Abstract
Every day, a large amount of food waste (FW) is generated that causes serious environmental problems such as the production of greenhouse gases and leachate. A possible treatment for this waste is anaerobic digestion (AD), but there are several problems associated with the accumulation of volatile fatty acids (VFA), foaming, or low buffer capacity. In order to resolve or mitigate this problem, FW was mixed with cabbage and cauliflower (CCF) leaves and stalks at different carbon/nitrogen ratios (C/N) to add value to this agricultural waste and benefit from the advantages of co-digestion. Under the study conditions, promising results were obtained during the co-digestion of FW and agricultural wastes at C/N = 45. These include a high biodegradability (98%), a methane yield of 475 mLSTP CH4/g VS, and an organic loading rate (OLR) of 0.06 kg of VS/m3 h for the CCF and FW mixture (CCF + FW). Anaerobic co-digestion of CCF + FW might be an interesting option for the simultaneous treatment of these types of organic waste, with the consequent social and environmental benefits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomass energy, also known as bio-energy, is a renewable form of energy produced from organic matter. This type of matter can include agricultural or municipal solid waste (MSW), and other organic substrates which are abundant around the world. Although waste is considered an unusable material and has a negative value because of its treatment management costs, this raw material could be a potentially recoverable resource due to its composition. In this regard, organic waste has recently been the subject of global discussions on how to improve its management because of the impacts that residual streams produce in soils, water and air, climate change, and other problems. Moreover, this waste is characterized by kinetics of rapid decomposition in humid climates which may involve risks to the environment and human health [1]. In particular, the organic fraction of MSW is a significant contributor to greenhouse gas emissions mainly because of its decomposition in landfill.
MSW is mainly comprised of FW [2] composed of high levels of organic substrates such as carbohydrates, proteins, and lipids, making it an ideal renewable resource [3]. The catering industry is one of the largest producers of (FW such as fruit, meat, and vegetables, which is produced daily and on a permanent basis, particularly in hotels and university restaurants [4]. Due to the high moisture content and high proportion of fruit and vegetables in kitchen waste, rapid decomposition occurs naturally a few days after collection, thus causing environmental problems, social issues, and health risks (bad odors, potential proliferation of pathogenic microorganisms). However, this type of waste might be desirable in biological valorization processes.
The treatment and valorization of biomass through AD have undeniable advantages in the fight against greenhouse effects and global warming. AD also provides a clean, renewable, and alternative source of energy to conventional fossil fuels, whose reserves are diminishing at a rapid rate. AD is the decomposition of organic matter in the absence of oxygen. It is a natural process carried out by highly diverse microbial populations present in a multitude of environments. During the transformation of organic matter, AD produces biogas (a gas similar to natural gas) consisting mainly of CH4 (40–70%) and CO2. In addition to energy recovery under controlled conditions, AD also produces digestate that can potentially be used as an organic soil conditioner with fertilizing properties for the benefit of farming communities [5,6,7]. However, there are still some problems that may limit the application of this technology. For example, imbalanced C/N ratios in the organic substrates might result in the accumulation of ammoniacal nitrogen and the subsequent inhibition of microbial activity [8]. At an adequate nutrients balance, carbon is the main source of energy for microorganisms, whereas nitrogen is an essential element for the synthesis of amino acids and proteins. In reduced form, nitrogen neutralizes the volatile acids (VA) produced by the fermenting bacteria and thus helps to maintain the neutral pH conditions necessary for cell growth. Consequently, microorganisms require a C/N ratio adapted to their metabolism [9].
FW has been reported to contain a high percentage of moisture (> 70%) and organic matter (measured through the concentration of volatile solids (VS) > 85%) [10, 11], while the C/N ratio generally varies between 12 and 21 [12, 13]. Consequently, FW is a promising substrate for renewable energy production through AD [10, 11, 13,14,15,16]. Despite the high potential of FW to produce biogas, it also has some limitations for AD, particularly the production of VFA in high concentrations, which can both influence the pH value in the digesters and become toxic for the development of microorganisms [17]. Furthermore, during anaerobic treatment, a complex set of biochemical interactions occurs among several microbial species. The high concentration or presence of certain nutrients could have an inhibitory or toxic effect on the anaerobic process, such as ammoniacal nitrogen in aqueous solution in the form of ammonium ion (NH4+) and un-ionized ammonia (free ammonia: NH3). For this reason, it is important to quantify the concentration of ammoniacal nitrogen and NH3 in the mixing liquor to ensure the stability of the AD process [18]. Ammoniacal nitrogen may be responsible for inhibition. Depending on the pH and temperature in the digester, the high protein content in FW may lead to a significant nitrogen concentration mainly in the form of NH4+ or NH3after hydrolysis. Previous studies have reported a 50% inhibition in methane production at 215 mg N-NH3/L for reactors at mesophilic temperature and at 468 mg N-NH3/L under thermophilic conditions. Other studies have shown inhibition at concentrations of ammoniacal nitrogen ranging from 650 to 1200 mg N-NH3/L [19,20,21].
Numerous studies have been conducted on the AD of FW. Among the relevant studies, most have focused on the use of FW as co-substrates, while others have sought to explore methods for pre-treating this type of organic substrate [6, 21,22,23,24,25]. Kuczman et al. [14] evaluated the AD of FW with a semi-continuous feeding system at 29 ± 0.5 °C, and a C/N ratio close to 16 and reported a methane yield as high as 444 ± 20 mL CH4/g VS. However, authors such as Xu et al. [26] reported several problems for the AD of FW. Therefore, to prevent the likely failure of FW digestion, an interesting approach might be the co-digestion of FW with other wastes that have a sufficient buffering capacity to increase the stability of the process. Co-digestion consists of the joint treatment of different residual materials and supplies the microorganisms several sources of carbon and other nutrients [27]. Although the availability of a single substrate could be sufficient to ensure the growth of the microbial population, many bacterial species are capable of simultaneously using more than one substrate, even if one of them is present at a very low concentration. This phenomenon, called “co-use,” allows microorganisms to grow at an increased rate, improves their adaptation to inhibitory substances, and dilutes them because the co-digestion leads to the right balance of nutrients [28]. Jian et al. [29] reported a positive effect of the co-digestion of fruit and vegetable waste with FW generated in China in terms of the waste methanogenic potential and process stability. No accumulation of VFA and NH3were observed, while at the optimal mixing ratio of 1:1, the methane yield increased to 0.49 m3 CH4/kg VS. In the same context, Disndale [30] and Pahl et al. [31] reported that the choice of co-digestion technology for kitchen waste and activated sludge was a major factor in the success of the experiment and helped to stabilize the process. In this context, the C/N ratio for anaerobic co-digestion is frequently chosen according to the characteristics of the raw material and the operational parameters, with values that may vary from less than 10 to over 90 for efficient treatment [32]. However, the most frequent C/N ratio values for AD have been reported to be within the range 15–45 depending on the type of bioreactor and nature of the substrates [33, 34].
The cultivation and manufacture of agricultural products such as cabbage and cauliflower generate a large amount of waste (residual leaves, stalks, and roots). Although this waste can be burned or blended when tilling soil, biochemical processes such as AD might be another interesting alternative for the sustainable management of these substrates due to their high moisture content. However, CCF is characterized by a high nitrogen concentration and hence a low C/N ratio (between 7 and 12) [35, 36], which might inhibit the biological process. It is worth noting that the annual production of cabbage and cauliflower has been reported to be 11.4 and 2.3 million tons in Europe and 0.81 and 0.45 million tons in North Africa, respectively. The amount of FW produced in developed countries is 100–170 kg per person and year (around 747 million inhabitants in Europe). Approximately, 80–90% of total FW is generated by food processing industries [37,38,39]. Consequently, their combined treatment might be of interest from the standpoint of sustainable development.
The main purpose of this study was to evaluate the feasibility of the anaerobic co-digestion of CCF and FW (CCF + FW) at different C/N ratios compared with mono-digestion. The percentages of the mixing ratio used in the co-digestion experiments were calculated as a function of the C/N ratio of each waste type. Two high CCF + FW mixing ratios were proposed (45 and 56) in order to increase the methane production yield and establish a carbon and nitrogen balance. The mixing ratios are equivalent, in dry weight, to 36.42% CCF + 63.57%FW and 14.38% CCF + 85.61% FW, respectively. Higher C/N ratios than those reported in the available literature were chosen given that FW is generated in higher amounts and continuous mode compared with CCF waste, which is available seasonally.
2 Materials and methods
2.1 Experimental set-up
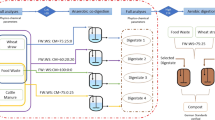
The experimental set-up used for the AD of CCF and FW consisted of four, 1-L Pyrex reactors operating in batch or discontinuous mode under mesophilic conditions through a thermostatic jacket of water at 37 °C. The continuous stirred tank reactors are equipped with four connections to load feedstock, ventilate the biogas, inject inert gas (nitrogen) to maintain the anaerobic conditions and remove effluent. The volume of methane produced during the process was measured using 1-L Boyle–Mariotte reservoirs connected to each reactor, and the experimental results were adjusted to standard conditions of temperature and pressure (STP, 0 °C and 1013.15 hPa). To remove the CO2 produced during the process, tightly closed bubblers containing NaOH solution (6 N) were connected between the two elements. The methane volume displaced an equal measurable volume of water from the reservoir, which was measured with the aid of a 1-L test tube (Fig. 1).
Experimental set-up used for the anaerobic digestion experiments. (1) digester; (2) thermostatic jacket; (3) agitation blade; (4) water circuit input; (5) water circuit output; (6) liquid substrate-feeding tube; (7) solid substrate-feeding tube; (8) sampling tube; (9) stirrer; (10) bubbler containing a NAOH solution (6 N) to remove CO2; (11) gasometer; (12) graduated tube
2.2 Chemical analyses
The following parameters were determined in the effluents of the reactors at the end of each load and in the wastes used as substrates: pH, alkalinity (Alk, mg CaCO3/L), volatile acidity (VADIS, mg C/L), conductivity (μS/cm), chemical oxygen demand (COD, mg O2/g dry weight), total solids (TS, g TS/kg sample), fixed solids (FS, g FS/kg sample), volatile solids (VS, g VS/kg sample), total Kjeldahl nitrogen (TKN, mg N/g sample), total soluble phosphorus (TP, mg P/g dry weight), total soluble organic carbon (TOC soluble, mg C/L), and total soluble nitrogen (TSN, mg N/L). C2–C6 VFA was analyzed by gas chromatography (VAGC, mg C2/L). All analyses were carried out in accordance with the Standard Methods of the APHA, except pH, conductivity, TOC soluble, and TSN, which were carried out according to the test methods of the US Department of Agriculture and the US Composting Council.
A Shimadzu model TOC-VCSH carbon analyzer was used to determine total soluble carbon (TC soluble), inorganic soluble carbon (IC soluble), and TOC, which was obtained as the difference between TC soluble (mg C/L) and IC soluble (mg C/L). The CO2 generated by the catalytic oxidation of the carbon in the sample was detected by infrared spectrometry.
The TSN was determined using a Shimadzu TOC-VCSH/CSN TOC/N analyzer, which allowed burning the sample at 720 °C to decompose the total N into gaseous NO that cools, dehumidifies, and is detected with a chemiluminescent gas analyzer.
In addition, the content of short-chain VFA (acetic, propionic, butyric, isobutyric, valeric, isovaleric, and caproic acid) was determined using a Hewlett-Packard HP-560 gas chromatograph. The chromatograph is equipped with a 15 m × 0.53 mm (i.d.) Nukol silica semi-capillary column, a flame ionization detector, and an oven to gradually increase the temperature from 100 to 150 °C at a rate of 4 °C/min.
2.3 Substrates
The CCF agricultural waste (leaves and stalks) was collected at a farm in Cordoba, Spain, in February 2017 and completely mixed. As cabbage and cauliflower are usually grown in the same season and cultivated in closely located places, their residual streams were used as co-substrate at a ratio of 50:50. After chopping to a particle size of < 2 mm, the CCF mixture was stored in plastic containers in a refrigerator at 4 °C for subsequent use. FW was collected from a restaurant at the Rabanales Campus of the University of Cordoba. The restaurant serves a variety of menus consisting of appetizers, burgers, pasta, salads, sandwiches, eggs, lentils, oranges, steak, chicken fingers, beef, fish and chips, and other foods. Samples were collected over 3 days in March 2017 in order to estimate the composition of organic waste contained in the residual streams.
The organic waste from the university restaurant was heterogeneous, so it was necessary to carry out a mechanical pretreatment using a blender to homogenize the waste and finally collect a representative sample, which was used as the raw material for this study. On the basis of the characterization of FW, and in order to increase the methanogenic potential of this waste, CCF was used as a co-substrate with FW at different C/N ratios (45 and 56). The characterization of CCF and the different mixtures evaluated are shown in Table 1. The C/N ratio was calculated using the following formula, considering the total concentration of VS and TKN in the substrates:
where 1.76 is the Wasksman number [40].
2.4 Anaerobic digesters: experimental procedure
The reactors were inoculated using granular biomass obtained from an anaerobic digester of the EMACSA wastewater treatment plant of Cordoba, Spain (VS = 10.27 ± 0.48 g/L; see Table 2), which has a methanogenic activity of 56.28 ± 5 mLSTP CH4/g COD·h (STP, 0 °C and 1 atm). The anaerobic reactors were initially loaded with 7 g VS/L of granular sludge. In order to activate the inoculum prior to the experiments with organic waste, the reactors were first fed with a synthetic medium (GAL medium) composed of glucose, sodium acetate, and lactic acid at concentrations of 50 g/L, 25 g/L, and 20.8 mL/L, respectively [41]. During this initial period, the load added to the reactors varied from 0.25, 0.50, 0.75, to 1.00 g VS of GAL/L over a 15-day period. After this initial stage, biomass acclimatization was carried out. The reactors were fed with 1.00 g VS/L composed of GAL and waste. The proportion of waste in the mixture varied from 25 to 100% after four loads. This acclimatization step was carried out in each reactor over a 15-day period. Following this adaptation period, each reactor was loaded with 100% of each selected waste. The added load was increased from 1.0, 1.5, to 2.0 g VS/L, and 8 replicates were performed for each load in semi-continuous mode, once biogas production completed in the loads. In all cases, the duration of each experiment was equal to the time interval required for maximum methane production. The volume of methane, under STP conditions, was measured as a function of time, and samples of the liquid digestate were taken and analyzed before and after feeding. The operational volume of the reactors remained constant by extracting the same volume of sample as the added volume. After sampling, the solid fraction of digestate (which included microorganisms and non-biodegraded substrate) was recovered from the samples and recirculated into the digesters after centrifugation at 2000 rpm. All the experiments, including the start-up, biomass acclimatization, and waste treatment, were carried out over a 60-day period.
2.5 Kinetic study
2.5.1 First-order kinetic model
In order to fit the experimental results obtained during AD, a first-order kinetic model was used to compare the methane production of the loads. According to this model, the volume of methane accumulated G (mLSTP CH4/L) with time can be calculated by the following equation [42]:
where Gm is the maximum theoretical volume of methane (mLSTP CH4/L) per liter of reactor measured under standard conditions (1 atm and 0 °C), k is an apparent kinetic constant that includes a specific constant and the concentration of biomass in the reactor (1/h), and t is time (h).
2.5.2 Sigmoidal kinetic model
The following expression represents the sigmoidal kinetic model used to analyze the cumulative methane production obtained for each experiment, particularly those with lag phase [43]:
Where G is the volume of methane accumulated (mLSTP/L) at a given time, Gm is the maximum methane production (mLSTP CH4/L), t is the time necessary for the anaerobic digestion of each load (h), and x0 and b are fitting parameters of the model.
2.5.3 Methane production rate
The methane production rate was calculated according to the mathematical model proposed previously (Eq. 2 and Eq. 3) as the time derivative of methane production (rCH4, mLSTP CH4/L·h), calculated by the following equations:
2.6 Software
The Sigma-Plot software (version 11.0) was used to create graphs, perform the statistical analysis (mean values and standard deviations), and fit the experimental data presented in this work.
3 Results and discussion
3.1 Characterization of substrates
The VS concentration in the CCF and FW was determined and reached similar values in both cases, while the TKN concentration was higher in the CCF (Table 1). In order to evaluate the viability and the methane yield of the methanogenic process and to prevent inhibition due to the marked presence of nitrogen in the case of CCF and high C/N ratio in the case of FW (C/N = 64), two different mixtures with FW were investigated at C/N ratios of 45 and 56 (FW provides carbon and compensates the excess of nitrogen in CCF). These values are within the range reported in the literature. Although Habiba et al. [44] and Fricke et al. [45] determined a C/N ratio of 25–30 as a reference value for AD, other authors such as Aiyuk et al. [46] have reported correct operation at C/N ratio values as high as 60. In addition, Giovanna et al. [47] studied the AD of water buffalo manure collected over a period of 3 years to investigate the effect of the substrate C/N ratio on biomethane production. The investigated samples showed a C/N ratio between 9 and 50, which is a wider interval than that typically considered in the literature as optimal for waste digestion processes.
The pH values of the different residual substrates were found to be acidic in a range between 4.61 ± 0.01and 5.54 ± 0.01. Although the pH of FW depends on its composition (according to dietary habits, country/restaurant of origin, etc.), the pH value of this waste was found to be similar to that obtained by Xu et al. [26], who reported a pH range of 3.3–5.7 for household and restaurant FW composed of banana skins, eggshells, and uneaten food such as plate waste. It is worth noting that the acidic pH of the evaluated wastes was neutralized by the mixing liquor of the digesters, as the microbial inoculum used had an initial pH value of 7.72 ± 0.01 (Table 2), which is close to the optimal pH of methanogenic archaeas (6.80–7.50) [48].
3.2 Co-digestion stability
The stability of the process was monitored at the end of each load through the variation in pH, the concentration of VAGC, and the VADIS/Alk ratio (eq acetic acid/eq CaCO3) in the reactor effluents.
As can be seen in Fig. 2a, the pH remained in a stable operating range between 7.50 and 7.82 with a mean value of 7.60 ± 0.10. There was a slight increase in the pH of the digester loaded with the CCF mixture, which was probably due to the nitrogen content of the substrate (7.78–7.81).In general, a high organic carbon concentration increases the formation of carbon dioxide and reduces the pH value, while the presence of nitrogen leads to the generation of NH4+ that could increase pH inside the reactors. Nevertheless, the pH range was within the optimal values for AD stability. Moreover, the FW led to lower pH values of the mixing liquor than the other substrates and was stable for higher loads, which could be due to the higher C/N ratio observed in the other residual substrates (allowing more organic matter to be available for acidification). In the case of the AD of CCF, an accumulation of nitrogen occurred (more than 150 mg NH3/L), which reached concentration values that inhibited methane production at loads higher than 1.0 g VS/L and for which a pH value of around 7.80, was observed.
In addition, the VA/Alk ratio, expressed as equivalents, reached values of 0.103 eq acetic acid/eq CaCO3 in the worst case for the digestion of CCF + FW (C/N = 45), as can be seen in Fig. 2b. A similar trend was also observed for substrates with a higher C/N ratio: FW and CCF + FW (C/N = 56). In all cases, the VA/Alk ratio increased with the load added to the reactors. However, the ratio values were markedly lower than the inhibition thresholds. Specifically, this ratio indicates the proper operation of the reactors, without risk of acidification, when its value is lower than 0.300–0.400 [49].
3.3 Biodegradability of substrates
The biodegradability of the single and co-digested substrates was another fundamental operational variable determined in the study (Fig. 3). According to the mean value of the methane production yield, the percentage of organic matter transformed into methane was quantified considering that 350 mLSTP CH4/g COD is the theoretical maximum CH4 produced per gram of COD [50].This value is proportional to the biodegradability, without taking into account the organic matter used for cellular growth and metabolism. Based on this information, the FW showed a maximum biodegradability value of around 60% for the 1.5 g VS/L load, while the biodegradability of CCF was lower due to the accumulation of nitrogen in the digesters. The percentage of biodegradability of FW makes it a favorable raw material for AD. However, maximum biodegradability was achieved using CCF + FW, specifically 85% and 98% for the different C/N ratios evaluated (56 and 45, respectively) when the added load was 1.5 g VS/L. These values are higher than those obtained by Zhang et al. [15] in a BMP test at 37 °C. Therefore, under the study conditions, the abovementioned load was considered the optimal operational load in terms of the biodegradability of the substrates. It is worth noting that the biodegradability of the mixture at C/N = 45 is markedly higher than the value obtained for the single digestion of FW under mesophilic conditions. Consequently, anaerobic co-digestion could be a viable treatment method for the types of waste evaluated.
3.4 Kinetics of methane production
As an example, Fig. 4 shows the evolution of the experimental methane production as a function of time in the case of CCF + FW with a C/N = 56. As can be seen, the behavior is not the same as when the load was increased from 1.0 and 1.5 to 2.0 g VS/L. The highest value was obtained at 1.5 g VS/L (598 ± 45 mLSTP CH4/L). However, a lag phase was observed at higher loads (2 g VS/L), which was possibly due to a slowdown of the metabolic pathway, since the hydrolytic phase is the limiting stage. In order to fit the experimental results obtained in this study and to facilitate comparison between different loads, the mathematical equation by non-linear regression of order one (Eq. 2) described in the “Kinetic study” section was used.
The K and Gm values were calculated numerically from the experimental data obtained for each load by non-linear regression using Sigma Plot (version 11.0; see Table 3).The kinetic constants diminished as the load added to the digesters was increased, which indicates inhibition by substrate. Furthermore, the sigmoidal model (Eq. 3), which includes a lag phase, was selected to analyze the cumulative methane production. A marked decrease in methane production was observed at the highest load (2 g VS/L), which was probably due to the presence of inhibitory factors.
In order to evaluate the accuracy of the proposed mathematical models, a linear relation between the maximum experimentally accumulated volume of methane (G) and the maximum theoretical volume (Gm) was investigated. The proposed model (Eq. 2) predicts 97% of the experimental maximum methane values with a confidence interval of 99% (Fig. 5). However, due to the lag phase at high loads that did not fit the mathematical model proposed for low loads, the methane production rate was investigated to compare the kinetics of different loads (Eq. 4 and Eq. 5).
Validation of mathematical model (Eq. 2) determined from the experimental maximum methane volume produced (G) and the maximum theoretical volume of methane (Gm) (mLSTP CH4/L)
As can be seen in Fig. 6a, b, and c, the methane production rate (Eq. 3) showed a similar trend along the loads. The highest methane production rate was achieved at 1.5 g VS/L for all the substrates studied, reaching the highest rates in the first 20 h. At 2.0 g VS/L, the methane production rate (Eq. 4) showed a growing trend until reaching a maximum, after which it declined due to the lag phase explained previously. In this second case, the highest rate was achieved at approximately 60 h. Similar results were observed during the anaerobic digestion of FW and CCF + FW (C/N = 45). Specifically, the highest rates increased slightly in the case of the co-digestion. According to these figures, the 1.5 g VS/L load showed the highest methane rate production due to an optimal microorganism/substrate ratio, and also achieved better biodegradability than at higher loads, where the accumulation of nitrogen might cause inhibitory effects.
3.5 Relation between methane yield production and C/N ratio
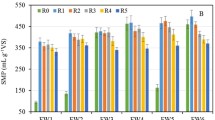
The methane yield coefficient (YCH4/S) was determined from the experimental maximum methane volume produced (Gexp) and the load added to the reactors (g VS added/L). The single AD of CCF and FW generally achieved a lower methane production yield (from 247 to 280 mLSTP CH4/g VS, respectively). These values are lower than those obtained by Kuczman et al. [14], who reported 444 mLSTP CH4/g VS when using FW as substrate at 29.0 ± 0.5 °C. However, in the co-digestion of CCF + FW at different C/N ratios, we noticed significant methane production for the 1.5 g VS/L load, especially for C/N = 45 (475 ± 9 mLSTP CH4/g VS). This increase in methane production might be due to an adequate nutrient balance and the adaptation of anaerobic microorganisms to the substrate used, which ensures the easy degradation of the mixture. According to Gil et al. [51], who evaluated various nutrients at different combinations of C/N ratios under mesophilic conditions (37 °C), although co-digestion might not always improve the methane production yield, it allows the joint treatment of different wastes.
Figure 7 shows the variation in methane yield production as a function of the C/N ratio of the substrates. As can be seen, the increase in the C/N ratio caused a decrease in methane yield for all the loads. Additionally, the co-digestion of FW with CCF showed better results than those obtained with the single digestion of FW. The best results obtained for CCF + FW (C/N = 45) were 475 ± 9 mLSTP CH4/g VS. This value is slightly higher than that obtained for the other C/N ratios and significantly higher than the value obtained for FW. The optimal results were achieved at 1.5 g VS/L, which correspond to a maximum OLR of 0.06 kg of CCF + FW/m3 h. These results are in concordance with Xu et al. [26], who reported a methane yield production of 460–530 mL/kg VS. The co-digestion of FW with CCF might improve methane yield production due to the dilution of toxic chemicals such as the high concentration of nitrogen, increase the micro-nutrients and microbiological activity [24], and also decrease treatment costs through the co-digestion of different raw materials.
The methanogenic potential of a mixture of substrates may be different from the sum of the methanogenic potentials of the constituent substrates in the mixture [52].In some cases, the methanogenic potential of a mixture of substrates is higher than the sum of the methanogenic potentials of the constituent substrates, reflecting synergy between the substrates in the mixture. In other cases, however, antagonism may be observed. In this context, synergism is considered an additional production of methane produced by co-digestion rather than mono-digestion. According to Mata-Alvarez et al. and Joute et al. [53, 54], the formulation of co-digestion substrate mixtures should allow the dilution of the constituting disadvantages of the substrates (presence of toxicity, high concentration of dry matter, low C/N ratio, etc.) and accumulate their strengths in order to constitute a methanogenic synergy. Table 4 summarizes this analysis for the co-digestion of CCF and FW compared with the mono-digestion of the residual substrates under study. The results illustrate the differences in methane yields for co-digestion and mono-digestion. The methanogenic potential obtained from the mono-digestion of FW was 287 mLSTP CH4/g VS for the load of 1.5 g VS/L, while it reached 247 mLSTP CH4/g VS for CCF. A simple mass balance allowed the calculation of the methane production that might be expected from CCF + FW at the mentioned load. As can be seen, an increase was observed in the experimental valued obtained. In absolute terms, the enhancement in the final volume of methane generated was even higher at the optimal load (1.5 g VS/L), with 475 and 433 mLSTP CH4/g VS for C/N ratios of 45 and 56, respectively. It is worth noting that these values were obtained with waste mixture at OLR of 0.06 kg/m3 h (at C/N = 45) and 0.11 kg/m3 h (C/N = 56). Therefore, compared with the mono-digestion of FW, the use of CCF as a co-substrate had a significant synergistic effect.
4 Conclusions
AD was stable in all cases. A slight increase in pH was observed for the CCF material, while in the case of FW, the pH was stable for higher loads. All of the VA/Alk ratios operated under optimal conditions.
Maximum biodegradability values were achieved when using the CCF + FW co-substrate with C/N ratios of 56 and 45 (85% and 98%, respectively).
The highest methane production was observed for co-digestion with the mixture at C/N = 45 during the 1.5 g VS/L load, which obtained a methane yield of 475 ± 9 mLSTP CH4/g VS.
The combination of CCF + FW (C/N = 45) with 0.06 kg of raw material/m3 h achieved the maximum OLR, with 1.5 g VS/L being the optimal load and mixture under the study conditions.
Abbreviations
- AD:
-
Anaerobic digestion
- Alk:
-
Alkalinity (mg CaCO3/L)
- BMP:
-
Biochemical methane potential
- CCF:
-
Cabbage and cauliflower mixture
- COD:
-
Chemical oxygen demand (mg O2/g dry weight)
- FA:
-
Free ammonia (mg NH3/L)
- FS:
-
Fixed solids (g FS/kg sample)
- FW:
-
Food waste
- G:
-
Methane volume (mLSTP CH4/L)
- Gexp :
-
Maximum experimental methane volume (mLSTP CH4/L)
- GAL:
-
Glucose, sodium acetate, and lactic acid
- Gm:
-
Maximum theoretical methane volume (mLSTP CH4/L)
- ICsoluble:
-
Inorganic soluble carbon (mg C/L)
- K:
-
Kinetic constant (1/h)
- OFMSW:
-
Organic fraction of municipal solid waste
- OLR:
-
Organic loading rate (kg VS/m3·h)
- rCH4 :
-
Methane production rate (mLSTP CH4/L·h)
- STP:
-
Standard temperature and pressure (0 °C, 1 atm)
- T:
-
Temperature (K)
- T:
-
Time (h)
- TCsoluble:
-
Total soluble carbon (mg C/L)
- TKN:
-
Total Kjeldahl nitrogen (mg N/g sample)
- TOC:
-
Total soluble organic carbon (mg C/L)
- TS:
-
Total solids (g TS/kg sample)
- TSN:
-
Total soluble nitrogen (mg N/L)
- V:
-
Volume (L; m3)
- VADIS :
-
Volatile acidity determined by titration (mg C/L)
- VAGC :
-
Volatile acidity determined by Gas Chromatography (mg C2/L)
- VFA:
-
Volatile fatty acid (mg C2/L)
- VS:
-
Volatile solids (g VS/kg sample)
- YCH4/S :
-
Methane yield coefficient (mLSTP CH4/g VS)
References
Amritha PK, Anilkumar PP (2016) Development of landscaped landfills using organic waste for sustainable urban waste management. Procedia Environ Sci 35:368–376. https://doi.org/10.1016/j.proenv.2016.07.016
Clercq D, Wen Z, Gottfried O, Schmidt F, Fei F (2017) A review of global strategies promoting the conversion of food waste to bioenergy via anaerobic digestion. Renew Sust Energ Rev 79:204–221. https://doi.org/10.1016/j.rser.2017.05.047
Zhao J, Liu Y, Wang D, Chen F, Li X, Zeng G, Zhang J, Li W, Lee J, Loh K, Dai Y, Tong Y (2017) Enhancement of biogas production in anaerobic co-digestion of food waste and waste activated sludge by biological co-pretreatment. Energy 137:479–486. https://doi.org/10.1016/j.energy.2017.02.163
Wang P, Wang H, Qiu Y, Ren L, Jiang B (2017) Microbial characteristics in anaerobic digestion process of food waste for methane production: a review. Bioresour Technol 248:29–36. https://doi.org/10.1016/j.biortech.2017.06.152
Li Y, Liu H, Yan F, Su D, Wang Y, Zhou H (2017) High-calorific biogas production from anaerobic digestion of food waste using a two-phase pressurized biofilm (TPPB) system. Bioresour Technol 224:56–62. https://doi.org/10.1016/j.biortech.2016.10.070
Liu C, Li H, Zhang Y, Liu C (2016) Improve biogas production from low-organic-content sludge through high-solids anaerobic co-digestion with food waste. Bioresour Technol 219:252–260. https://doi.org/10.1016/j.biortech.2016.07.130
Fedailaine M, Moussi K, Abada S, Saber M, Tirichine N (2015) Modeling of the anaerobic digestion of organic waste for biogas production. Proc Comput Sci 52:730–737. https://doi.org/10.1016/j.procs.2015.05.086
Dioha IJ, Ikeme CH, Nafi’u T, Soba NI, Yusuf MBS (2013) Effect of carbon to nitrogen ratio on biogas production. Int Res J Nat Sci 1(3):1–10
Hassan AN, Nelson BK (2012) Invited review: anaerobic fermentation of dairy food wastewater. J Dairy Sci 95(11):6188–6203. https://doi.org/10.3168/jds.2012-5732
Xiao L, Deng Z, Fung KY, Ng KM (2013) Biohydrogen generation from anaerobic digestion of food waste. Int J Hydrog Energy 38(32):13907–13913. https://doi.org/10.1016/j.ijhydene.2013.08.072
Lin Y, Wu S, Wang D (2013) Hydrogen-methane production from pulp & paper sludge and food waste by mesophilic–thermophilic anaerobic co-digestion. Int J Hydrog Energy 38(35):15055–15062. https://doi.org/10.1016/j.ijhydene.2012.01.051
Gueri MVD, Kuczman O, De Souza SNM, Schirmer WN, Alves HJ, Secco D, Buratto WG, Ribeiro CB, Hernandes FB (2018) Food waste anaerobic digestion of a popular restaurant in southern Brazil. J Clean Prod:S0959–652631641-X
Zhang C, Xiao G, Peng L, Su H, Tan T (2013) The anaerobic co-digestion of food waste and cattle manure. Bioresour Technol 129:170–176. https://doi.org/10.1016/j.biortech.2012.10.138
Kuczman O, Gueri MVD, De Souza SNM, Schirmer WN, Alves HJ, Secco D, Buratto WG, Ribeiro CB, Hernandes FB (2018) Food waste anaerobic digestion of a popular restaurant in Southern Brazil. J Clean Prod 196:382–389. https://doi.org/10.1016/j.jclepro.2018.05.282
Zhang L, Leeb YW, Jahnga D (2011) Anaerobic co-digestion of food waste and piggery wastewater: focusing on the role of trace elements. Bioresour Technol 102(8):5048–5059. https://doi.org/10.1016/j.biortech.2011.01.082
Veeken AH, Hamelers BV (2000) Effect of substrate-seed mixing and leachate recirculation on solid state digestion of biowaste. Water Sci Technol 41(3):255–262
Zhang Y, Banks CJ, Heaven S (2012) Anaerobic digestion of two biodegradable municipal waste streams. J Environ Manag 104(0):166–174. https://doi.org/10.1016/j.jenvman.2012.03.043
El Hadj TB, Astals S, Gali A, Mace S, Mata-Alvarez J (2009) Ammonia influence in anaerobic digestion of OFMSW. Water Sci Technol 59(6):1153–1158. https://doi.org/10.2166/wst.2009.100
Mata-Álvarez J (2003) Anaerobic digestion of the organic fraction of municipal solid wastes. IWA publishing, London
Hartmann H, Ahring BK (2005) A novel process configuration for anaerobic digestion of source-sorted household waste using hyper-thermophilic post-treatment. Biotechnol Bioeng 90(7):830–837. https://doi.org/10.1002/bit.20485
Ebner JH, Labatut RA, Lodge JS, Williamson AA, Trabold TA (2016) Anaerobic co-digestion of commercial food waste and dairy manure: characterizing biochemical parameters and synergistic effects. Waste Manag 52:286–294. https://doi.org/10.1016/j.wasman.2016.03.046
Dennehy C, Lawlor PG, Croize T, Jiang Y, Morrison L, Gardiner GE, Zhan X (2016) Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Manag 56:173–180. https://doi.org/10.1016/j.wasman.2016.06.032
Dhamodharan K, Ajay SK (2014) Pre-treatment and anaerobic digestion of food waste for high rate methane production – a review. J Environ Chem Eng 2:1821–1830. https://doi.org/10.1016/j.jece.2014.07.024
Ariunbaatar J, Panico A, Frunzo L, Esposito G, Lens PNL, Pirozzi F (2014) Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J Environ Manag 146:142–149. https://doi.org/10.1016/j.jenvman.2014.07.042
Jun W, Yuan W (2013) Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag 33:813–819. https://doi.org/10.1016/j.wasman.2012.11.013
Xu F, Li Y, Ge X, Yang L, Li Y (2018) Anaerobic digestion of food waste – challenges and opportunities. Bioresour Technol 247:1047–1058. https://doi.org/10.1016/j.biortech.2017.09.020
Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sust Energ Rev 38:383–392. https://doi.org/10.1016/j.rser.2014.05.038
Álvarez J, Otero L, Lema J (2010) A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial. Bioresour Technol 101:1153–1158. https://doi.org/10.1016/j.biortech.2009.09.061
Jia L, Jiane Z, Lili G, Peng L, Fenglin L, Kaijun W, Lei C, Hainan G (2011) Effect of mixture ratio on anaerobic co-digestion of fruit and vegetable waste and food waste of China. J Environ Sci 23(8):1403–1408
Disndale RM, Premier GC, Hawkes FR, Hawkes DL (2000) Two stage anaerobic co-digestion of activated sludge and fruit vegetable waste using inclined tubular digesters. Bioresour Technol 72:159–168. https://doi.org/10.1016/S0960-8524(99)00105-4
Pahl O, Firth A, MacLeod I, Baird J (2008) Anaerobic co-digestion of mechanically treated municipal waste with primary sewage sludge – a feasibility study. Bioresour Technol 99:3354–3364. https://doi.org/10.1016/j.biortech.2007.08.027
Marchaim U (1992) Food and Agriculture Organization of the United Nations. Food & Agriculture Org. - Science - 232 pages
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sust Energ Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Atandi E, Rahman S (2012) Prospect of anaerobic co-digestion of dairy manure: a review. Technol Rev 1:127–135. https://doi.org/10.1080/09593330.2012.698654
Yan H, Zhao C, Zhang J, Zhang R, Xue C, Liu G, Chen C (2017) Study on biomethane production and biodegradability of different leafy vegetables in anaerobic digestion. AMB Express 7:27. https://doi.org/10.1186/s13568-017-0325-1
Mane AB, Rao B, Rao A (2015) Characterization of fruit and vegetable waste for maximizing the biogas yield. Int J Adv Technol Eng Sci 3(special issue):01
Kafle K, Bhattarai G, Hun Kim S, Chen L (2014) Effect of feed to microbe ratios on anaerobic digestion of Chinese cabbage waste under mesophilic and thermophilic conditions: biogas potential and kinetic study. J Environ Manag 133:293–301. https://doi.org/10.1016/j.jenvman.2013.12.006
FAO (2011) Global food losses and food waste. http://www.fao.org/docrep/014/mb060e/mb060e00.pdf. Accessed on 15.09.2017
Dung T, Sen B, Chen C, Kumar G, Lin C (2014) Food waste to bioenergy via anaerobic processes. Energy Procedia 61:307–312. https://doi.org/10.1016/j.egypro.2014.11.1113
Waskman SA (1935) Chemical nature of organic matter or humus in sols, peat bogs and composts. J Chem Educ:511–519
Serrano A, Siles JA, Martín MA, Chica AF, Estévez-Pastor FS, Toro-Baptista E (2016) Improvement of anaerobic digestion of sewage sludge through microwave pre-treatment. J Environ Manag 177:231–239. https://doi.org/10.1016/j.jenvman.2016.03.048
Borja R, Martín A, Banks CJ, Alonso V, Chica A (1995) A kinetic study of anaerobic digestion of olive mill wastewater at mesophilic and thermophilic temperatures. Environ Pollut 88:13–18. https://doi.org/10.1016/0269-7491(95)91043-K
Chanakya HN, Khuntia HK, Mukherjee N, Aniruddha R, Mudakavi JR, Thimmaraju P (2015) The physicochemical characteristics and anaerobic degradability of desiccated coconut industry waste water. Environ Monit Assess 187:1–12. https://doi.org/10.1007/s10661-015-4991-7
Habiba L, Hassib B, Moktar H (2009) Improvement of activated sludge stabilisation and filterability during anaerobic digestion by fruit and vegetable waste addition. Bioresour Technol 100:1555–1560. https://doi.org/10.1016/j.biortech.2008.09.019
Fricke K, Santen H, Wallmann R, Hüttner A, Dichtl N (2007) Operating problems in anaerobic digestion plants resulting from nitrogen in MSW. Waste Manag 27:30–43. https://doi.org/10.1016/j.wasman.2006.03.003
Aiyuk S, Forrez I, Lieven DK (2006) Anaerobic and complementary treatment of domestic sewage in regions with hot climates – a review. Bioresour Technol 97:2225–2241. https://doi.org/10.1016/j.biortech.2005.05.015
Giovanna G, Claudia C, Filomena D, Stefania P, Biagio M, Mario M (2016) Does the C/N ratio really affect the bio-methane yield? A three years investigation of Buffalo manure digestion. Chem Eng Trans 49:463–468. https://doi.org/10.3303/CET1649078
Martí N (2006) Phosphorus precipitation in anaerobic digestion process. Thesis Disertation. Boca Raton, Florida, EEUU. ISBN: 1-58112-332-9
WPCF (Water Pollution Control Federation) (1967) Anaerobic sludge digestion, manual of practice N° 16
Gutiérrez MC, Siles JA, Diz J, Chica AF, Martín MA (2017) Modelling of composting process of different organic waste at pilot scale: biodegradability and odor emissions. Waste Manag 59:48–58. https://doi.org/10.1016/j.wasman.2016.09.045
Gil A, Siles JA, Serrano A, Chica AF, Martín MA (2019) Effects of variation in the C/[N+P] ratio on anaerobic digestion. Environ Prog Sustain Energy 38(1):228–236. https://doi.org/10.1002/ep.12922
Labatut RA, Angenent LT, Scott NR (2011) Biochemical methane potential and biodegradability of complex organic substrates. Bioresour Technol 102:2255–2264. https://doi.org/10.1016/j.biortech.2010.10.035
Mata-Alvarez J, Macé S, Astals S (2009) Codigestion of solid wastes: a review, dans international workshop on anaerobic digestion: an old story for today and tomorrow. Laboratoire de Biotechnologie de l’Environnement, Narbonne, pp 83–89
Joute J, El bari H, Belhadj S, Karouach F, Gradi Y, Stelte W, Bjerre AB (2016) Semi-continuous anaerobic co-digestion of cow manure and banana waste: effects of mixture ratio. Appl Ecol Environ Res 14(2):337–349. https://doi.org/10.15666/aeer/1402_337349
Acknowledgements
We wish to acknowledge the University of Cordoba, Spain, for providing the laboratory facilities to carry out this research study.
Funding
This study is supported by the European Union under the Erasmus + Program KA107 UCO-UIT-2015 and the Ministry of Economy, Industry and Competitiveness through project CTM2017-88723-R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beniche, I., Hungría, J., El Bari, H. et al. Effects of C/N ratio on anaerobic co-digestion of cabbage, cauliflower, and restaurant food waste. Biomass Conv. Bioref. 11, 2133–2145 (2021). https://doi.org/10.1007/s13399-020-00733-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00733-x