Abstract
Most of the lignocellulosic biomass residues are decomposed and transformed by a variety of microbes in the natural environment. The xylanase production from Aspergillus niger strain BG has been produced using wheat bran under solid-state fermentation (SSF). One factor at a time approach (OFAT) was used to optimize the effect of the incubation period, initial pH, moisture content, and cultivation temperature on the xylanase production. Furthermore, experiments were designed with a Box–Behnken design (BBD) on the same variables using response surface methodology (RSM). Analysis of variance (ANOVA) was carried out and the xylanase production was expressed with a mathematical equation depending on the factors. Maximum xylanase yield after OFAT approach and RSM optimization was significant with maximum values of 4008.25 ± 3.73 U/g of dry substrate (U/gds) and 5427.51 ± 4.4 U/gds which have been recorded respectively compared with the initial conditions (1899.02 ± 1.6 U/gds) after 7 days of fermentation. The effects of individual, interaction, and square terms on xylanase production were represented using the non-linear regression equations with significant R2 and p values. The optimum conditions established by RSM method for the maximum xylanase production were obtained with a pH media of 2.5 at 37 °C using wheat bran as 84% humidified substrate after 66 h of incubation, this conditions resulted in 65.01% increased level of the xylanase production than produced in the initial conditions. Xylanase production from Aspergillus niger strain BG using RSM is considered advantageous for bioconversion of the agriculture residues.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world’s present economic system is highly reliant on diverse fossil energy sources such as oil, coal, and natural gas [1]. Several alternative energies can change fossil fuels in the future, such as biomass, solar, wind, hydro, and ocean thermal energy. Within these energy sources, biomass is the only carbon-based sustainable energy used around the world [2]. Lignocellulose is the principle element of plant biomass. It consists of three main components: cellulose, hemicelluloses, and lignin. Lignocellulose and its derivatives are the principal constituents of agricultural waste used as solid substrates in both energy and environmental sectors. Xylan is the most abundant hemicellulose in the cell wall of higher plants [3]. Xylanase (endo-1,4-β-D-xylanase; EC 3.2.1.8) is the term generic which utilized to indicate enzyme particularly important to catalyze the cleavage of internal β-(1,4)-linked of long-chain xylan into short xylooligosaccharides, which are further hydrolyzed to D-xylose by β-1,4-xylosidases (β-D-xyloside xylohydrolase; EC 3.2.1.37) [4, 5]. This group of enzyme is produced by various organisms including crustaceans, protozoans, seeds, snails, insects, marine algae, bacteria, fungi, and yeast. Owing to secreting high-level xylanase in the culture medium, among microorganisms, filamentous fungi are within the most potent producers of xylanase. Furthermore, Aspergillus genus principally Aspergillus niger is commonly known as xylanase producer in industrial plants such as in paper manufacturing, animal feed, bread-making, juice, and wine industries [6,7,8]. In this context, the valorization of lignocellulosic biomass for the production of the enzymes by either submerged or solid-sate fermentation has been highly studied [9, 10], and the solid-state fermentation has successfully applied for biofuels, biotransformation, biological detoxification, and bioremediation, in solving an energy crisis and environmental pollution, and the application in environmental protection is to enhance their nutritional value [11]. Optimization of the fermentation conditions to achieve high enzyme yield. This uni-dimensional approach is simple and easy but did not respect different factor interactions. On the other hand, in the RSM method, it is possible to identify interaction effects between input parameters [12]. Wheat bran, the agricultural residue is a low-cost resource available in Algeria. In the present work, wheat bran is used as substrate to reduce the cost of xylanase production. The aim of this present study was carried out to optimize fermentation conditions, using OFAT approach and the RSM experiment based on the BBD design to select an optimal range of physicochemical parameters for maximizing xylanase production by A. niger strain BG using wheat bran in the solid-state fermentation (Fig. 1). The optimization of xylanase production is believed to open new horizons for a wide range of industrial applications.
2 Materials and methods
2.1 Substrate and chemicals
Carboxy-methyl cellulose (CMC), Beechwood xylan, and analytical grade reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA). All other chemicals were of the analytical grade unless otherwise stated.
2.2 Microorganisms
The xylanase-producing microorganism was isolated from soil decaying abundant on olive tree collected from Akbou area, Bejaia, located in North-east of Algeria. Carboxy-methyl cellulose (CMC) agar medium supplemented with 0.01% gentamycin was used for isolation of the fungal strain, a soluble cellulose derivative such as CMC was used as the sole source of carbon and energy, and was able to induce cellulases and xylanases production [13]; 1 gram of sample was transferred to the aliquots of 9 ml of physiological water. It was shaken vigorously with vortex at a constant rate for 15 min. The suspension was then subjected to serial dilutions than inoculated in Petri dishes. The Petri dishes were incubated for 7 days at 28 °C. The grown has rapidly appeared, and single colonies were picked up, sub-cultivated on the medium potato dextrose agar (PDA was purchased from Thermo Fisher Scientific), and were incubated at 28 °C for 7 days, then stored at 4 °C; the culture conserved as a suspension of spores and mycelium in 25% (v/v) sterile glycerol at − 20 °C [13, 14]. The isolate was identified based on its morphological characteristics, and the internal transcribed spacer (ITS) region of the rDNA gene and its internal transcribed spacer (ITS) sequences were amplified using a pair of universal primers designed by White et al. [15], ITS1(5′-TCCGTAGGTGAACCTGCGG-3′) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′). PCR analysis was performed according to the method described by Gonzalez-Mendoza et al. [16].
2.3 Preparation of spore suspensions
The sample which contains fungi was left at room temperature (25 °C), then inoculated in PDA medium at pH 5.0, and incubated at 28 °C for 7 days, for xylanase production and optimization under solid-state fermentation (SSF). Spore was harvested delicately to prevent detachment of the mycelium by adding 10 ml of sterile distilled water containing 1% (v/v) tween 80 and then collected in sterilized bottles for use as an inoculum for enzyme production, the suspension was diluted by 1:100 (v/v), and the spore count was performed in a counting chamber (Malassez REF 06 106 10 MARIENFELD, Germany) [17, 18].
2.4 Xylanase production under solid-state fermentation
Wheat bran a lignocellulosic substrate was purchased from the local market of Akbou (Algeria) and was thoroughly washed in distilled water, dried at 80 °C for 24 h, and then stored for further use. Aspergillus niger strain BG was cultivated using solid-state fermentation in a 500-ml flask containing wheat bran (10 g) dry substrates moistened at 70% in Mandels medium [19], and the initial pH medium was adjusted to 5.0. A spore suspension (107 spores/g) of Aspergillus niger strain BG was inoculated in a 500-ml flask and incubated at 28 °C for 7 days. Thereafter, the enzymatic extract was harvested by mixing the contents of the flasks in 100 ml distilled water with a glass rod and then shaking on an orbital shaker at 100 rpm for 10 min at room temperature. The filtrate was centrifuged at 10000g for 10 min at 4 °C. Cell-free supernatant served as the crude extracellular enzyme, was stored at 4 °C, and used for further investigations [20].
2.5 Enzyme assay
Xylanase activity was determined according to Bailey et al. [21], using Beechwood xylan as substrate. The liberation of reducing sugars was estimated by the dinitro-salicylic-acid (DNS) method [22]. A 100-μl culture supernatant was added to 900 μl xylan solution (2%, pH 4.8, 50 mM sodium citrate buffer) and incubated at 50 °C. After 10 min, then, 1.5 ml of 3,5-dinitrosalicylic acid reagent was added to stop the reaction, and the amount of reducing sugars released in the reaction was estimated by measuring the absorbance at 540 nm [22] and compared with a standard curve of xylose (0 to 1 μmol/ ml). One unit of the enzyme was defined as the amount of the enzyme per 1 ml needed to liberate 1 μmol of reducing sugar from a substrate per minute under the stated assay conditions [23, 24].
2.6 OFAT approach for enhanced xylanase production
Various cultivation conditions were adjusted for optimal xylanase production, using OFAT approach. Optimized conditions were then used sequentially in the subsequent fermentation [24]. In these experiments, the conditions for xylanase production by Aspergillus niger strain BG were optimized. Standard inocula (107spores/g) were inoculated in each 500-ml Erlenmeyer flask containing 10 g of wheat bran as substrate.
2.6.1 Effect of the incubation period
The effect of incubation period was studied by carrying a fermentation using Aspergillus niger strain BG in Erlenmeyer flasks (500 ml) containing 10-g wheat bran powder as substrate at 28 °C with 70% of moisture. The initial pH was adjusted to 5.0. The enzyme activity was measured for 7 days with 24 h as intervals, at the desired initial pH (5.0), moisture (70%), and temperature (28 °C). The effect of incubation period was studied by carrying a fermentation experiment for up to 7 days, where the enzyme production was measured at 24-h intervals.
2.6.2 Effect of initial pH value
To estimate the effects of initial culture pH of solid-state fermentation on xylanase production, the initial pH values were assessed by adjusting the initial pH of production medium at 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5, and 10.0 with hydrochloric acid (HCl) or sodium hydroxide (NaOH) solutions. The production of xylanase by Aspergillus niger strain BG was studied by incubating the flasks at 28 °C for the optimum time determined before, and then xylanase activity was determined.
2.6.3 Effect of moisture level
The effect of moisture level on xylanase production was studied by varying initial moisture contents at 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, and 95. The prepared flasks were incubated under optimum pH and incubation period.
2.6.4 Effect of incubation temperature
Incubation temperature was optimized by varying the temperature at 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, and 40 °C and incubating the inoculated flasks for optimum humidity, pH, and optimal incubation time, the enzyme activity was assayed.
2.7 Statistical analysis
In this study, all assays were performed in triplicate measurements and expressed as mean values ± SD. The data was subjected to one-way analysis of variance (ANOVA), using XLSTAT software (version 2009.1.02), and a p value < 0.05 is deemed as significant.
2.8 RSM approach for enhanced xylanase production using BBD exploratory model
Optimization of the solid-state fermentation for xylanase production focused a single-factor-test to determine the preliminary range of the extraction variables including A, fermentation time; B, effect of moisture level; C, initial pH; and D, incubation temperature (Table 1). Based on the OFAT experimental results, high influence factors and their levels were confirmed. Twenty-seven experiments with triplicates of the central point were employed (Table 2) to fit the polynomial model which is based on a Box–Behnken design (BBD, 4 variables) achieved by Design Expert 10®software (Version10.0.5.0.USA) and was conducted to optimize the processes. A three-level and four factors, BBD experimental design was tested and the number of the trial (N) intended was defined according to Eq (1):
k is the number of factors and C0 is the number of central points (3). For each assay, SSF employed 10 g of wheat bran and the initial pH (C) was adjusted according to the levels presented in Table 1
The substrate was uniformly distributed into 500-ml Erlenmeyer flasks and sterilized by autoclave at 121 °C for 20 min at 15 psi. When the medium reached room temperature, a suspension of 107spores/g was spread evenly over the surface of each sample and incubated for three levels at different incubation temperatures (A). Regression analysis of the data to fit a second-order polynomial equation (quadratic model) was carried out according to the following general equation (Eq. (2)) which was, then, used to predict the optimum conditions of the xylanase production.
R represents the response surfaces, β0 is the constant term (intercept), βA, βB, βC, and βD represent the coefficients of the linear parameters, A, B, C, and D represent the variables, βAA, βBB, βCC, and βDD represent the coefficients of the quadratic parameter, βAB, βAC, βAD, βBC, βBD, and βCD represent the coefficients of the interaction parameters, and ε is the residual associated with the experiments, the prediction error, represents the difference between measured R values and predicted R and quantifies the random variability in this design of the experiment. R was used to perform regression analyses and ANOVA for the regression.
The equation model was put to experimental data to output the proposed model. The corresponding coefficients of variables, interaction variables, and response surface graphs were drawn by Design Expert10. Statistical analysis of the significance of the coefficient estimations was performed via Fisher F test. The optimum values of the selected variables were obtained by solving the regression equation and by analyzing the response surface plots, the proposed model was validated by performing new assays in triplicate [17, 23].
3 Result and discussion
3.1 Strain identification
Based on morphological observations, Aspergillus niger strain BG had dark brown to black conidial heads, which is indicative of the genus Aspergillus. To identify this strain at the molecular level, the ITS sequence was amplified and analyzed. The alignment of this ITS fragment with all related sequences in the NCBI database by the BLASTN program showed that it was identical to those of strain Aspergillus niger (100% identity). The results reported in this research for the nucleotide sequence of the 5.8 S rDNA gene (413 bp) were deposited in the GenBank databases under accession number MG792343.
3.2 Xylanase optimization using OFAT approach
In this context, wheat bran was used as substrate for xylanase production by Aspergillus niger strain BG in solid-state fermentation, 1899.02 ± 1.6 U/gds activity was registered under standard condition. Furthermore, varying operating conditions such as incubation time, effect of moisture level, initial pH, and temperature were optimized xylanase enzymes in solid-state fermentation.
The time of incubation plays a vital role in the production of enzymes and was considered as significant factor affecting on xylanase production (p value < 0.05). Figure 2a illustrates the effect of incubation time on the production of xylanase enzyme. The optimal production of xylanase enzyme was achieved after 2 days (3478.85 ± 0.6 U/gds) of fermentation; Okafor et al., Abdel-Sater and El-Said, Thomas et al., and Singh et al., achieved maximum production of xylanase in 4, 8, 4, and 7 days with Penicillium chrysogenum PCL501, Trichoderma harzianum, and Aspergillus sp. SH-1 and SH-2 strains respectively [19, 25,26,27].
To check the optimum initial medium pH for xylanase production, the experiments showed that maximum enzyme production (3894.80 ± 2.78 U/gds) was obtained in pH 2 (Fig. 2b). Different investigations reported that initial medium pH of 4.5, 6.0, and 6.5 was best for fungal xylanase production [11, 27, 28]; these reports indicating that most of the fungi exhibit an acidic environment for their growth [11].
Xylanase production was significantly affected by the initial pH with a p value < 0.05, and as shown in Fig. 2b, the pH profile strongly indicates that enzyme production is influenced by variations in the pH of the medium. In particular, for an extracellular enzyme, its cell release into the medium relies on the mechanism of membrane transport, which is regulated by the concentration of the hydrogen in the medium and may be the hydrogen ion effect on the stability of enzymes [29].
The effect of medium humidity on xylanase biosynthesis was studied for a humidity range of 30–95% and was statistically significant (p value < 0.05). The optimal humidity was 85% (Fig. 2c), and the maximum xylanase production 4008.25 ± 3.73 U/gds was obtained. Nutrient availability might influence xylanase production by varying moisture level [20, 30]. Forty-three percent and 83% were the optimum moisture level for xylanase production, which were reported by strains Aspergillus niger and Paecilomyces thermophila respectively [31, 32].
The results of the test made at different temperature values showed that the optimal temperature for xylanase activity (4084.75 ± 0.30 U/gds) was 36 °C (Fig. 2d). The effect of incubation temperature was statistically significant (p value < 0.05) in xylanase production, and the results showed that the enzyme production decreased when the temperature increases above 36 °C. Many workers have reported different temperatures for maximum xylanase production. Fusarium oxysporum in shake flask cultures also produces maximum xylanase yield at an incubation temperature of 30 °C. Gautam et al. and Irfan et al. [33, 34] registered the maximum xylanase production at 30 °C by Aspergillus foetidus and Aspergillus flavus ARC-12 respectively, also Zehra et al. [35] reported the maximum xylanase production at 35 °C by Aspergillus fumigatus MS16. At lower temperature, the production of xylanase decreased owing to lower transfer of substrates in the cell membrane; a higher temperature could induce to reduce growth due to the denaturation of enzymes, which results in the higher preservation energy for cellular growth and lower metabolites generation [36].
A classical optimization approach for xylanase production by Aspergillus niger strain BG on wheat bran was measuring xylanase activity at the varied incubation conditions. Production was characterized by short incubation time (two days), acidity pH (pH 2), large moistening level (85%), and mesophilic temperature (36 °C); however, the strain was able to produce higher amounts of xylanase with an activity of 4008.25 ± 3.73 U/gds.
3.3 Model establishment
3.3.1 Modeling and fitting the model using RSM
BBD matrix and the corresponding results of RSM experiments are shown in Table 2, which are the best design for response surface optimization. The production was selected with four factors including incubation temperature (A), effect of moisture level (B), initial pH (C), and incubation time (D). All factors were kept constant at the optimum level that they obtained in the OFAT trial. Afterward, the Box–Behnken design was used to optimize the level of each of these factors giving the highest production and to study their interaction.
The ANOVA for the model is grouped in Table 3. This last demonstrated that the model is highly significant with a p < 0.0001, and the model F value of 13.41 implies the model is significant. There is only a 0.01 % chance that an F value this large could occur due to noise. The values of “Prob > F” less than 0.05 are indicated model terms are significant and in these cases, A, B, C, D, AD, A2, B2, C2, and D2 are significant model terms.
Values greater than 0.1 indicate that model terms are not significant if there are many insignificant model terms (not counting those required to support hierarchy). The lack-of-fit F value of 4.81 signifies the lack-of-fit is insignificant relative to the pure error. There is an 18.44 % chance that a lack of fit F value this large could occur due to noise. Non-significant lack-of-fit is suitable; it is desired the model to fit. According to Bezerra et al. [37], significant regression and a non-significant lack-of-fit present in the model was well fitted to the experiments.
Figure 3 shows a good correlation between the actual and predicted xylanase production and linear distribution is indicative of a well-fitted model and demonstrates that the model is quite realistic since it shows no significant differences between experimental and predicted values [38].
The generated models were employed subsequently to study the effect of various parameters and their interactions on the xylanase activity, and the model for the predicted response could be expressed by the quadratic polynomial equations (in the form of coded factors) and was achieved as follows Eq (3):
A is the incubation temperature, B is the effect of moisture level, C is the initial pH, and D is the incubation time. The positive sign in front of the terms designates a synergistic effect, while the negative sign indicates an antagonistic effect. Negative values of a coefficient estimate denote a negative influence of parameters on the xylanase enzyme product. This quadratic model was found to have a coefficient of determination value (R2) of 0.9399 confirming the effectiveness of the model, which means that 93.99% of the total variation in the observed results was assigned to the independent variables. Therefore, the high value of R2 obtained in this regression model indicates a good agreement between predicted and real xylanase activity. The value of the coefficient of variation (CV%) was 19.31% and the “Adequate Precision” ratio of 12.21 suggested that the model was reliable and reproducible agreeing previous reports compare the range of predicted values at design points with the average prediction error [39, 40]. Ratios greater than four indicate adequate model discrimination [41]. It is interesting to examine in Fig. 4 that the interaction plots of A, B, C, and D on xylanase activity, as can be seen from Table 3, there is a significant interactions between incubation temperature vs. incubation time (AD) (p 0.02 < 0.05). By contrast, a no significant interaction is observed between; incubation temperature vs. effect of moisture level (AB) (p 0.53 > 0.05), incubation temperature vs. initial pH (AC) (p 0.13 > 0.05), effect of moisture level vs. initial pH (BC) (p 0.79 > 0.05), effect of moisture level vs. incubation time (BD) (p 0.10 > 0.05), and initial pH vs. incubation time (CD) (p 0.84 > 0.05).
Interaction plots of the experimental factors on the xylanase production by solid-state fermentation with Aspergillus niger strain BG on wheat bran. Interaction between the variables, incubation temperature vs. effect of moisture level (a), incubation temperature vs. initial pH (b), incubation temperature vs. incubation time (c), effect of moisture level vs. initial pH (d), effect of moisture level vs. incubation time (e), and initial pH vs. incubation time (f)
3.3.2 Analysis of response surfaces model
The relationship between parameters and responses can be explained by studying the contour plot (two dimensional 2D) response surface plots for xylanase activity; this response was generated from the predicted quadratic model. The 2D response surface plot can also be used to determine the optimum level of each variable for xylanase activity (Fig. 5a–f). While maintaining other variables at their optimal level, any two variables were constructed in the response surface plot.
Response surface analysis for xylanase production by solid-state fermentation of Aspergillus niger strain BG on wheat bran and the contour plot showing interaction between incubation temperature and effect of moisture level (a), incubation temperature and initial pH (b), incubation temperature and incubation time (c), effect of moisture level and initial pH (d), effect of moisture level and incubation time (e), and initial pH and incubation time (f)
Figures 5a–b illustrate combinations of the effects of independent variables on the xylanase activity, with data obtained from the surface response graph, a tendency can be observed of the xylanase activity as a function of A and B (Fig. 5a) and the interaction between A and C (Fig. 5b). These parameters are insignificantly influenced xylanase production. Optimization of fermentation parameters allowed obtaining values of temperature, effect of moisture level, and initial pH in studies conducted by Nishio et al., and Yadav [42, 43] reported that the moisture content and temperature were considered relevant to SSF process and they were the major parameters related with the best enzymes production in SSF using fungi [17, 44].
Figure 5c illustrates the effect of A and D on the xylanase activity when the initial pH and effect of moisture level were fixed at level 0. The xylanase activity increases significantly (p < 0.05) with increasing the temperature and fermentation time, these parameters to an increase in xylanase activity. The xylanase activity mainly depends on the temperature and fermentation time as its quadratic and linear effects were highly significant (p < 0.001), confirming the single-factor experiment results (Table 3). These two parameters may have influenced the metabolism responsible for xylanase production. Temperature is a very significant factor in the fermentation process. High- and low-temperature activity may result in the reduction of microbial growth and consequently lower production of the enzyme, but Behnam et al. [45] reported that the interaction term of temperature and time was insignificant in the optimization of xylanase production by strain Mucor indicus through SSF. According to studies conducted by dos Santos et al., Pathania et al., and Yegin et al. [46,47,48], an interaction between an incubation time and temperature has an impact on maximum xylanase production by strains Aureobasidium pullulans Y-2311-1, Aspergillus niger, and Rhizopus delemar F2, respectively. Similarly, Behnam, Karimi, and Khanahmadi [49] reported that the interaction term of moisture agent level and incubation time was the significant effect with strain M. indicus and was insignificant effect with remaining strains of M. hiemalis and Rhizopus oryzae for the xylanase and cellulase produced through SSF on wheat bran.
Figure 5d–f show the effect of B and C (Fig. 5d), B and D (Fig. 5e), and C and D (Fig. 5f) on the xylanase activity. These interactions are not significantly influenced by the response. It indicates a not significant mutual interaction between these parameters. According to studies conducted by de Almeida Antunes Ferraz et al. and Azzouz et al. [50, 51], an interaction between the moisture agent level and an incubation time has proved to be insignificant on the biomass and xylanase productions by strains Penicillium roqueforti ATCC 10110 and Trichoderma afroharzianum isolate az 12 using yellow mombin residue and wheat bran under solid-state fermentation respectively. Also, Behnam et al. and Cao et al. [45, 52] reported that the interaction term of moisture content, incubation time, and pH was insignificant in the optimization of xylanase production.
3.4 Validation of the developed models
Response surface equations were validated by the contrast between experimental values and the estimated values derived from the response regression; the RSM model and the regression equation was validated with the optimal conditions proposed by the model. In this study, after establishing the optimum conditions and predicting the response with these conditions, a new position of the experiment was calculated with the selected optimal conditions to predict and verify the precision of the mathematical model. A new range and level of the variables were studied to further determine the maximum xylanase production at different variable levels. The ranges of variable levels were set up based on the maximum production of xylanase recorded in the full model; the ranges of used values are presented in Table 4.
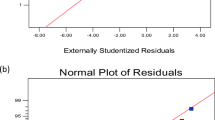
Analyses of residuals emerge to be a very functional and really simple tool in model construction and model criticism, and are an important role in adjudicating model adequacy. The residuals from the regression model are shown in Fig. 6, which presents plot residuals versus the predicted response. A random model on these plots would show model adequacy.
4 Concluding remarks
Importance of these optimization studies was related to the valorization of xylanase production using economical carbon sources such as wheat bran as a substrate, and an increase in xylanase production with saving time of fermentation. For the first time, classical optimization following OFAT was applied for xylanase production at the varied incubation conditions. Production was characterized by short incubation time (2 days), acidic pH (pH 2), high moisture level (85%), and mesophilic temperature (36 °C); however, the strain was able to produce higher amounts of xylanase with an activity of 4008.25 ± 3.73 U/gds compared with the initial conditions (1899.02 ± 1.6 U/gds). Also, RSM proved to be a powerful tool for the optimization of culture conditions, using wheat bran as the solid substrate at 37 °C temperature, pH 2.5, 84% effect of moisture level, and 66-h fermentation time under these conditions, the xylanase activity was 5427.51 ± 4.4 U/gds, and the xylanase activity was enhanced by 65.01% compared with the standard conditions. However, RSM was established to be more satisfactory and effective than other methods due to its efficacy to study many variables simultaneously with a low number of observations, saving time and costs.
Abbreviations
- OFAT:
-
One factor at a time
- BBD:
-
Box–Behnken design
- RSM:
-
Response surface methodology
- ANOVA:
-
Analysis of variance
- U/gds:
-
Unit per gram of dry substrate
- SSF:
-
Solid-state fermentation
- PDA:
-
Potato dextrose agar
References
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27. https://doi.org/10.1016/j.renene.2011.06.045
Faraco V (2013) Lignocellulose conversion: enzymatic and microbial tools for bioethanol production. Springer Science & Business Media, London
Harris AD, Ramalingam C (2010) Xylanases and its application in food industry: a review. JES Life Sci 1:1–11
Cantarel BI, Coutinho PM, Rancurel C et al (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:233–238. https://doi.org/10.1093/nar/gkn663
Davies G, Gilbert H, Henrissat B et al (2018) Ten years of CAZypedia: a living encyclopedia of carbohydrate-active enzymes. Glycobiology 28:3–8. https://doi.org/10.1093/glycob/cwx089
Loureiro DB, Romanini D, Tubio G (2016) Structural and functional analysis of Aspergillus niger xylanase to be employed in polyethylenglycol/salt aqueous two-phase extraction. Biocatal Agric Biotechnol 5:204–210. https://doi.org/10.1016/j.bcab.2015.12.008
Takahashi Y, Kawabata H, Murakami S (2013) Analysis of functional xylanases in xylan degradation by Aspergillus niger E-1 and characterization of the GH family 10 xylanase XynVII. Springerplus 2:1–11. https://doi.org/10.1186/2193-1801-2-447
Costa JR, Tonon RV, Gottschalk LMF et al (2019) Enzymatic production of xylooligosaccharides from Brazilian Syrah grape pomace flour: a green alternative to conventional methods for adding value to agricultural by-products. J Sci Food Agric 99:1250–1257. https://doi.org/10.1002/jsfa.92977
Chandel A, Da Silva SS (2013) Sustainable degradation of lignocellulosic biomass: techniques, applications and commercialization. BoD-Books on Demand
Wang K, Cao R, Wang M et al (2019) Biotechnology for biofuels a novel thermostable GH10 xylanase with activities on a wide variety of cellulosic substrates from a xylanolytic Bacillus strain exhibiting significant synergy with commercial Celluclast 1. 5 L in pretreated corn stover hydroly. Biotechnol Biofuels:1–13. https://doi.org/10.1186/s13068-019-1389-8
Chen H (2013) Modern solid state fermentation: theory and practice. Springer, Dordrecht
Tang XJ, He GQ, Chen QH et al (2004) Medium optimization for the production of recombinant nattokinase by Bacillus subtilis using response surface methodology. Biotechnol Prog 93:175–181. https://doi.org/10.1016/j.biortech.2003.10.013
Abu Bakar NK, Abd-Aziz S, Hassan MA et al (2010) Isolation and selection of appropriate cellulolytic mixed microbial cultures for cellulases production from oil palm empty fruit bunch. Biotechnology 9:73–78. https://doi.org/10.3923/biotech.2010.73.78
Gautam SP, Bundela PS, Pandey AK et al (2011) Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol Res Int 2011:1–8. https://doi.org/10.4061/2011/810425
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic P, New York, pp 315–322
Gonzalez-Mendoza D, Moreno AQ, Zapata-Perez O (2008) An improved method for the isolation of total RNA from Avicennia germinans leaves. Zeitschrift fur Naturforsch - Sect C J Biosci 63:124–126. https://doi.org/10.1515/znc-2008-1-222
Handa CL, Couto UR, Vicensoti AH et al (2014) Optimisation of soy flour fermentation parameters to produce b-glucosidase for bioconversion into aglycones. Food Chem 152:56–65. https://doi.org/10.1016/j.foodchem.2013.11.101
Ang SK, Yahya A, Aziz SA, Salleh M (2015) Isolation , screening , and identification of potential cellulolytic and xylanolytic producers for biodegradation of untreated oil palm trunk and its application in saccharification of lemongrass leaves. Prep Biochem Biotechnol 45:279–305. https://doi.org/10.1080/10826068.2014.923443
Mandels M, Weber J (1969) The production of cellulases. Food Microbiol 23:391–414. https://doi.org/10.1021/ba-1969-0095.ch023
Singh S, Tyagi CH, Dutt D, Upadhyaya JS (2009) Production of high level of cellulase-poor xylanases by wild strains of white-rot fungus Coprinellus disseminatus in solid-state fermentation. New Biotechnol 26:4–9. https://doi.org/10.1016/j.nbt.2009.09.004
Bailey MJ, Biely P, Kaisa P (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270. https://doi.org/10.1016/0168-1656(92)90074-j
Miller GL (1959) Use of dinitrosaiicyiic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Dai X, Liu M, Jin H, Jing M (2011) Optimisation of solid-state fermentation of Aspergillus niger JL-15 for xylanase production and xylooligosaccharides preparation. Czech J Food Sci 29:557–567. https://doi.org/10.17221/103/2010-CJFS
Fatokun EN, Nwodo UU, Okoh AI (2016) Classical optimization of cellulase and xylanase production by a marine Streptomyces Species. Appl Sci 6:1–14. https://doi.org/10.3390/app6100286
Okafor U, Okochi V, Onyegeme-okerenta B, Nwodo-Chinedu S (2007) Xylanase production by Aspergillus niger ANL 301 using agro - wastes. Afr J Biotechnol 6:1710–1714
Thomas L, Parameswaran B, Pandey A (2016) Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp for bioethanol production. Renew Energy 98:9–15. https://doi.org/10.1016/j.renene.2016.05.011
Fadel M (2001) High-level xylanase production from sorghum flour by a newly isolate of Trichoderma harzianum cultivated under solid state fermentation. Ann Microbiol 51:61–78
Irfan M, Nadeem M, Syed Q (2014) One-factor-at-a-time ( OFAT ) optimization of xylanase production from Trichoderma viride -IR05 in solid-state fermentation. J Radiat Res Appl Sci 7:317–326. https://doi.org/10.1016/j.jrras.2014.04.004
Bibi Z, Ansari A, Zohra RR et al (2014) Production of xylan degrading endo-1, 4-β-xylanase from thermophilic Geobacillus stearothermophilus KIBGE-IB29. J Radiat Res Appl Sci 7:478–485. https://doi.org/10.1016/j.jrras.2014.08.001
da Silva Menezes B, Rossi DM, Squina F, Marco AZA (2018) Comparative production of xylanase and the liberation of xylooligosaccharides from lignocellulosic biomass by Aspergillus brasiliensis BLf1 and recombinant Aspergillus nidulans XynC. Int J Food Sci Technol 53:2110–2118. https://doi.org/10.1111/ijfs.13798
Kheng PP, Omar IC (2005) Xylanase production by a local fungal isolate, Aspergillus niger USM AI 1 via solid state fermentation using palm kernel cake ( PKC ) as substrate. J Sci Technol 27:325–336
Yang SQ, Yan QJ, Jiang ZQ et al (2006) High-level of xylanase production by the thermophilic Paecilomyces themophila J18 on wheat straw in solid-state fermentation. Bioresour Technol 97:1794–1800. https://doi.org/10.1016/j.biortech.2005.09.007
Kuhad RC, Manchanda M, Singh A (1998) Optimization of xylanase production by a hyperxylanolytic mutant strain of Fusarium oxysporum. Process Biochem 33:641–647. https://doi.org/10.1016/S00329592(98)00025-9
Javed U, Aman A, Ali S, Qader U (2017) Utilization of corncob xylan as a sole carbon source for the biosynthesis of endo-1 , 4-β xylanase from Aspergillus niger KIBGE - IB36. Bioresour Bioprocess 4:1–7. https://doi.org/10.1186/s40643-017-0149-5
Zehra M, Syed MN, Sohail M (2020) Banana peels: a promising substrate for the coproduction of pectinase and xylanase from Aspergillus fumigatus MS16. Pol J Microbiol 69:19–26. https://doi.org/10.33073/pjm-2020-002
Pal A, Khanum F (2010) Bioresource technology production and extraction optimization of xylanase from Aspergillus niger DFR-5 through solid-state-fermentation. Bioresour Technol 101:7563–7569. https://doi.org/10.1016/j.biortech.2010.04.033
Bezerra MA, Santelli RE, Oliveira EP et al (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
He X, Song C, Li Y et al (2018) Ecotoxicology and environmental safety efficient degradation of Azo dyes by a newly isolated fungus Trichoderma tomentosum under non-sterile conditions. Ecotoxicol Environ Saf 150:232–239. https://doi.org/10.1016/j.talanta.2008.05.019
Chen W, Wang W-P, Zhang H-S, Huang Q (2012) Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from Boletus edulis mycelia using response surface methodology. Carbohydr Polym 87:614–619. https://doi.org/10.1016/j.carbpol.2011.08.029
Khusro A, Kaliyan BK, Al-Dhabi NA et al (2016) Statistical optimization of thermo-alkali stable xylanase production from Bacillus tequilensis strain ARMATI. Electron J Biotechnol 22:16–25. https://doi.org/10.1016/j.ejbt.2016.04.002
Kraber S (2013) How to get started with Design-Expert software. Stat-Ease,Inc:1–53
Nishio N, Tai K, Nagai S (1979) Hydrolase production by Aspergillus niger in solid-state cultivation. Eur J Appl Microbiol Biotechnol 8:263–270. https://doi.org/10.1007/BF00508790
Yadav JS (1988) SSF of wheat straw with alcaliphilic Coprinus. Biotechnol Bioeng 31:414–417. https://doi.org/10.1002/bit.260310504
Lonsane BK, Saucedo-castaneda G, Ghildyal NP et al (1992) Scale-up strategies for solid state fermentation systems. Process Biochem 27:259–273. https://doi.org/10.1016/0032-9592(92)85011-P
Behnam S, Karimi K, Khanahmadi M et al (2016) Optimization of xylanase production by Mucor indicus , Mucor hiemalis, and Rhizopus oryzae through solid state fermentation. Biol J Microorg 4:1–10
dos Santos TC, dos Santos Reis N, Silva TP et al (2017) Production, optimisation and partial characterisation of enzymes from filamentous fungi using dried forage cactus pear as substrate. Waste Biomass Valor 9:571–579. https://doi.org/10.1007/s12649-016-9810-z
Pathania S, Sharma N, Handa S (2017) Optimization of culture conditions using response surface methodology for synergism production of cellulase, xylanase and pectinase by Rhizopus delemar F2 under solid state fermentation. Pharmacognosy Phytochem 6:1872–1878
Yegin S, Oguz A, Sayit B, Yekta S (2016) Exploitation of agricultural wastes and by-products for production of Aureobasidium pullulans Y-2311-1 xylanase : screening, bioprocess optimization and scale up. Waste Biomass Valor. https://doi.org/10.1007/s12649-016-9646-6
Behnam S, Karimi K, Khanahmadi M (2019) Cellulase production under solid-state fermentation by ethanolic zygomycetes fungi: application of response surface methodology. Nutr Food Sci Res 6:27–34. https://doi.org/10.29252/nfsr.6.1.27
de Almeida Antunes Ferraz JL, Oliveira Souza L, Gustavo de Araújo Fernandes A et al (2020) Optimization of the solid-state fermentation conditions and characterization of xylanase produced by Penicillium roqueforti ATCC 10110 using yellow mombin residue (Spondias mombin L.). Chem Eng Commun 207:31–42. https://doi.org/10.1080/00986445.2019.1572000
Azzouz Z, Bettache A, Boucherba N et al (2020) Optimization of xylanase production by newly isolated strain Trichoderma afroharzianum isolate az 12 in solid state fermentation using response surface methodology. Cellul Chem Technol 54:451–462. https://doi.org/10.35812/CelluloseChemTechnol.2020.54.46
Cao Y, Meng D, Lu J, Long J (2008) Statistical optimization of xylanase production by Aspergillus niger AN-13 under submerged fermentation using response surface methodology. Afr J Biotechnol 7:631–638
Acknowledgments
The authors would like to express their acknowledgment to Pr. Maria Fernández-Lobato and Mr. Peter Elias Kidibule in the Centre for Molecular Biology Severo Ochoa (CSIC UAM), University Autonomous from Madrid (Spain) for their technical assistance to identify the fungal strain.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Optimization of the xylanase production by wheat bran biodegradation on SSF.
• Xylanase production was enhanced at the different conditions using OFAT and RSM approaches.
• RSM is underline with optimum OFAT conditions to select the optimal range.
• The xylanase production has been enhanced to 26.15% using RSM.
Rights and permissions
About this article
Cite this article
Azzouz, Z., Bettache, A., Djinni, I. et al. Biotechnological production and statistical optimization of fungal xylanase by bioconversion of the lignocellulosic biomass residues in solid-state fermentation. Biomass Conv. Bioref. 12, 5923–5935 (2022). https://doi.org/10.1007/s13399-020-01018-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01018-z