hydrolysis.
5.1.3 Physicochemical pretreatment
Ammonia treatment
Ammonia is an important pretreatment reagent, an effectual swelling reagent with many useful properties for LC biomass. It can be recovered and reused due to its high volatile nature. Ammonia is a non-polluting as well as non-corrosive chemical. In the lignin-carbohydrate complex, the ether-ester bonds and C–O–C bonds can be cleaved during the reaction of aqueous ammonia with lignin [97]. Ammonia recycle percolation is an effective process that has been developed for pretreatment. In this process, the feedstock maintained at 170 °C is used through which ammonia is pumped. This is the selective process to achieve up to 85% delignification as well as the yield of glucose in enzymatic hydrolysis [98]. Most of the glucan and xylan can be preserved by soaking the feedstock in aqueous ammonia (SAA) pretreatment at very moderate temperatures ranging from 40 to 90 °C [97].
Steam explosion
Steam explosion or steam-based pretreatment is the main among the most important used physicochemical methods and high explosion on feedstock that need for further hydrolysis [99]. Steam treatment is usually a mixture of mechanical forces (pressure drop) and chemical effects (autohydrolysis of the acetyl group of hemicellulose) where the biomass is treated at high pressure (0.7–4.8 MPa) saturated steam for release of hemicellulose. Major components that have an impact on this treatment are temperature, residence time, moisture content, and biomass size [100]. The treatment of hardwoods and agricultural residues mostly prefers steam explosion and was found to be more effective. The advantages of this method are it requires low energy, limiting chemical utility, no cost for recycling, and most importantly it is environment friendly.
Liquid hot water (LHW)
This method is referred to as hot compressed water which is mostly similar to the steam pretreatment method, but the LHW process utilizes water medium at high temperature (170–230 °C) and pressure (up to 5 MPa) instead of steam. This method results in hydrolysis of hemicellulose and making cellulose more accessible for a rupture with simultaneous removal of lignin. LHW can be carried out in 3 distinct ways based on biomass present in the reactor and the direction of the flow of water.
-
Co-current LHW pretreatment - Both water and slurry of biomass are heated at a particular temperature, various conditions needed for pretreatment for residence time before being cooled.
-
Countercurrent LHW - The biomass is pumped by hot water under controlled conditions.
-
Flow-through pretreatment - In this process, the hot water flows through the biomass which acts as a stationary bed, and the fractions that are hydrolyzed are carried out in the reactor.
The main advantages of LHW are:
-
Requires low temperature.
-
It forms minimum inhibitory compounds.
-
The solvent is affordable and cheap.
Despite that, downstream processing demands a large amount of energy because of the considerable quantity of water involved [99].
Wet oxidation
Wet oxidation treatment is one of the convenient processes of lignocellulosic pretreatment, i.e., more appropriate for lignin-enriched biomass. The enriched biomass is treated with air/oxygen as well as water or hydrogen peroxide medium at high temperature (above 120 °C for 30 min) [101]. The wet oxidation process is more capable and mainly depends upon three various factors such as temperature, oxygen pressure, and reaction time. In this process, water behaves like acid with an elevation of temperature above 170 °C where it catalyzes hydrolytic reactions. The hemicellulose fraction is divided into its pentose and hexose monomers, and the lignin part undergoes oxidation, while the wet oxidation treatment affects less the cellulose fraction.
CO2 explosion
This technique performs the pretreatment of biomass through supercritical fluid CO2 where the gas acts as a solvent. In this process, the supercritical CO2 undergoes through a high-pressure vessel containing the biomass [102]. The vessel is then heated to the necessary temperature and left for a few minutes at high temperatures [16]. CO2 penetrates inside the feedstock at high pressure which forms carbonic acid that hydrolyses the fraction of hemicellulose. The pressurized gas when released breaks the feedstock by increasing the surface area [103]. This method is mainly appropriate for feedstock having minimum moisture content. The higher the moisture contents in the biomass, the higher the hydrolytic yield [102]. The benefits of this process are it requires less temperature, low cost of CO2, high solid capacity, and no formation of the toxin. On the other hand, due to expensiveness of the reactor for a tolerance of high-pressure conditions, it is becoming a barrier in its application on large scale [99].
Oxidative treatment
This process mainly involves various types of an oxidizing agent such as hydrogen peroxide, ozone, oxygen, or air [104]. Above all, the oxidizing agent that is employed frequently is hydrogen peroxide. It has been observed that hydrolysis of hydrogen peroxide results in the generation of hydroxyl radicals which are responsible for the degradation of lignin and formation of low molecular weight products. Removal of lignin from lignocellulose reveals cellulose and hemicellulose resulting in increasing enzymatic hydrolysis that could reach up to 95% [105]. During oxidative pretreatment, a lot of valuable chemical reactions such as electrophilic substitution, side-chain displacements, and oxidative cleavage of an aromatic ring, ether linkages may take place. This process acts as an inhibitor by the delignification process and converting lignin to acids. So, these acids need to be removed [106]. A major drawback of this pretreatment is that it harms a substantial amount of hemicellulose making it inefficient for the fermentation process [107].
5.1.4 Biological pretreatment
Biological pretreatment provides major benefits such as low energy and chemical use, but an effective and rapid process has not yet been found. On the basis of some strict conditions such as corrosion-resistant equipment, dumping of chemical wastes and proper washing in the chemical treatment has various disadvantages. This pretreatment is a secure and eco-friendly method for the removal of lignin and degrading the component present in the lignocellulose as mentioned in Table 7. The white-rot fungi of Basidiomycetes are the most useful and propitious microorganism used in biological pretreatment [117]. These are the four popular white-rot fungi species (Phanerochaete chrysosporium, Trametes versicolor, Ceriporiopsis subvermispora, and Pleurotus ostreatus) utilized during biological treatment of rice straw. This method is mostly based on the quantitative and morphological alteration in the lignocellulose components of the pretreated rice straw as well as susceptibility to enzymatic hydrolysis [117]. Suhara et al. [108] determined that pretreatment of bamboo culms with Punctularia sp. (white-rot basidiomycetes) improved the effect of enzymatic hydrolysis and degraded 50% of lignin content. The white-rot fungus, P. Ostreatus, is the best agent for degrading the lignin part of rice straw than the holocellulose component. The total loss of weight and the degree of Klason lignin (insoluble residue portion after remaining ash) degraded were 25% and 41%, respectively, which has been done by pretreatment with P. ostreatus for 60d and those untreated rice straws contained 83% and 52% of cellulose and hemicellulose, respectively. Commercial cellulase enzyme preparation for 48 h, 52% holocellulose and 44% cellulose, in the pretreated rice straw was solubilized by enzymatic hydrolysis process. The net sugar yields of untreated rice straw were 33% from holocellulose and 32% for glucose obtained from cellulose [117]. So, by this pretreatment method, porosity increase in rice straw has been done by loosening in their cell structure. Pretreatment by using P. ostreatus increases the rice straw sensitivity towards enzymatic hydrolysis which has been shown by the scanning electron microscope (SEM). This sensitivity has been done due to a break in lignin bonds, which is responsible for opposing the entry of cellulase in the rice as described above. Du et al. [109] developed a biological pretreatment with I. lacteus which yields glucan (82%) by hydrolysis. The fungal pretreatment by Ceriporiopsis subvermispora of corn stover increases the glucose yield [115]. It was reported by Cianchetta et al. when wheat straw was pretreated by fungi Ceriporiopsis subvermispora strain minimizes the loss of cellulose content with the highest sugar yield to 44%. In the biological pretreatment of rice husk, Phanerochaete chrysosporium, the white-rot fungus, was utilized for reducing sugar production.
5.2 Combined pretreatment methods
To increase the efficiency of the treatment process due to the drawbacks available in single pretreatment methods, many researchers make an effort to combine those methods to overcome the challenges. Nowadays, many studies have been conducted by a combination of various pretreatment methods. For example, in fungal pretreatment, the main disadvantage is its longer operation time. In that context, combining the fungal pretreatment with some other physical and chemical treatment methods improves the efficiency of the process [118]. In another survey, when hydrogen peroxide is combined with the steam pretreatment, the yield of xylose and glucose yield increased by 34% and 12%, respectively, but further there is no rise in the yield of arabinose and mannose significantly. Hence, hydrogen peroxide has not improved the formation of lignocellulose-derived by-products throughout the pretreatment process [96]. In recent studies, alkaline hydrogen peroxide is utilized due to its effectiveness for a broad range of LCBs with the high performance of enzymatic hydrolysis when utilized solitarily or combined [119]. Some recent studies of bioethanol production from LCB using combined methods is depicted in Table 8. The combined microwave alkali acid pretreatment found to have more cellulose content was increased to 60.07% [120]. Another pretreatment with a combination of phosphoric acid and hydrogen peroxide on wheat straw for ethanol conversion of 88.2% [122]. A productive approach of pretreatment on wheat straw using alkaline NaOH and alkaline hydrogen peroxide where 92.4% of sugar conversion takes place [124] but when the wheat straw pretreated with alkaline NaOH followed by steam pretreatment results in 80% of glucose and 65% of xylose [123]. Yuan et al. [125] suggested that when corn stover pretreated with sodium hydroxide methanol solution (SMs) that enhances the enzyme accessibility of corn stover. Another study of Vergara et al. [126] investigated that corn stover pretreated with ethanol-water (EW) and diluted sulfuric acid (DSA) showed a combined effect of delignification and ultimately enhances the enzymatic cellulose hydrolysis.
5.3 Chemicals from pretreatment processes
There is a generation of various chemical intermediates in different pretreatment processes in several industrial sectors such as food, paper and timber, and fibers. It can be petroleum substitute’s products or might have new functionalities or numerous superior properties than the ancient manufactured petroleum products such as:
-
(i).
Liquid hydrocarbon fuels [129, 130] and chemicals like furfural [131], 5-hydroxymethyl furfural (HMF) [132], 2,5-furan dicarboxylic acid (FDCA), g-Valero lactone (GVL) [133, 134], polymers [135, 136], and organic acids [137, 138] from components of carbohydrates that are hemicellulose and cellulose.
-
(ii).
Phenolic lignin compounds emerge with several utilities such as agricultural chemicals, thermal and electrical energy, diesel fuel, carbon fiber, adhesives, additives, dispersants, resins, textile dyes, and aromatics [139, 140].
- (iii).
5.4 Novel and emerging pretreatment technologies
5.4.1 Hydrothermal pretreatment
Hydrothermal pretreatment uses a high temperature of subcritical water (< 374 °C) which denatures plant cell walls and degrades hemicellulose and transformation of lignin into sugars/syngas [143]. This technique can be categorized depending on the target product into 3 various classes such as (Fig 8):
-
Carbonization - This process is carried out at a temperature ranging between 200 and 270 °C, and the product formed is solid char that is mostly rich in carbon.
-
Liquefaction - This process which mostly produces water-soluble constituents, bio-oil, char, and a gas phase that comprises carbon dioxide works out under processing conditions between 250 and 400 °C [144].
-
Gasification - In gasification hydrothermal treatment, the temperature must be greater than 400 °C, and the product obtained in this process is fuel gas.
In gasification hydrothermal, the temperature is much high which ultimately results in greater reaction rates as compared to reaction rates that are obtained in hydrothermal liquefaction and carbonization.
Advantages of hydrothermal pretreatment:
6 Enzymatic hydrolysis
By the use of an enzyme, polymers of cellulose and hemicellulose are cleaved. Hemicellulose contains some components like xylan, mannan, glucan, and galactan, whereas cellulose contains glucans only. Ultimately, hydrolysis product of hemicellulose and cellulose results in various pentoses, hexoses, and glucose, respectively [146]. The high amount of lignin can block enzyme accessibility, inhibit the end product, and diminish the rate and yield of hydrolysis. The strong inhibitors of cellulase are lignin, cellobiose, and glucose [147]. Several factors affecting the products for the formation of monomeric sugars are liquid to solid ratio, specificities of various acid used, temperature, reaction time, size of the particle of the biomass as well as the length of the macromolecules, polymerization degree of cellulose, arrangement of the cellulose chain, and connection of cellulose with other polymeric structures present within the plant cell wall such as lignin, pectin, hemicellulose, proteins, and mineral elements (Fig. 9a, b).
7 Scope of applications
In this review, this topic is portrayed in short and brief, but this subject is beyond the scope of this review. Multiple valuable products can be obtained and formed through lignocellulosic biomass. Among which biofuel and green chemicals are widely publicized and broadly reviewed [148]. Table 9 shows the advantages and disadvantages of different type of pretreatments.
7.1 Biofuels
Numerous biofuels are obtained through lignocellulose biomass in different forms such as bio-oil, bioethanol, biohydrogen, biogas, and syngas.
-
Bio-oil - It is produced by depolymerization of lignocellulosic components, viz., carboxylic acids, hydroxyl aldehydes, sugars, hydroxyl ketones, and phenols. Through the pyrolysis process, bio-oil is obtained with by-products such as biochar, tar, and gases [156, 157].
-
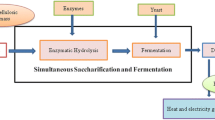
Bioethanol - It is a well-known second-generation biofuel. There are five different methods such as separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), consolidated bioprocessing (CBP), and integrated bioprocessing (IBP) for the production of bioethanol [155, 158]. SSF is most potential and promising between these processes as it is cost-effective and high product yield. IBP is one more favorable process that involves treatment in a single step with several microorganisms [159].
-
Biohydrogen - It can be generated through thermo-chemical (gasification and pyrolysis) or biological routes [160]. In the pyrolysis process, hydrogen can be produced through fast or flash pyrolysis [161]. By partial oxidation and steam reformation followed by water-gas shift reaction, hydrogen can be produced through gasification. There are two different routes to produce biohydrogen, that is, one through biological routes known as photo fermentation, i.e., light-dependent, and dark fermentation, i.e., light-independent [162].
-
Biogas and syngas both have almost similar types of composition (CO2, CO, CH4, H2, and N2) but are produced through 2 different routes. Biogas is generated through an anaerobic digestion method which comprises 4 steps like hydrolysis, acidogenesis, acetogenesis, and methanogenesis [163], while syngas is produced by the process of gasification that is produced at a lower temperature due to the high reactivity of biomass. Biomass gasification has three types of processes: (1) pyrolysis which involves anaerobic decomposition of biomass at high temperatures; (2) a limited quantity of oxygen is required for partial oxidation; and (3) steam gasification which involves the reaction of water with biomass.
7.2 Bioproducts
The chemical that is obtained from lignocellulosic biomass originated either from the part of the carbohydrate portion or through the lignin area.
-
Dehydration of C5 and C6 sugars through acid-catalyzed reactions, the chemical derived from carbohydrate part is furfural and 5-hydroxymethylfurfural (HMF) [164]. By hydrogenation of hexose and pentose, sugar-based alcohols are obtained such as sorbitol and xylitol [165]. By hydrogenolysis of sugar alcohol such as sorbitol and xylitol which helps in sweetening the products used in medication, candy, gums, etc. The most widely used glycerol can be produced mainly applicable for preparing biosolvents, polymers, surfactants, etc. [166]. Also, lactic acid is mainly used in organic synthesis industries and succinic acid mostly used in the beverage industry, and these two chemicals can be obtained from biological conversion caused by bacteria.
-
In earlier days, lignin has been used to generate heat. But in recent times, lignin has been a significant and valuable source of products as it consists of long-chain phenolic compounds. Depolymerization is the main fundamental process for the conversion of lignin to phenolic compounds. Numerous methods obtained for conversions of lignin components to their respective phenolic compounds are liquefaction [167], oxidation [168], solvolysis [169], hydrocracking [170], and hydrolysis [171]. It has also been utilized for the development of sophisticated products for energy storage, transportation facilities, medical applications, biosensing, environmental remediation, etc. [172,168,174].
8 Advantages of bioethanol
-
A number of other chemicals such as ethyl esters, ethyl acetate, extractants, antifreeze, and intermediates in the synthesis of various organic chemicals are formed by ethanol.
-
The second-generation fuel, i.e., bioethanol, is environmental friendly, biodegradable, and less toxic than the ancient nonrenewable fossil fuels.
-
It is mainly originated from renewable sources; i.e., any by-products obtained from agricultural biomass can be used for the production provided; it contains sugar and starch such as rice straw, wheat straw, corn stover, corncob, and sugarcane bagasse.
-
During bioethanol production, it reduces greenhouse gases and neutralizes the carbon availability in the atmosphere.
-
Bioethanol has mostly been used as a biofuel as it is blended with petrol at 5%, and these fuel spills are ecofriendly or diluted to nontoxic concentrations.
-
The gases of ethanol that are discharged are much cleaner and undergo complete combustion, and the effect of ethanol utilization results in a decrease in the depletion of the ozone layer in the atmosphere which is important and beneficial for environmental concerns.
9 Conclusion
The existence of lignin in the biomass hinders the hydrolysis of cellulose and hemicellulose. Consequently, broad research has been completed for creating different pretreatment procedures for the delignification of biomass. Mostly combination of two different types of pretreatments indicated favorable and advantageous effects on improving chemical yield and process of enzymatic hydrolysis of LBs, but it increases the cost of operation. Acid treatment, steam explosion, and hydrothermal processes all together show a comparatively high effect on eliminating hemicelluloses fraction from the structure of biomass. Alkali, oxidative, and organosolv pretreatments are more efficient in removing and degrading of lignin portion. On the other hand, the operational expenses and maintenance costs of organosolv and oxidative delignification processes are much higher than alkali pretreatment. Biological pretreatment exhibits many merit output on eliminating lignin fraction from LBs. So far, alkali pretreatments are considered; it is still the most attractive and inexpensive method to expel out lignin. According to the comparison, dilute acid process, hydrothermal and steam explosion combined alkali pretreatment is the most favorable combination for pretreating LBs. On the contrary, advanced hydrothermal pretreatment (based on the biorefinery platform) aimed at generating value-added products from lignin and other components (currently focusing on the production of energy). Nonetheless, basic investigation of pretreatment techniques carries us to an end that pretreatment strategy is a “customized” process for each biomass which ought to be fastidiously chosen and arranged dependent on the trademark properties of biomass. Likewise, it tends to be presumed that to date a solitary pretreatment technique has not been built up which can do finish the delignification of biomass in a monetary and condition agreeable way. However, consolidated pretreatment techniques have been effective to a degree; still, a great deal of research should be done in creating joined pretreatment strategies to their maximum capacity. There are two different conditions for various type of feedstocks such as the biomass that is applicable for biochemical conversion should have high moisture content (>30%), rich in cellulose and hemicellulose content, and carbon to nitrogen ratio less than 30, whereas biomass containing moisture of < 30%, rich in lignin content, and a carbon to nitrogen ratio greater than 30 are mostly preferred for thermochemical conversion and subsequent treatment for biofuel production. This basic audit involving physical, chemical, physicochemical, and biological pretreatment forms alongside their focal points and impediments will help the specialist in arranging, determinating and improvement of the pretreatment process for different lignocellulosic biomass.
Data availability
Not applicable.
References
Kang Q, Appels L, Baeyens J, Dewil R, Tan T (2014) Energy-efficient production of cassava-based bio-ethanol. Advances in Bioscience and Biotechnology 5(12):925. https://doi.org/10.4236/abb.2014.512107
Bioethanol production: insight into past, present and future perspectives Shreyas Niphadkar, Praful Bagade and Shadab Ahmed. biofuels 2017
Paul S, Dutta A (2018) Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour Conserv Recycl 130:164–174. https://doi.org/10.1016/j.resconrec.2017.12.005
Lei J, Bi Y, Shen L (2011, 2011) Performance and emission characteristics of diesel engine fueled with ethanol-diesel blends in different altitude regions. Biomed Res Int:1–10. https://doi.org/10.1155/2011/417421
Chen F, Srinivasa Reddy MS, Temple S, Jackson L, Shadle G, Dixon RA (2006) Multisite genetic modulation of monolignol biosynthesis suggests new routes for formation of syringyl lignin and wallbound ferulic acid in alfalfa (Medicagosativa L.). Plant J 48(1):113–124. https://doi.org/10.1111/j.1365-313X.2006.02857.x
Kang Q, Huybrechts J, Van der Bruggen B, Baeyens J, Tan T, Dewil R (2014) Hydrophilic membranes to replace molecular sieves in dewatering the bio-ethanol/water azeotropicmixture. Separationand purification Technology 136:144–149. https://doi.org/10.1016/j.seppur.2014.09.009
Werther J, Saenger M, Hartge EU, Ogada T, Siagi Z (2000) Combustion of agricultural residues. Prog Energy Combust Sci 26(1):1–27. https://doi.org/10.1016/S0360-1285(99)00005-2
Bååth H, Gällerspång A, Hallsby G, Lundström A, Löfgren P, Nilsson M, Ståhl G (2002) Remote sensing, field survey, and long-term forecasting: an efficient combination for local assessments of forest fuels. Biomass Bioenergy 22(3):145–157. https://doi.org/10.1016/S0961-9534(01)00065-4
Fischer G, Schrattenholzer L (2001) Global bioenergy potentials through 2050. Biomass and bioenergy 20(3):151–159. https://doi.org/10.1016/S0961-9534(00)00074-X
Demirbas MF, Balat M, Balat H (2009) Potential contribution of biomass to the sustainable energy development. Energy Convers Manag 50(7):1746–1760. https://doi.org/10.1016/j.enconman.2009.03.013
Vertes AA, Qureshi N, Yukawa H, Blaschek HP (2011) Biomass to biofuels: strategies for global industries. Wiley
Raven PH, Evert RF, Eichhorn SE (1999) Biology of plants WH freedman and company worth publishers 347-368
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. Journal of radiation research and applied sciences 7(2):163–173
Singh H, Sapra PK, Sidhu BS (2013) Evaluation and characterization of different biomass residues through proximate & ultimate analysis and heating value. Asian Journal of Engineering and Applied Technology 2(2):6–10
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annual review of plant biology 61(1): 263–289. https://doi.org/10.1146/annurev-arplant-042809-112315
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Brandão PC, Souza TC, Ferreira CA, Hori CE, Romanielo LL (2010) Removal of petroleum hydrocarbons from aqueous solution using sugarcane bagasse as adsorbent. J Hazard Mater 175(1–3):1106–1112. https://doi.org/10.1016/j.jhazmat.2009.10.060
Adekiigbe A (2012) Determination of heating value of five economic trees residue as a fuel for biomass heating system. Nature and science 10(10):26–29
Shen J, Zhu S, Liu X, Zhang H, Tan J (2010) The prediction of elemental composition of biomass based on proximate analysis. Energy Convers Manag 51(5):983–987. https://doi.org/10.1016/j.enconman.2009.11.039
Raj T, Kapoor M, Gaur R, Christopher J, Lamba B, Tuli DK, Kumar R (2015) Physical and chemical characterization of various Indian agriculture residues for biofuels production. Energy & Fuels 29(5):3111–3118. https://doi.org/10.1021/ef5027373
Bridgeman TG, Jones JM, Shield I, Williams PT (2008) Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 87(6):844–856. https://doi.org/10.1016/j.fuel.2007.05.041
Kumar S, Paritosh K, Pareek N, Chawade A, Vivekanand V (2018) De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: an overview. Renewable and Sustainable Energy Reviews 90:160–170. https://doi.org/10.1016/j.rser.2018.03.049
Worasuwannarak N, Sonobe T, Tanthapanichakoon W (2007) Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. Journal of Analytical and Applied Pyrolysis 78(2):265–271. https://doi.org/10.1016/j.jaap.2006.08.002
Vassilev SV, Baxter D, Andersen LK, Vassileva CG (2010) An overview of the chemical composition of biomass. Fuel 89(5):913–933. https://doi.org/10.1016/j.fuel.2009.10.022
Channiwala SA, Parikh PP (2002) A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 81(8):1051–1063. https://doi.org/10.1016/S0016-2361(01)00131-4
Tsai WT, Lee MK, Chang YM (2006) Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J Anal Appl Pyrolysis 76(1–2):230–237. https://doi.org/10.1016/j.jaap.2005.11.007
Demirbas A (2004) Combustion characteristics of different biomass fuels. Prog Energy Combust Sci 30(2):219–230. https://doi.org/10.1016/j.pecs.2003.10.004
Telmo C, Lousada J, Moreira N (2010) Proximate analysis, backwards stepwise regression between gross calorific value, ultimate and chemical analysis of wood. Bioresour Technol 101(11):3808–3815. https://doi.org/10.1016/j.biortech.2010.01.021
Zhang H, Zhang P, Ye J, Wu Y, Liu J, Fang W, Zeng G (2018) Comparison of various pretreatments for ethanol production enhancement from solid residue after rumen fluid digestion of rice straw. Bioresource technology 247:147–156. https://doi.org/10.1016/j.biortech.2017.09.065
Liu H, Zhang Y, Hou T, Chen X, Gao C, Han L, Xiao W (2018) Mechanical deconstruction of corn stover as an entry process to facilitate the microwave-assisted production of ethyl levulinate. Fuel Processing Technology 174:53–60. https://doi.org/10.1016/j.fuproc.2018.02.011
Irmak S, Meryemoglu B, Sandip A, Subbiah J, Mitchell RB, Sarath G (2018) Microwave pretreatment effects on switchgrass and miscanthus solubilization in subcritical water and hydrolysate utilization for hydrogen production. Biomass Bioenergy 108:48–54. https://doi.org/10.1016/j.biombioe.2017.10.039
Savoo S, Mudhoo A (2018) Biomethanationmacrodynamics of vegetable residues pretreated by low-frequency microwave irradiation. Bioresour Technol 248:280–286. https://doi.org/10.1016/j.biortech.2017.05.200
Sun R, Tomkinson J (2002) Comparative study of lignins isolated by alkali and ultrasound-assisted alkali extractions from wheat straw. Ultrason Sonochem 9(2):85–93. https://doi.org/10.1016/S1350-4177(01)00106-7
Montalbo-Lomboy M, Johnson L, Khanal SK, van Leeuwen JH, Grewell D (2010) Sonication of sugary-2 corn: a potential pretreatment to enhance sugar release. Bioresour Technol 101(1):351–358. https://doi.org/10.1016/j.biortech.2009.07.075
Fan LT, Gharpuray MM, Lee YH (1987) Enzymatic hydrolysis. In: Cellulose hydrolysis. Springer, Berlin, Heidelberg, pp 21–119
Mafei TD, Neto FS, Peixoto G, de Baptista NÁ, Monti R, Masarin F (2020) Extraction and characterization of hemicellulose from Eucalyptus by-product: assessment of enzymatic hydrolysis to produce Xylooligosaccharides. Applied Biochemistry and Biotechnology 190(1):197–217. https://doi.org/10.1007/s12010-019-03076-0
Ferraz A, Baeza J, Rodriguez J, Freer J (2000) Estimating the chemical composition of biodegraded pine and eucalyptus wood by DRIFT spectroscopy and multivariate analysis. Bioresour Technol 74(3):201–212. https://doi.org/10.1016/S0960-8524(00)00024-9
Masarin F, Gurpilhares DB, Baffa DC, Barbosa MH, Carvalho W, Ferraz A, Milagres AM (2011) Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnology for biofuels 4(1):55. https://doi.org/10.1186/1754-6834-4-55
Mendes FM, Siqueira G, Carvalho W, Ferraz A, Milagres AM (2011) Enzymatic hydrolysis of chemithermomechanically pretreated sugarcane bagasse and samples with reduced initial lignin content. Biotechnol Prog 27(2):395–401. https://doi.org/10.1002/btpr.553
Kuchelmeister C, Bauer S (2015) Rapid small-scale determination of extractives in biomass. BioEnergy Research 8(1):68–76. https://doi.org/10.1007/s12155-014-9493-x
Sluiter JB, Ruiz RO, Scarlata CJ, Sluiter AD, Templeton DW (2010) Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J Agric Food Chem 58(16):9043–9053. https://doi.org/10.1021/jf1008023
Anderson S (2004) Soxtec. In: Soxtec: its principles and applications. Critical Issues and Competitive Studies, Oil Extraction and Analysis
Sitholé BB, Vollstaedt P, Allen LH (1991) Comparison of Soxtec and Soxhlet systems for determining extractives content. TAPPI J 74(11):187–191
Kim TH, Taylor F, Hicks KB (2008) Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresour Technol 99(13):5694–5702. https://doi.org/10.1016/j.biortech.2007.10.055
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686. https://doi.org/10.1016/j.biortech.2004.06.025
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresources and Bioprocessing 4(1):7
Hideno A, Inoue H, Tsukahara K, Fujimoto S, Minowa T, Inoue S, Sawayama S (2009) Wet disk milling pretreatment without sulfuric acid for enzymatic hydrolysis of rice straw. Bioresour Technol 100(10):2706–2711. https://doi.org/10.1016/j.biortech.2008.12.057
Xiong J, Ye J, Liang WZ, Fan PM (2000) Influence of microwave on the ultrastructure of cellulose I. Journal of South China University Technology 28(1):84–89
Azuma J, Tanaka F, Koshijima T (1984) Enhancement of enzymatic susceptibility of lignocellulosic wastes by microwave irradiation. J Ferment Technol 62(4):377–384
Ooshima H, Aso K, Harano Y, Yamamoto TJBL (1984) Microwave treatment of cellulosic materials for their enzymatic hydrolysis. Biotechnol Lett 6(5):289–294
Intanakul P, Krairiksh M, Kitchaiya P (2003) Enhancement of enzymatic hydrolysis of lignocellulosic wastes by microwave pretreatment under atmospheric pressure. Journal of wood chemistry and technology 23(2):217–225. https://doi.org/10.1081/WCT-120021926
Zhu S, Wu Y, Yu Z, Wang C, Yu F, Jin S, Zhang Y (2006) Comparison of three microwave/chemical pretreatment processes for enzymatic hydrolysis of rice straw. Biosyst Eng 93(3):279–283. https://doi.org/10.1016/j.biosystemseng.2005.11.013
Yachmenev V, Condon B, Klasson T, Lambert A (2009) Acceleration of the enzymatic hydrolysis of corn stover and sugar cane bagasse celluloses by low intensity uniform ultrasound. Journal of Biobased Materials and Bioenergy 3(1):25–31. https://doi.org/10.1166/jbmb.2009.1002
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729. https://doi.org/10.1021/ie801542g
Shafizadeh F, Bradbury AGW (1979) Thermal degradation of cellulose in air and nitrogen at low temperatures. J Appl Polym Sci 23(5):1431–1442. https://doi.org/10.1002/app.1979.070230513
Hsu TC, Guo GL, Chen WH, Hwang WS (2010) Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour Technol 101(13):4907–4913. https://doi.org/10.1016/j.biortech.2009.10.009
Zhu S, Wu Y, Yu Z, Liao J, Zhang Y (2005) Pretreatment by microwave/alkali of rice straw and its enzymic hydrolysis. Process Biochemistry 40(9):3082–3086. https://doi.org/10.1016/j.procbio.2005.03.016
Abedinifar S, Karimi K, Khanahmadi M, Taherzadeh MJ (2009) Ethanol production by Mucorindicus and Rhizopusoryzae from rice straw by separate hydrolysis and fermentation. Biomass and bioenergy 33(5):828–833. https://doi.org/10.1016/j.biombioe.2009.01.003
Park JY, Shiroma R, Al-Haq MI, Zhang Y, Ike M, Arai-Sanoh Y, Tokuyasu K (2010) A novel lime pretreatment for subsequent bioethanol production from rice straw–calcium capturing by carbonation (CaCCO) process. Bioresour Technol 101(17):805–6811. https://doi.org/10.1016/j.biortech.2010.03.098
Amiri H, Karimi K, Zilouei H (2014) Organosolvpretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour Technol 152:450–456. https://doi.org/10.1016/j.biortech.2013.11.038
Nguyen TAD, Kim KR, Han SJ, Cho HY, Kim JW, Park SM, Sim SJ (2010) Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Bioresour Technol 101(19):7432–7438. https://doi.org/10.1016/j.biortech.2010.04.053
Oberoi HS, Vadlani PV, Brijwani K, Bhargav VK, Patil RT (2010) Enhanced ethanol production via fermentation of rice straw with hydrolysate-adapted Candida tropicalis ATCC 13803. Process Biochem 45(8):1299–1306. https://doi.org/10.1016/j.procbio.2010.04.017
Oberoi HS, Babbar N, Sandhu SK, Dhaliwal SS, Kaur U, Chadha BS, Bhargava VK (2012) Ethanol production from alkali-treated rice straw via simultaneous saccharification and fermentation using newly isolated thermotolerant Pichiakudriavzevii HOP-1. J Ind Microbiol Biotechnol 39(4):557–566
Singh A, Bishnoi NR (2012) Enzymatic hydrolysis optimization of microwave alkali pretreated wheat straw and ethanol production by yeast. Bioresour Technol 108:94–101. https://doi.org/10.1016/j.biortech.2011.12.084
Bak JS (2014) Electron beam irradiation enhances the digestibility and fermentation yield of water-soaked lignocellulosic biomass. Biotechnology Reports 4 3:0–33. https://doi.org/10.1016/j.btre.2014.07.006, 30
Gong G, Liu D, Huang Y (2010) Microwave-assisted organic acid pretreatment for enzymatic hydrolysis of rice straw. Biosystems engineering 107(2):67–73. https://doi.org/10.1016/j.biosystemseng.2010.05.012
Yu H, Xiao W, Han L, Huang G (2019) Characterization of mechanical pulverization/phosphoric acid pretreatment of corn stover for enzymatic hydrolysis. Bioresour Technol 282:69–74. https://doi.org/10.1016/j.biortech.2019.02.104
De Wild PJ, Huijgen WJJ, Heeres HJ (2012) Pyrolysis of wheat straw-derived organosolv lignin. Journal of Analytical and Applied Pyrolysis 93:95–103. https://doi.org/10.1016/j.jaap.2011.10.002
Wen JL, Sun SL, Xue BL, Sun RC (2013) Quantitative structural characterization of the lignins from the stem and pith of bamboo (Phyllostachyspubescens). Holzforschung 67(6):613–627. https://doi.org/10.1515/hf-2012-0162
Wildschut J, Smit AT, Reith JH, Huijgen WJ (2013) Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresource technology 135:58–66. https://doi.org/10.1016/j.biortech.2012.10.050
Shimizu K, Usami K (1978) Enzymatic hydrolysis of wood. III Pretreatment of woods with acidic methanol–water mixture MOkuzaigakkaishi 24(9):632–637
Oliet M, Garcıa J, Rodrıguez F, Gilarrranz MA (2002) Solvent effects in autocatalyzed alcohol–water pulping: comparative study between ethanol and methanol as delignifying agents. Chemical Engineering Journal 87(2):157–162. https://doi.org/10.1016/S1385-8947(01)00213-3
Gáspár M, Kálmán G, Réczey K (2007) Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem 42(7):1135–1139. https://doi.org/10.1016/j.procbio.2007.04.003
Zhang Q, Cai W (2008) Enzymatic hydrolysis of alkali-pretreated rice straw by Trichoderma reesei ZM4-F3. Biomass Bioenergy 32(12):1130–1135. https://doi.org/10.1016/j.biombioe.2008.02.006
Tarkow H, FEIST WC (1969) A mechanism for improving the digestibility of lignocellulosic materials with dilute alkali and liquid ammonia. https://doi.org/10.1021/ba-1969-0095.ch012
SumphanwanichJ LN, Srinorakutara T, Akaracharanya A (2008) Evaluation of dilute-acid pretreated bagasse, corn cob and rice straw for ethanol fermentation by Saccharomyces cerevisiae. Ann Microbiol 58(2):219–225
Zhao X, Cheng K, Liu D (2009) Organosolvpretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82(5):815–827
Park N, Kim HY, Koo BW, Yeo H, Choi IG (2010) Organosolvpretreatment with various catalysts for enhancing enzymatic hydrolysis of pitch pine (Pinusrigida). Bioresour Technol 101(18):7046–7053. https://doi.org/10.1016/j.biortech.2010.04.020
Kokorin A (2011) Ionic liquids: applications and perspectives. BoD–Books on Demand
Kim TH, Lee YY, Sunwoo C, Kim JS (2006) Pretreatment of corn stover by low-liquid ammonia recycle percolation process. Appl Biochem Biotechnol 133(1):41–57
Mora-Pale M, Meli L, Doherty TV, Linhardt RJ, Dordick JS (2011) Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol Bioeng 108(6):1229–1245. https://doi.org/10.1002/bit.23108
Moulthrop JS, Swatloski RP, Moyna G, Rogers RD (2005) High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions. Chem Commun 12:1557–1559. https://doi.org/10.1039/B417745B
Alriols MG, Tejado A, Blanco MA, Mondragon I, Labidi J (2009) Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process. Chemical Engineering Journal 148(1):106–114. https://doi.org/10.1016/j.cej.2008.08.008
Ichwan M, Son TW (2011) Study on organosolv pulping methods of oil palm biomass. In: International seminar on chemistry, pp 364–370
Bajpai P (2016) Pretreatment of lignocellulosic biomass for biofuel production. Springer Singapore, Singapore, p 87
Satlewal A, Agrawal R, Bhagia S, Sangoro J, Ragauskas AJ (2018) Natural deep eutectic solvents for lignocellulosic biomass pretreatment: recent developments, challenges and novel opportunities. Biotechnol Adv 36(8):2032–2050. https://doi.org/10.1016/j.biotechadv.2018.08.009
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114(21):11060–11082. https://doi.org/10.1021/cr300162p
Sarmad S, Xie Y, Mikkola JP, Ji X (2017) Screening of deep eutectic solvents (DESs) as green CO 2 sorbents: from solubility to viscosity. New J Chem 41(1):290–301. https://doi.org/10.1039/C6NJ03140D
Pandey A, Dhingra D, Pandey S (2017) Hydrogen bond donor/acceptor cosolvent-modified choline chloride-based deep eutectic solvents. J Phys Chem B 121(16):4202–4212. https://doi.org/10.1021/acs.jpcb.7b01724
Vigier KDO, Chatel G, Jérôme F (2015) Contribution of deep eutectic solvents for biomass processing: opportunities, challenges, and limitations. ChemCatChem 7(8):1250–1260. https://doi.org/10.1002/cctc.201500134
Li W, Zheng P, Guo J, Ji J, Zhang M, Zhang Z, Abbas G (2014) Characteristics of self-alkalization in high-rate denitrifying automatic circulation (DAC) reactor fed with methanol and sodium acetate. Bioresour Technol 154:44–50. https://doi.org/10.1016/j.biortech.2013.11.097
Marcotullio G, De Jong W (2010) Chloride ions enhance furfural formation from D-xylose in dilute aqueous acidic solutions. Greenchemistry 12(10):1739–1746. https://doi.org/10.1039/B927424C
Kamireddy SR, Li J, Tucker M, Degenstein J, Ji Y (2013) Effects and mechanism of metal chloride salts on pretreatment and enzymatic digestibility of corn stover. Ind Eng Chem Res 52(5):1775–1782. https://doi.org/10.1021/ie3019609
Kang KE, Park DH, Jeong GT (2013) Effects of NH4Cl and MgCl2 on pretreatment and xylan hydrolysis of miscanthus straw. Carbohydrate polymers 92(2):1321–1326. https://doi.org/10.1016/j.carbpol.2012.10.019
Sun Y, Lu X, Zhang S, Zhang R, Wang X (2011) Kinetic study for Fe (NO3) 3 catalyzed hemicellulose hydrolysis of different corn stover silages. Bioresour Technol 102(3):2936–2942. https://doi.org/10.1016/j.biortech.2010.11.076
Verardi A, Blasi A, Marino T, Molino A, Calabrò V (2018) Effect of steam-pretreatment combined with hydrogen peroxide on lignocellulosic agricultural wastes for bioethanol production: analysis of derived sugars and other by-products. Journal of energy chemistry 27(2):535–543. https://doi.org/10.1016/j.jechem.2017.11.007
Kim TH, Lee YY (2007) Pretreatment of corn stover by soaking in aqueous ammonia at moderate temperatures. In: Applied biochemistry and biotechnology. Humana press, pp 81–92
Drapcho CM, Nhuan NP, Walker TH (2008) Biofuels engineering process technology (no. Sirsi) i9780071487498. McGraw-Hill, New York
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685. https://doi.org/10.1016/j.biotechadv.2011.05.005
Rabemanolontsoa H, Saka S (2016) Various pretreatments of lignocellulosics. Bioresour Technol 199:83–91. https://doi.org/10.1016/j.biortech.2015.08.029
Varga E, Schmidt AS, Réczey K, Thomsen AB (2003) Pretreatment of corn Stover using wet oxidation to enhance enzymatic digestibility. Appl Biochem Biotechnol 104(1):37–50
Kim KH, Hong J (2001) Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour Technol 77(2):139–144. https://doi.org/10.1016/S0960-8524(00)00147-4
Zheng Y, Lin HM, Wen J, Cao N, Yu X, Tsao GT (1995) Supercritical carbon dioxide explosion as a pretreatment for cellulose hydrolysis. Biotechnol Lett 17(8):845–850
Nakamura Y, Daidai M, Kobayashi F (2004) Ozonolysis mechanism of lignin model compounds and microbial treatment of organic acids produced. Water Sci Technol 50(3):167–172. https://doi.org/10.2166/wst.2004.0188
Hammel KE, Kapich AN, Jensen KA Jr, Ryan ZC (2002) Reactive oxygen species as agents of wood decay by fungi. Enzyme and microbial technology 30(4):445–453. https://doi.org/10.1016/S0141-0229(02)00011-X
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101(13):4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Lucas M, Hanson SK, Wagner GL, Kimball DB, Rector KD (2012) Evidence for room temperature delignification of wood using hydrogen peroxide and manganese acetate as a catalyst. Bioresource technology 119:174–180. https://doi.org/10.1016/j.biortech.2012.05.086
Suhara H, Kodama S, Kamei I, Maekawa N, Meguro S (2012) Screening of selective lignin-degrading basidiomycetes and biological pretreatment for enzymatic hydrolysis of bamboo culms. International Biodeterioration& Biodegradation 75:176–180. https://doi.org/10.1016/j.ibiod.2012.05.042
Du W, Yu H, Song L, Zhang J, Weng C, Ma F, Zhang X (2011) The promoting effect of byproducts from Irpexlacteus on subsequent enzymatic hydrolysis of bio-pretreated cornstalks. Biotechnology for biofuels 4(1):37
Cianchetta S, Di Maggio B, Burzi PL, Galletti S (2014) Evaluation of selected white-rot fungal isolates for improving the sugar yield from wheat straw. Appl Biochem Biotechnol 173(2):609–623
Taha M, Shahsavari E, Al-Hothaly K, Mouradov A, Smith AT, Ball AS, Adetutu EM (2015) Enhanced biological straw saccharification through coculturing of lignocellulose-degrading microorganisms. Appl Biochem Biotechnol 175(8):3709–3728
CastoldiR BA, de Morais GR, Baesso ML, RCG C, Peralta RA, Peralta RM (2014) Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: study of degradation patterns and saccharification kinetics. Chemical Engineering Journal 258:240–246. https://doi.org/10.1016/j.cej.2014.07.090
Potumarthi R, Baadhe RR, Nayak P, Jetty A (2013) Simultaneous pretreatment and sacchariffication of rice husk by Phanerochetechrysosporium for improved production of reducing sugars. Bioresource technology 128:113–117. https://doi.org/10.1016/j.biortech.2012.10.030
Song L, Yu H, Ma F, Zhang X (2013) Biological pretreatment under non-sterile conditions for enzymatic hydrolysis of corn stover. BioResources 8(3):3802–3816
Wan C, Li Y (2011) Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feedstocks. Bioresource technology 102(16):7507–7512. https://doi.org/10.1016/j.biortech.2011.05.026
Dhiman SS, Haw JR, Kalyani D, Kalia VC, Kang YC, Lee JK (2015) Simultaneous pretreatment and saccharification: green technology for enhanced sugar yields from biomass using a fungal consortium. Bioresource technology 179:50–57. https://doi.org/10.1016/j.biortech.2014.11.059
Taniguchi M, Suzuki H, Watanabe D, Sakai K, Hoshino K, Tanaka T (2005) Evaluation of pretreatment with Pleurotusostreatus for enzymatic hydrolysis of rice straw. Journal of bioscience and bioengineering 100(6):637–643. https://doi.org/10.1263/jbb.100.637
Shirkavand E, Baroutian S, Gapes DJ, Young BR (2016) Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment–a review. Renewable and Sustainable Energy Reviews 54:217–234. https://doi.org/10.1016/j.rser.2015.10.003
Dutra ED, Santos FA, Alencar BRA, Reis ALS, de Souza RDFR, da Silva Aquino KA et al (2018) Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: status and perspectives. Biomass Conversion and Biorefinery 8(1):225–234
Akhtar N, Goyal D, Goyal A (2017) Characterization of microwave-alkali-acid pre-treated rice straw for optimization of ethanol production via simultaneous saccharification and fermentation (SSF). Energy Convers Manag 141:133–144. https://doi.org/10.1016/j.enconman.2016.06.081
Molaverdi M, Karimi K, Mirmohamadsadeghi S, Galbe M (2019) High titer ethanol production from rice straw via solid-state simultaneous saccharification and fermentation by Mucorindicus at low enzyme loading. Energy Convers Manag 182:520–529. https://doi.org/10.1016/j.enconman.2018.12.078
Qiu J, Tian D, Shen F, Hu J, Zeng Y, Yang G, Zhang J (2018) Bioethanol production from wheat straw by phosphoric acid plus hydrogen peroxide (PHP) pretreatment via simultaneous saccharification and fermentation (SSF) at high solid loadings. Bioresour Technol 268:355–362. https://doi.org/10.1016/j.biortech.2018.08.009
Yuan Z, Li G, Hegg EL (2018) Enhancement of sugar recovery and ethanol production from wheat straw through alkaline pre-extraction followed by steam pretreatment. Bioresour Technol 266:194–202. https://doi.org/10.1016/j.biortech.2018.06.065
Yuan Z, Wen Y, Li G (2018) Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour Technol 259:228–236. https://doi.org/10.1016/j.biortech.2018.03.044
Yuan W, Gong Z, Wang G, Zhou W, Liu Y, Wang X, Zhao M (2018) Alkaline organosolvpretreatment of corn stover for enhancing the enzymatic digestibility. Bioresource technology 265:464–470. https://doi.org/10.1016/j.biortech.2018.06.038
Vergara P, Ladero M, García-Ochoa F, Villar JC (2018) Pre-treatment of corn stover, Cynaracardunculus L. stems and wheat straw by ethanol-water and diluted sulfuric acid: comparison under different energy input conditions. Bioresource technology 270:449–456. https://doi.org/10.1016/j.biortech.2018.09.058
Hilares RT, Kamoei DV, Ahmed MA, da Silva SS, Han JI, dos Santos JC (2018) A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrasonicssonochemistry 43:219–226. https://doi.org/10.1016/j.ultsonch.2018.01.016
Wang Z, Dien BS, Rausch KD, Tumbleson ME, Singh V (2018) Fermentation of undetoxified sugarcane bagasse hydrolyzates using a two stage hydrothermal and mechanical refining pretreatment. Bioresour Technol 261:313–321. https://doi.org/10.1016/j.biortech.2018.04.018
BondJQ UAA, Olcay H, Tompsett GA, Jae J, Xing R, Foster A (2014) Production of renewable jet fuel range alkanes and commodity chemicals from integrated catalytic processing of biomass. Energy Environ Sci 7(4):1500–1523
de Beeck BO, Dusselier M, Geboers J, Holsbeek J, Morré E, Oswald S, Sels BF (2015) Direct catalytic conversion of cellulose to liquid straight-chain alkanes. Energy Environ Sci 8(1):230–240
Cai CM, Zhang T, Kumar R, Wyman CE (2014) Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J Chem Technol Biotechnol 89(1):2–10. https://doi.org/10.1002/jctb.4168
Wang T, Nolte MW, Shanks BH (2014) Catalytic dehydration of C 6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem 16(2):548–572. https://doi.org/10.1039/C3GC41365A
Tang X, Zeng X, Li Z, Hu L, Sun Y, Liu S, Lin L (2014) Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renewable and Sustainable Energy Reviews 40:608–620. https://doi.org/10.1016/j.rser.2014.07.209
Shuai L, Questell-Santiago YM, Luterbacher JS (2016) A mild biomass pretreatment using γ-valerolactone for concentrated sugar production. Green Chem 18(4):937–943. https://doi.org/10.1039/C5GC02489G
Pang J, Zheng M, Sun R, Wang A, Wang X, Zhang T (2016) Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem 18(2):342–359. https://doi.org/10.1039/C5GC01771H
Hassanin AH, Hamouda T, Candan Z, Kilic A, Akbulut T (2016) Developing high-performance hybrid green composites. Compos Part B 92:384–394. https://doi.org/10.1016/j.compositesb.2016.02.051
Pileidis FD, Titirici MM (2016) Levulinic acid biorefineries: new challenges for efficient utilization of biomass. ChemSusChem 9(6):562–582. https://doi.org/10.1002/cssc.201501405
Koutinas AA, Vlysidis A, Pleissner D, Kopsahelis N, Garcia IL, Kookos IK, Lin CSK (2014) Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem Soc Rev 43(8):2587–2627. https://doi.org/10.1039/C3CS60293A
Strassberger Z, Tanase S, Rothenberg G (2014) The pros and cons of lignin valorisation in an integrated biorefinery. RSC Adv 4(48):25310–25318. https://doi.org/10.1039/C4RA04747H
Li SH, Liu S, Colmenares JC, Xu YJ (2016) A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chemistry 18(3):594–607. https://doi.org/10.1039/C5GC02109J
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Progress in energy and combustion science 42:35–53. https://doi.org/10.1016/j.pecs.2014.01.001
Joelsson E, Erdei B, Galbe M, Wallberg O (2016) Techno-economic evaluation of integrated first-and second-generation ethanol production from grain and straw. Biotechnology for biofuels 9(1):1
Zhang Y, Fu X, Chen H (2012) Pretreatment based on two-step steam explosion combined with an intermediate separation of fiber cells-optimization of fermentation of corn straw hydrolysates. Bioresour Technol 121:100–104. https://doi.org/10.1016/j.biortech.2012.07.006
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36(5):2328–2342. https://doi.org/10.1016/j.energy.2011.03.013
Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Pandey A (2010) Bioethanol production from rice straw: an overview. Bioresour Technol 101(13):4767–4774. https://doi.org/10.1016/j.biortech.2009.10.079
Taherzadeh MJ, Niklasson C (2004) Ethanol from lignocellulosic materials: pretreatment, acid and enzymatic hydrolyses, and fermentation. https://doi.org/10.1021/bk-2004-0889.ch003
Knauf M, Moniruzzaman M (2004) Lignocellulosic biomass processing: a perspective. International sugar journal 106(1263):147–150
Kumar A, Kumar N, Baredar P, Shukla A (2015) A review on biomass energy resources, potential, conversion and policy in India. Renew Sust Energ Rev 45:530–539. https://doi.org/10.1016/j.rser.2015.02.007
Zhuang X, Wang W, Yu Q, Qi W, Wang Q, Tan X, Yuan Z (2016) Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour Technol 199:68–75. https://doi.org/10.1016/j.biortech.2015.08.051
Ji G, Gao C, Xiao W, Han L (2016) Mechanical fragmentation of corncob at different plant scales: impact and mechanism on microstructure features and enzymatic hydrolysis. Bioresource technology 205:159–165. https://doi.org/10.1016/j.biortech.2016.01.029
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. https://doi.org/10.1016/j.biortech.2015.08.061
García A, Alriols MG, Labidi J (2014) Evaluation of different lignocellulosic raw materials as potential alternative feedstocks in biorefinery processes. Ind Crop Prod 53:102–110. https://doi.org/10.1016/j.indcrop.2013.12.019
de Carvalho DM, Sevastyanova O, Penna LS, da Silva BP, Lindström ME, Colodette JL (2015) Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid and alkaline pretreatments. Ind Crop Prod 73:118–126. https://doi.org/10.1016/j.indcrop.2015.04.021
Meng X, Ragauskas AJ (2014) Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Current opinion in biotechnology 27:150–158. https://doi.org/10.1016/j.copbio.2014.01.014
Jagmann N, Philipp B (2014) Design of synthetic microbial communities for biotechnological production processes. Journal of biotechnology 184:209–218. https://doi.org/10.1016/j.jbiotec.2014.05.019
Ji-Lu Z (2007) Bio-oil from fast pyrolysis of rice husk: yields and related properties and improvement of the pyrolysis system. J Anal Appl Pyrolysis 80(1):30–35. https://doi.org/10.1016/j.jaap.2006.12.030
Lu Q, Yang XL, Zhu XF (2008) Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J Anal Appl Pyrolysis 82(2):191–198. https://doi.org/10.1016/j.jaap.2008.03.003
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renewable energy 37(1):19–27. https://doi.org/10.1016/j.renene.2011.06.045
Chandel AK, Gonçalves BC, Strap JL, da Silva SS (2015) Biodelignification of lignocellulose substrates: an intrinsic and sustainable pretreatment strategy for clean energy production. Crit Rev Biotechnol 35(3):281–293. https://doi.org/10.3109/07388551.2013.841638
Balat M (2009) Production of hydrogen via biological processes. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 31(20):1802–1812.10.1080/15567030802463109
Putro JN, Soetaredjo FE, Lin SY, Ju YH, Ismadji S (2016) Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv 6(52):46834–46852. https://doi.org/10.1039/C6RA09851G
Sivagurunathan P, Kumar G, Bakonyi P, Kim SH, Kobayashi T, Xu KQ, Bélafi-Bakó K (2016) A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int J Hydrog Energy 41(6):3820–3836. https://doi.org/10.1016/j.ijhydene.2015.12.081
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9(9):1621–1651. https://doi.org/10.3390/ijms9091621
Delidovich I, Hausoul PJ, Deng L, Pfützenreuter R, Rose M, Palkovits R (2016) Alternative monomers based on lignocellulose and their use for polymer production. Chemical reviews 116(3):1540–1599. https://doi.org/10.1021/acs.chemrev.5b00354
Romero A, Alonso E, Sastre Á, Nieto-Márquez A (2016) Conversion of biomass into sorbitol: cellulose hydrolysis on MCM-48 and d-glucose hydrogenation on Ru/MCM-48. Microporous and Mesoporous Materials 224:1–8. https://doi.org/10.1016/j.micromeso.2015.11.013
Choi S, Song CW, Shin JH, Lee SY (2015) Biorefineries for the production of top building block chemicals and their derivatives. Metab Eng 28:223–239. https://doi.org/10.1016/j.ymben.2014.12.007
Kang S, Li X, Fan J, Chang J (2013) Hydrothermal conversion of lignin: a review. Renew Sust Energ Rev 27:546–558. https://doi.org/10.1016/j.rser.2013.07.013
Ma R, Xu Y, Zhang X (2015) Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem 8(1):24–51. https://doi.org/10.1002/cssc.201402503
Gellerstedt G, Li J, Eide I, Kleinert M, Barth T (2008) Chemical structures present in biofuel obtained from lignin. Energy Fuel 22(6):4240–4244. https://doi.org/10.1021/ef800402f
Yoshikawa T, Yagi T, Shinohara S, Fukunaga T, Nakasaka Y, TagoT MT (2013) Production of phenols from lignin via depolymerization and catalytic cracking. Fuel Process Technol 108:69–75. https://doi.org/10.1016/j.fuproc.2012.05.003
Roberts VM, Stein V, Reiner T, Lemonidou A, Li X, Lercher JA (2011) Towards quantitative catalytic lignin depolymerization. Chem Eur J 17(21):5939–5948. https://doi.org/10.1002/chem.201002438
Brinchi L, Cotana F, Fortunati E, Kenny JM (2013) Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr Polym 94(1):154–169. https://doi.org/10.1016/j.carbpol.2013.01.033
Wang L, Mu G, Tian C, Sun L, Zhou W, Yu P, Fu H (2013) Porous graphitic carbon nanosheets derived from cornstalk biomass for advanced supercapacitors. ChemSusChem 6(5):880–889. https://doi.org/10.1002/cssc.201200990
Yang J, Christiansen K, Luchner S (2013) Renewable, low-cost carbon fiber for light weight vehicles. Detroit, US Department of Energy
Funding
The authors acknowledge the funding provided by the BPCL OUAT Biofuel project implemented in OUAT, Bhubaneswar, for providing financial support in the preparation of this paper.
Author information
Authors and Affiliations
Contributions
All authors equally contributed towards the conceptions design, interpretation of data, drafting, revising, and approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, N., Jena, P.K., Padhi, D. et al. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Conv. Bioref. 13, 1503–1527 (2023). https://doi.org/10.1007/s13399-021-01294-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01294-3