Abstract
This study was conducted to investigate the efficiency of lignin extraction from oil palm empty fruit bunch fiber (OPEFBF) by using aqueous sodium hydroxide and aqueous ammonia solutions. In this study, the aqueous ammonia treatment was conducted via three different modes, namely low temperature-long time soaked treatment (LTLt-NH3), high temperature-short time soaked treatment (HTSt-NH3), and low temperature-long time replenished treatment (LTLt-Rep-NH3). Low- and high-temperature treatments were conducted at 50 and 100 °C, respectively. The efficiency of lignin extraction upon aq. NaOH treatment at 120 °C and solid content of 1:17 (OPEFBF-to-liquid) for 180 min was not significantly different with the LTLt-Rep-NH3 treatment at 50 °C and solid content of 1:7 (OPEFBF-to-ammonia) for 4 days. Among the tested methods, LTLt-Rep-NH3 treatment was proved to be the best lignin extraction method. Under the optimum conditions, approximately 64% of Klason lignin had been removed from the OPEFBF and about 33.0 ± 2.2% of lignin was successfully recovered, with minimal lignin structural deconstruction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The world is currently facing serious challenges in sustaining higher demand for food supplies, energy, and clean water, following a rapid increase of human population. In order to bridge the gap between economic growth and environmental sustainability, new technologies such as biorefineries proved to be an attractive solution. The biorefinery concept has the potential to help mitigate the threat of climate changes and boundless demand of energy, fuels, chemicals, and materials in the population. The biorefinery concept is a fully integrated system of sustainable, environmental, and resource-friendly technologies for the comprehensive utilization and exploitation of biomass in a spectrum of valuable bio-based product production [1, 2].
Oil palm biomass in Malaysia, especially its oil palm empty fruit bunch fiber (OPEFBF), is an excellent and readily available feedstock for biorefineries. According to the statistical report from Malaysia Palm Oil Board (MPOB), approximately 19 million tons of crude palm oil (CPO) has been produced in the year of 2017 [3]. Approximately a tonnage of wet OPEFBF (with ~ 60% of moisture) is generated from every tonnage of CPO production [4, 5]. Thus, more research and innovation on the utilization of this biomass are urgently needed to ensure the sustainable development of the oil palm industry.
Research on the development and improvement of the existing green technologies to maximize the capacity of bio-energy recovery from the lignocellulosic biomass has been investigated effortlessly. Among the three main components of lignocellulose (cellulose, hemicellulose, and lignin), cellulose had gained the most extensive attention, as it covered the highest percentage of biomass composition and is the primary feedstock for various biofuel production processes. In the upstream process of cellulose recovery, hemicellulose and lignin have been identified as two main physical barriers. Biomass recalcitrance is the main factor that caused the project of biorefineries to reach to bottleneck [6]. Pretreatment is a crucial step to overcome the recalcitrance of biomass [5]. Lignin removal is one of the most noteworthy pretreatment mechanisms with the aim to improve the efficiency of cellulose extraction/degradation in the downstream process. Lignin removal would not only destroy the physical barrier against the accessibility of cellulase but also reduce the tendency of non-productive adsorption of the cellulolytic enzyme onto the lignin matrix [7, 8]. Among various pretreatment methods, alkaline pretreatment was commonly reported as the most effective treatment in delignification. Many research had reported the superiority of the use of aqueous sodium hydroxide and ammonia solution in improving the hydrolysability of biomass in the enzymatic saccharification process [9,10,11,12,13]. Aqueous alkaline medium promotes saponification of intermolecular ester bonds that cross-link carbohydrate and lignin in the biomass [14]. By-products such as lignin that are retrieved from the pretreatment stream should be effectively utilized in order to maximize the revenues of biorefineries and broaden the price margin between the feedstock and products, hence making pretreatment steps economically attractive [6].
Lignin is the second most abundant renewable resource of lignocellulosic biomass. Various lignin fractionation processes produced lignin with different physicochemical properties, and thus diverse applications are possible. Lignin is the most abundant aromatic polymer and possesses great potential in a vast spectrum of lignin-derived product conversion. Instead of being used to produce fuel, lignin-derived products, such as vanillin, vanillic acid, quinones, guaiacol, phenol, resorcinol, polyelectrolyte, and polymer alloy and polymer blends such as starch-lignin blends, protein-lignin blends, and epoxy-lignin composites, offer greater commercial values [15,16,17,18].
In order to maximize lignin retrieval from OPEFBF biorefinery process, the efficiency of lignin extraction and recovery from the black liquor stream were the main focus of this study. Due to the prohibition of direct discharge of black liquor, treatment of black liquor prior discharge is necessary to reduce and/or remove its chemical oxygen demand (COD), biological oxygen demand (BOD), and other contaminants. Studies by Jin et al. [19] and Zaied and Bellakhal [20] reported the success of COD and BOD reduction of black liquor following lignin removal. Maximum lignin removal from solid lignocellulose structure and the recovery of dissolved lignin from black liquor makes this study highly significant. The former contributes to the optimum sugar recovery from biomass, while the later maximizes the by-product recovery from the waste stream of the pretreatment process. Due to the excellent performance of aqueous sodium hydroxide (NaOH) and aqueous ammonia (NH3) in delignifying lignocellulose [10, 21, 22], these alkalis had been chosen for the current study. The efficiency of NaOH and NH3 in lignin removal from OPEFBF was investigated and compared. Then, the chemical changes of lignin and OPEFBF after different treatments were studied through ATR-FTIR analysis. On top of that, the surface morphological changes of OPEFBF after lignin removal were also observed under a scanning electron microscope (SEM).
2 Materials and methodologies
2.1 Materials
The OPEFBF was provided by Seri Ulu Langat Sdn. Bhd., Dengkil, Selangor, Malaysia. The fresh OPEFBF was washed with tap water to remove foreign particles and then sun-dried until the moisture content reached approximately 10–11% (dry basis). The OPEFBF was cut into the size of 5–8 cm, then kept in sealed plastic bag and stored at ~ 4 °C until further usage. The aqueous sodium hydroxide solution was prepared from sodium hydroxide pellets to a concentration of 50 g L−1, whereas aqueous ammonia solution was prepared from 25%w/w aqueous ammonia stock solution (density of 0.91 g cm−3). All the chemicals were analytical grade supplied by Merck, Germany.
2.2 Methodology
2.2.1 Aqueous sodium hydroxide treatment
For the aqueous sodium hydroxide (aq. NaOH) treatment, OPEFBF was soaked in 300 mL of 50 g L−1 aqueous NaOH solution. The treatments were conducted at a different solid content (at a solid-to-liquid ratio of 1:50, 1:17, and 1:10) in a 500-mL Schott bottle at 120 °C in an autoclave for different treatment times (60, 120, and 180 min). Then, the OPEFBF was separated from the black liquor through vacuum filtration by using Muslin cloth. The black liquor was kept for total dissolved lignin (TDL) and total recovered lignin (TRL) analysis. While the treated OPEFBF was washed with tap water until the pH of final washing water reached 6–7. The treated OPEFBF was air-dried at room temperature (~ 25 °C) [5]. Total composition loss (TCL) of OPEFBF after treatment was determined based on the formula as below:
Total Klason lignin of the treated OPEFBF was also determined [23]. Total Klason lignin loss (TLL) of OPEFBF after treatment was determined by the differences of Klason lignin content of OPEFBF before and after treatment. The overall experimental procedures are summarized in Fig. 1.
2.2.2 Aqueous ammonia treatment
Aqueous ammonia-soaked treatments of OPEFBF were conducted in three different modes, namely low temperature-long time (LTLt-NH3), high temperature-short time (HTSt-NH3), and low temperature-long time replenishment (LTLt-Rep-NH3). Initially, the effect of aqueous ammonia concentration on the efficiency of lignin extraction was studied by treating OPEFBF (at a solid-to-liquid ratio of 1:15) with 6.25, 12.5, and 25 wt% aqueous ammonia (at a OPEFBF-to-ammonia ratio of 1:3.5, 1:7, and 1:14), respectively, at 50 °C for 24 h in a water bath. The experiments were conducted in a 500-mL tightly capped Schott bottle. Then, TCL, TLL, TDL, and TRL analyses were performed on OPEFBF and black liquor, respectively. The concentration of aqueous ammonia that contributes to maximum lignin removal from OPEFBF was selected to proceed with LTLt-NH3, HTSt-NH3, and LTLt-Rep-NH3 treatment.
For LTLt-NH3 treatment, OPEFBF was soaked in an aqueous ammonia solution at a solid-to-liquid ratio of 1:15 at 50 °C for 24, 48, 72, 96, and 120 h, respectively, in the water bath. All experiments were conducted in a 500-mL tightly capped Schott bottle. The same experimental protocol was applied in the LTLt-Rep-NH3 treatment, except the black liquor was removed every 24 h and replaced with the fresh aqueous ammonia solution. For HTSt-NH3 treatment, the experiment was conducted in a sealed-pressurized reactor. OPEFBF at a solid-to-liquid ratio of 1:15 was treated with aqueous ammonia at 100 °C for 1, 2, and 3 h, respectively.
The treated OPEFBF was separated from the black liquor through vacuum filtration using Muslin cloth. The experimental procedures for lignin extraction with aq. ammonia solution are summarized in Fig. 2.
2.2.3 Determination of total Klason lignin
Klason lignin content of OPEFBF before and after treatment was determined according to standard biomass analysis protocol by National Renewable Energy Laboratory (NREL) [23]. OPEFBF was oven-dried to moisture lower than 5% before proceeding to a two-step acid hydrolysis. The black residues that remained after acid hydrolysis are the Klason lignins of OPEFBF. TLL of OPEFBF was determined based on the formula as below:
where
- %KLIni:
Klason lignin content of OPEFBF before treatment
- WIni:
Weight OPEFBF used in treatment (dry basis)
- %KLFinal:
Klason lignin content of OPEFBF after treatment
- WFinal:
Weight OPEFBF after treatment (dry basis)
2.2.4 Determination of total dissolved lignin
Total dissolved lignin of black liquor was determined according to protocols described in Tang et al. [5]. The absorbance of black liquor at a wavelength of 205 nm was measured with a UV-Vis spectrophotometer (Thermo Fisher Scientific Inc., MA) using a quartz cuvette. TDL of the black liquor was calculated as follows:
where
- Abs205nm:
Absorbance at wavelength 205 nm
- DF:
Dilution factor
- VTotal:
Total volume of hydrolysate
- b:
Cell path length (1 cm)
- a:
Absorptivity (110 mg mL−1 cm−1)
- Wsample:
Sample weight (dry basis)
2.2.5 Determination of total recovered lignin
The dissolved lignin of black liquor from NaOH treatment was recovered following the procedures by Sun et al. [24]. The collected black liquor was acidified to pH 2.0 by drop-wise addition of concentrated sulfuric acid. The dissolved lignin formed a precipitate at pH 2.0. The lignin precipitate was separated through centrifugation at 10,000 rpm for 10 min using centrifuge Eppendorf 5804 (Hamburg, Germany).
For aqueous ammonia liquor, ammonia removal was conducted prior to acidification. The aqueous ammonia solution was heated in a boiling water bath until the boiling point of the solution reached 96–98 °C at atmospheric condition. The hot solution was then acidified with concentrated sulfuric acid until pH 2.0. The resulted colloidal suspension of lignin was concentrated with a rotary evaporator to the half of the initial volume. Lignin precipitate was recovered through centrifugation at 10,000 rpm for 10 min [25].
The recovered lignin precipitate was repeatedly washed with distilled water until the pH of washing water reached 6–7. The lignin was oven-dried at 105 °C overnight and the weight was recorded. Total recovered lignin was calculated based on the formula as below:
2.2.6 Fourier transform infrared analysis
The Fourier transform infrared (FTIR) spectra of lignin and OPEFBF were measured with a PerkinElmer Spectrum 400 FTIR spectrometer (MA, USA) fitted with a universal attenuated total reflectance (ATR) accessory. The scanning range was from 650 to 4000 cm−1 with a resolution of 1 cm−1. The samples were initially dried in an oven at 80 °C overnight until constant weight, and then the spectra were recorded by Spectrum software (PerkinElmer, MA, USA).
2.2.7 Scanning electron microscopy analysis
Surface morphology study of OPEFBF after lignin removal was conducted by using a scanning electron microscope model LEO 1450VP. The OPEFBF was coated with gold-palladium by using a BioRad SC500 sputter coater (Quorum Technologies Ltd., UK) at a current of 15 mA for 2 min. The coated samples were then observed under a scanning electron microscope at electron high tension of 15–20 kV and a magnification range of ×500–2500.
2.2.8 Statistical analysis
All experiments were conducted in triplicate and the data were expressed as mean ± standard deviation. The significance (p < 0.05) between mean values was compared by one-way analysis of variance (ANOVA), followed by Duncan multiple range test by using SPSS 20.0 software (SPSS, Chicago, USA).
3 Results and discussion
3.1 Lignin extraction via aq. NaOH treatment
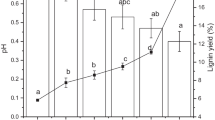
Figure 3 indicates the effect of substrate concentration and treatment time on the TCL and TLL of OPEFBF after aq. NaOH treatment. The results revealed that low-substrate concentration and longer treatment time led to higher total composition and Klason lignin loss from OPEFBF. Our finding was in agreement with the study by Zhao et al. [26]. In their study, the total composition loss was high when the crofton weed stem was treated at low-substrate loading and long treatment time. High-substrate loading may lead to system inhomogeneity, hence reduces the treatment efficiency of the biomass.
In our study, the highest TCL and TLL of OPEFBF were reported at 52.1 ± 1.3% and 70.9 ± 0.8%, respectively, in the treatment with 1:50 (OPEFBF-to-liquid) substrate content for 180 min. These results revealed that our strategy to improve the efficiency of lignin extraction from OPEFBF was more efficient than most of the previous reports. According to the study by Sun et al. [24], only about 40.2% of total lignin was successfully removed from OPEFBF in the treatment using 50 g L−1 sodium hydroxide for 120 min. Furthermore, a study by Kim et al. [27] reported that about 70.7% lignin was removed from OPEFBF through sequential acid/alkali treatment by using 8 wt% sulfuric acid (121 °C, 1 h) and 10 N sodium hydroxide (ambient temperature, 4 h). However, OPEFBF was found to be less susceptible to NaOH treatment, compared to switchgrass which is one of the most extensively studied biomasses. About 85.8% of lignin was successfully removed from the switchgrass via 1-h treatment using 2 wt% sodium hydroxide at 121 °C [22], but only 63.0% of lignin had been removed from OPEFBF in the 1-h treatment using 5 wt% sodium hydroxide at 120 °C. This finding suggested that the efficiency of lignin removal is strongly influenced by the biomass susceptibility to the chemical used and not merely its concentration. A similar finding was reported by Soares Rodrigues et al. [21] in their study on the delignification of switchgrass and miscanthus by using different types of alkali.
In spite of that, the yield of lignin was also further analyzed in the black liquor. Figure 4 indicates the TDL and TRL in the black liquors produced from treatments at different substrate content and treatment time.
The results demonstrated that the black liquor produced from treatment with a substrate content of 1:50 (OPEFBF-to-liquid) contained higher TDL than treatment with substrate contents of 1:17 and 1:10 (OPEFBF-to-liquid). Nonetheless, the highest yield of TRL [296.4 ± 17.6 mg g−1 OPEFBF (d.b)] was produced from the black liquor of treatment with a substrate content of 1:17 (OPEFBF-to-liquid) for 180 min. This finding was found in parallel with the results of Fig. 3, which shows high TCL and TLL in the treatment with low-substrate content and long treatment time. Furthermore, the obtained results also indicated that the yield of TDL and TRL has increased with the increase of treatment time at a substrate content of 1:10 (OPEFBF-to-liquid) treatment but not for the treatment with a substrate content of 1:50 (OPEFBF-to-liquid). The TDL and TRL in the black liquor of treatment with a substrate content of 1:10 (OPEFBF-to-liquid) were increased from 122.7 ± 3.1 mg g−1 OPEFBF (d.b) and 137.3 ± 14.8 mg g−1 OPEFBF (d.b), respectively, at 60-min treatment to 185.0 ± 0.7 mg g−1 OPEFBF (d.b) and 236.6 ± 38.2 mg g−1 OPEFBF (d.b), respectively, at 120-min treatment. While the yield of TRL did not affect the time factor in the treatment with a substrate content of 1:50 (OPEFBF-to-liquid). This finding suggested that there was a strong correlation between the substrate content and treatment time that affects both TDL and TRL yield. Perhaps, longer treatment time may be required for a less homogenous high-substrate system to achieve effective delignification, compared to the low-substrate system which is more homogeneous.
In spite of that, results in Fig. 4 also showed that the black liquor containing the highest TDL [420.3 ± 32.4 mg g−1 OPEFBF (d.b)] did not correspond with the highest TRL yield. This finding indicated that the content of TDL in the black liquor was not directly proportional to the yield of TRL. In this study, the yield of TDL was quantified via spectrophotometric method at wavelength 205 nm. This method is significantly affected by the lignin molecular structures. Absorption at wavelength 205 nm indicates the π→π* electronic transition in the aromatic ring. Even though most of the lignin analysis was reported to conduct at wavelength 280 nm, yet wavelength 205 nm was more suitable in this analysis. Absorption at wavelength 280 nm indicates the maximum absorption of the guaicyl unit and it is corresponding to the π→π* electronic transition in the aromatic ring of unconjugated phenolic units. Moreover, absorption at this wavelength is also indicative of free and etherified hydroxyl group [28]. Therefore, the use of wavelength 280 nm in this analysis might cause strong interference, as the produced black liquor contained a high amount of broad range phenolic compounds such as hydrocinnamic acids. According to Hatfield and Fukushima [29], wavelength selection in the spectrophotometric analysis of dissolved lignin is strongly dependent on the properties of the analyzed samples. Thus, in order to avoid overestimation of the yield of TDL, a wavelength at 205 nm which possesses minimum interference from other potential phenolic compounds had been selected. Besides, Shi et al. [30] also proposed that molecular weight and structure/functional groups of lignin could affect the efficiency of lignin recovery from the black liquor through acidification. Yet, this shortcoming will be less significant than compound interferences in the spectrophotometric method, particularly for black liquors which were produced from similar treatments. Therefore, the amount of recovered lignin was used as the primary benchmark for the determination of lignin yield in the following study.
According to Hatfield and Fukushima [29], lignin quantification can be conducted through different protocols, as long as the results are consistent and hold the promise of producing a true reflection of lignin yield. All three different lignin measurements (Klason lignin, dissolved lignin, and recovered lignin) in our study played a key role in the determination of an optimum condition for lignin extraction from OPEFBF. TLL estimated the extent of Klason lignin removal from OPEFBF, TDL measured the amount of soluble lignin in the black liquor generated from OPEFBF treatment, whereas TRL ascertained the amount of soluble lignin that can be recovered. Even though the optimum parameters that were obtained from three analyses were different, the total Klason lignin loss of OPEFBF treated at a substrate content of 1:17 (OPEFBF-to-liquid) for 180 min (68.9 ± 0.6%) was found just slightly lower than the treatment with a substrate content of 1:50 (OPEFBF-to-liquid) (70.9 ± 0.8%). Therefore, treatment with a substrate content of 1:17 (OPEFBF-to-liquid) for 180 min, which produced the highest yield of recovered lignin, had been selected as the optimum condition of aq. NaOH treatment for lignin extraction.
3.2 Lignin extraction via aqueous ammonia treatment
Besides sodium hydroxide, aqueous ammonia is also a promising delignifying agent. Due to its mild alkali nature, ammonia is highly selective for lignin degradation by cleaving the ester bonds that cross-link lignin and carbohydrate, while preserving the carbohydrate fragment of the biomass. There are two common types of aqueous ammonia treatment, namely ammonia recycled percolation (ARP) and ammonia-soaked treatment (SAA). ARP had the features of high severity and low contact time, whereas SAA exerts low severity to the biomass at a long contact time [11, 31]. Here, the chemical circulation effect of ARP running in specially designed percolation reactor was partially simulated by replenishing the ammonia solution in the reaction medium consistently.
3.2.1 Effects of aqueous ammonia concentration
Table 1 shows the results of TCL, TLL, TDL, and TRL of OPEFBF and black liquor, respectively, after treatments at a different aqueous ammonia concentration.
Based on the results obtained, the yields of TDL and TRL from the black liquor were being affected significantly (p < 0.05) by the concentration of aqueous ammonia (aq. NH3). Black liquor produced from the treatment at the ratio of 1:14 (OPEFBF-to-ammonia) contained the highest TDL [93.3 ± 1.4 mg g−1 OPEFBF (d.b)], whereas the highest TRL yield [64.6 ± 2.6 mg g−1 OPEFBF (d.b)] was produced from the black liquor of treatment at the ratio of 1:7. Notwithstanding, the concentration of aq. NH3 did not affect the TCL and TLL of OPEFBF significantly (p > 0.05). This finding was contraindicated with the study by Kim et al. [32]. In their study, the promising effect of aq. NH3 concentration on the efficiency of delignification of rice straw had been demonstrated. The failure of the gravimetric method to detect significant changes of the total composition and Klason lignin content in the treated OPEFBF suggested that the methodologies used in the quantification of the total composition and Klason lignin were not sensitive enough to identify minor changes. The impact of aq. NH3 concentration on the severity level may not be obvious in the mild treatment at 50 °C for 24 h. Nonetheless, the decision on the optimum aq. NH3 concentration still can be made based on the yield of TDL and TRL in the black liquor.
Based on the results in Table 1, we decided to choose the ratio of 1:7 (OPEFBF-to-ammonia), which produced the highest yield of TRL as the optimum concentration of aq. NH3 for the subsequent study.
3.2.2 Effects of treatment mode and time
OPEFBF delignification using aq. NH3 solution was further studied via LTLt-NH3, LTLt-Rep-NH3, and HTSt-NH3 mode at the solid ratio of 1:7 (OPEFBF-to-ammonia). HTSt-NH3 treatment at 100 °C was conducted in a sealed-pressurized chamber at a recorded pressure ~ 5.5 bar. Tables 2 and 3 denote the TCL, TLL, TDL, and TRL of OPEFBF and black liquor, respectively, after LTLt-NH3, LTLt-Rep-NH3, and HTSt-NH3 treatments at a different time.
Results in Tables 2 and 3 reveal that all the measured responses (TCL, TLL, TDL, and TRL) were increased with the increase of treatment time for all modes of treatments (LTLt-NH3, LTLt-Rep-NH3, and HTSt-NH3). Our findings were supported by Yoo et al. [10], where lignin removal from the barley straw had been promoted by extending the treatment time of aq. NH3 treatment. However, the rate of delignification decelerated after reaching an optimum treatment duration. A similar observation was also noticed in the LTLt-NH3 treatment of this study, where the Klason lignin was lost drastically when the reaction time was increased from 24 h (21.5 ± 1.0%) to 48 h (46.0 ± 1.3%).
Likewise, the results also elicit that the optimum treatment times based on the highest lignin recovery for LTLt-NH3, LTLt-Rep-NH3, and HTSt-NH3 treatment were 96, 96, and 2 h, respectively. By comparing with the NaOH treatment, longer treatment time was required to achieve effective lignin removal in the treatment using aq. NH3 solution at a low temperature (50 °C). Our finding was in accordance with the studies by Chaudhary et al. [11] and Kim and Lee [12]. In their study, effective aq. NH3 treatment was achieved at treatment time up to 40 and 60 days, respectively. Among the investigated modes, LTLt-Rep-NH3 treatment has been proved to be the best technique for lignin extraction from OPEFBF, followed by LTLt-NH3 and lastly HTSt-NH3. The results obtained manifest that aq. NH3 replenishment in the LTLt-Rep-NH3 treatment improved the yield of TRL drastically by threefold, compared to LTLt-NH3 treatment. The outstanding performance of LTLt-Rep-NH3 treatment in lignin extraction can be described based on the ARP mechanism. During ARP operation, aq. NH3 is circulated continuously to separate the lignin from the lignocellulose surface, and lignin re-condensation can be prevented effectively [31]. While LTLt-Rep-NH3 treatment effectively prevents lignin re-condensation by replenishing the black liquor containing dissolved lignin with the fresh aq. NH3 solution daily. Kim et al. [33] signified that ARP is a highly effective method to remove lignin from lignocellulose. In the conventional ARP process (170 °C), about 70–85% lignin can be removed from the biomass within the first 20 min. However, the LTLt-Rep-NH3 treatment which was operated at a low temperature (50 °C) may not perform as efficient as the conventional ARP operation. At a low temperature, the time and amount of aq. NH3 that are needed to achieve effective lignin removal from OPEFBF should be higher.
Under the optimum condition of LTLt-NH3 treatment, about 46.6 ± 0.7% of Klason lignin was lost and approximately 116.6 ± 18.4 mg g−1 OPEFBF (d.b) of lignin was successfully recovered. The same finding was reported in the study by Jung at al. [34]. In their study, about 40.9% of Klason lignin was removed from OPEFBF (25–120 mesh size) via aq. NH3 treatment using 14 wt% aq. NH3 solution at 60 °C for 12 h. Apart from that, about 64.0 ± 0.4% of Klason lignin was lost and 330.3 ± 21.9 mg g−1 OPEFBF (d.b) of lignin was recovered via the LTLt-Rep-NH3 treatment. For HTSt-NH3 treatment, only about 22.3 ± 0.2% of Klason lignin was lost and 76.8 ± 9.2 mg g−1 OPEFBF (d.b) of lignin was recovered. The aq. NH3 concentration and temperature are strongly believed to be the limiting factors that lead to the poor performance of HTSt-NH3 treatment in lignin extraction from OPEFBF. According to Liu et al. [9], lignin removal from miscanthus was improved significantly with the increase of aq. NH3 concentration (10–30 wt%) and temperature (100–180 °C). In their study, the impact of temperature on the efficiency of lignin removal was found to be more significant than ammonia concentration. Due to the volatility nature of aq. NH3 solution, the pressure built up in the reactor will be affected by both factors of aq. NH3 concentration and temperature. The specification limitation of our current pressurized reactor (maximum pressure limit at 10 bar) restricts the experiments to proceed at higher temperature and aq. NH3 concentration.
3.3 FTIR characterization
3.3.1 Lignin
Figure 5 illustrates the FTIR spectra of lignin recovered from the black liquor of NaOH, LTLt-NH3, and HTSt-NH3 treatments. The spectra present minor changes in the peak intensities, which indicate the “core” of the lignin structure did not change dramatically under different treatments. All lignin spectra show broad band between 3200 and 3300 cm−1, which attributed to the hydroxyl groups in phenolic and aliphatic structures [35]. The band 2920 cm−1 arises from the C-H stretching in aromatic methoxyl groups and methylene groups of the side chains, while band 2850 cm−1 assigned to C-H stretching in the methyl groups [28, 35]. Furthermore, the band at 1715 cm−1 originated from the non-conjugated carbonyl group stretching [36]. Absence or weak signal of 1715 cm−1 indicated partial degradation of non-conjugated carbonyl groups. The absence of band 1715 cm−1 in NaOH- and LTLt-NH3-lignin and weak signal in the HTSt-NH3-lignin indicated that majority of the ester groups of lignin was cleaved during the extraction process. A similar result was also observed in the NaOH-lignin extracted from bamboo in the study by Li et al. [28]. The band 1650 cm−1 in the spectra is attributed to conjugated carbonyl groups in the lignin structures. The lowest intensity of the band at 1650 cm−1 in LTLt-NH3-lignin and NaOH-lignin suggested that most of the conjugated carbonyl groups in lignin structures were cleaved during the extraction process. In addition, the bands at 1590, 1505, 1460, and 1420 cm−1 in the spectra were assigned to aromatic skeleton vibrations. Band 1505 cm−1 elicits the vibration involved C-C stretching and changes at H-C-C bond angle [37]. Furthermore, the syringyl ring breathing with C-O stretching and guaicyl ring breathing with C=O stretching also can be seen clearly at the band 1330 and 1280 cm−1, respectively [37, 38]. Besides, the syringyl ring also gives the signal at 1220 cm−1 [28]. The C=O stretching in p-coumaric conjugated ester group was proved with the presence of the weak signal at shoulder 1160 cm−1. This is the typical signal usually observed in HGS lignin [38, 39]. The intensive band at 1100 and 1030 cm−1 indicates the C-O stretching of the phenolic hydroxyl group and guaicyl type C-H in-plane deformation, respectively. Moreover, the aromatic C-H out of bending in the lignin structure was showed at band 840 cm−1 [37, 38].
By comparing the FTIR spectrum of three different types of lignin, it was found that the intensity of bands was in the range of 1590–1220 cm−1, which indicate the aromatic structures of lignin were the highest in HTSt-NH3-lignin. This finding suggested that the lignin extracted via HTSt-NH3 treatment has the highest purity and least contaminated by the carbohydrate fragment, compared to NaOH- and LTLt-NH3-lignin. The intensity of these bands in NaOH-lignin was the lowest. Such finding was in agreement with the study by Gupta and Lee [40]. Furthermore, the detection of band 890 and 1000 cm−1 in the spectrum of NaOH-lignin indicates that the lignin produced is contaminated with hemicellulose fragments. This finding implied that the extracted NaOH-lignin was augmented with the lignin-carbohydrate complex (LCC) [13, 39]. This result is supported by Garcia et al. [41].
3.3.2 Oil palm empty fruit bunch fiber
The FTIR spectra of OPEFBF before and after lignin extraction were illustrated in Fig. 6. According to Fig. 6, the intensities of bands 2900 and 890 cm−1 that are attributed to C-H stretching and β-glycosidic linkages, respectively, increased after treatment. Signal intensities of bands 2900 and 890 cm−1 in the untreated OPEFBF were lower than the treated OPEFBF.
An increase in signal intensities of these bands indicates that the methyl and methylene group of cellulose and β-glycosidic linkages between the sugar units in the OPEFBF were more apparent after lignin removal [13, 42]. The presence of hemicellulose in OPEFBF after lignin extraction also can be detected at band 1030 cm−1 [13], while the absence of band 1720 cm−1 (carbonyl C=O stretching) after extraction indicates that the lignin side chains had been cleaved. Moreover, the band 1720 cm−1 also signifies the presence of hemicellulose acetyl and uronic ester linkages in the lignin or the existence of ferulic acid and p-coumaric acid [42]. Based on Fig. 6, it is clearly shown that band 1720 cm−1 was absent in all OPEFBFs after alkali treatments.
In spite of that, HTSt-NH3-treated OPEFBF was also found to contain a significant amount of hemicellulose compared to the rest. This finding suggested that the aq. NH3 is more specific in lignin removal while preserving the carbohydrate portion in the biomass, as proposed in the previous study [11, 31, 34]. The highest signal intensity of band 2900 and 890 cm−1 in HTSt-NH3-treated OPEFBF implied that aq. NH3 treatment facilitated lignin removal while preserving the cellulose portion in the OPEFBF structure. This finding is also supported by our result shown in Fig. 1 that claimed the least carbohydrate contamination in the HTSt-NH3-lignin. However, the long-time LTLt-NH3 treatment had been found to promote higher hemicellulose loss than the short time HTSt-NH3 treatment (with lower signal intensity of band 1030 cm−1). In addition, the bands 1648, 1422, 1320, 1160, and 1120 cm−1 which indicate the conjugated carbonyl groups, aromatic skeleton vibration, C-O stretching of syringyl ring breathing, conjugated C=O ester group, and aromatic C-H in-plane deformation, respectively, also had been detected. These bands elicit the lignin structure of OPEFBF [38, 39].
Results of the FTIR spectrum suggested that aq. NH3 treatment was a better option than NaOH treatment in lignin removal. The cellulose and hemicellulose of OPEFBF have been preserved during the aqueous ammonia delignification process. The efficiency of these methods in lignin removal was further verified through SEM. Surface morphological changes of OPEFBF after different extraction methods were shown in Fig. 7. From left to right, the surface morphology of the untreated and treated OPEFBFs was observed at increasing magnification power (×500 to ×2500). Through the surface morphological observation, OPEFBF underwent more severe structural destruction after LTLt-NH3 treatment (Fig. 7c) than NaOH (Fig. 7b) and HTSt-NH3 treatment (Fig. 7d). More cellulose strands were exposed on the surface of LTLt-NH3-treated OPEFBF. This observation explained the reason why the band 890 cm−1 which indicated the β-glycosidic linkages was the lowest in the FTIR spectrum of LTLt-NH3-treated OPEFBF. Besides, Fig. 7d also showed that there were agglomerated particles deposited on the irregular surface of HTSt-NH3-treated OPEFBF. When these agglomerates were observed closely at a higher magnification power under SEM, the glossy particles were believed to be lignin. Lignin re-deposition was suspected to happen during the HTSt-NH3 treatment. During the lignocellulose delignification process, lignin is recycled between the solid and liquid phase through a complex mechanism that involved phase transition, reaction, and solubilization. At the high-temperature treatment, lignin droplets formed as a result of lignin transition from the glassy state to rubbery state, followed by coalescence, migration, and extrusion from the cell wall. However, these lignin droplets are hardened and lead to deposition on the biomass surface upon cooling. High-temperature treatment was also proven to cause a higher extent of lignin deconstruction, hence promotes re-polymerization and re-deposition of lignin onto the biomass surface [43]. This mechanism had explained the reason why lignin removal through HTSt-NH3 treatment was less effective in our study. Moreover, Fig. 7c demonstrated that there was no obvious lignin droplet deposition on the surface of LTLt-NH3-treated OPEFBF. The glass transition temperature of lignin is in the range of 80–193 °C [43]. Therefore, the rate of lignin re-deposition was believed to be low at low-temperature (50 °C) treatment. However, extraction at low temperature had resulted in low lignin yield in LTLt-NH3 treatment. Notwithstanding, the lignin yield of low-temperature aq. NH3 treatment was successfully improved through LTLt-Rep-NH3 treatment. Although higher lignin yield has been achieved through LTLt-Rep-NH3 treatment, no obvious surface morphology difference was observed between the LTLt-NH3-treated and LTLt-Rep-NH3 OPEFBF. During LTLt-Rep-NH3 treatment, OPEFBF was delignified repeatedly. High lignin yield was achieved by avoiding the removed lignin from coming into contact with the OPEFBF surface again. Thus, the rate of lignin re-deposition has remained low in the LTLt-Rep-NH3 treatment. However, when comparing the surface morphology of NaOH- and LTLt-NH3-treated OPEFBF, the surface deconstruction of NaOH-treated OPEFBF was less severe than LTLt-NH3-treated OPEFBF. Yet, results in Fig. 4 showed that the yield of recovered lignin from NaOH treatment was higher than LTLt-NH3 treatment. According to Trajano et al. [43], carbohydrates influence the solubility of lignin. Covalent bonds between lignin and hemicellulose may improve lignin solubility during lignin deconstruction by preventing lignin precipitation on the biomass surface. The lignin is released into the solution in the form of LCC, and the bonds within LCC are cleaved to produced lignin and carbohydrate fragments once in the solution. The formation of LCC during lignin removal had also been proved in the FTIR spectrum of NaOH-lignin, where peak 890 cm−1 which represents β-glycosidic linkage was found present in the recovered lignin fragment.
4 Conclusion
Overall, the findings of this study revealed that the efficiency of lignin extraction from OPEFBF was influenced by the types of alkali used (aq. NaOH vs. aq.NH3), substrate concentration (at the range of 1:10–1:50 for aq. NaOH and 1:3.5–1:14 for aq. NH3 treatment), time (at the range of 60–180 min for aq. NaOH and 1–120 h for aq.NH3 treatment), and the mode of experiment setting (LTLt-, HTSt-soaked and LtLt-Rep NH3 treatment). Through our study, LTLt-Rep-NH3 treatment was recognized as the best method for the extraction of lignin from OPEFBF. Under its optimum condition (at 1:7 OPEFBF-to-ammonia, 50 °C and 96 h), about 64.0 ± 0.4% of Klason lignin had been removed from the OPEFBF and 330.3 ± 21.9 mg g−1 OPEFBF (d.b) of lignin was successfully recovered from the black liquor. Furthermore, the results of FTIR analysis also illustrated that NaOH-lignin was highly contaminated with carbohydrate fragments while the HTSt-NH3-lignin has the highest purity in comparison with the LTLt-NH3- and LTLt-Rep-NH3-lignin. In spite of that, both LTLt-NH3- and LTLt-Rep-NH3-lignin had also been found to possess similar chemical characteristic. Further investigation through a scanning electron microscope proved that lignin particle re-deposition on the surface of the fiber is the cause that leads to low lignin yield in the HTSt-NH3 treatment. Due to the relatively high purity of HTSt-NH3-lignin, future study is recommended to further investigate the strategies to improve the efficiency of HTSt-NH3 treatment for lignin extraction from OPEFBF. A new pressurized reactor with a higher pressure limit and equipped with an agitator should be invested to extend the range of studied parameters such as temperature, aq. NH3 concentration, pressure, speed of agitation, and substrate concentration. Response surface methodology is proposed to simultaneously optimize the studied parameters to maximize the lignin yield via HTSt-NH3 treatment.
References
World Economic Forum (2010) The future of industrial biorefineries. World Economic Forum, Geneva. https://www.iwbio.de/fileadmin/Publikationen/IWBio-Publikationen/WEF_Biorefineries_Report_2010.pdf. Accessed 18 December 2018
Kamm B, Kamm M (2004) Principles of biorefineries. Appl Microbiol Biotechnol 64:137–145
MPOB (2018) Production of crude palm oil 2017. Malaysia: Malaysia Palm Oil Board. http://bepi.mpob.gov.my/index.php/en/statistics/production/177-production-2017/792-production-of-crude-oil-palm-2017.html. Accessed 18 June 2018
Fauziah S, Nurhayati A, Heiko G, Adilah S (2010) A perspective of oil palm and its wastes. J Phys Sci 21:67–77
Tang PL, Osman H, Jamaliah MJ, Wan-Aida WM, Mohamad-Yusof M (2014) Fibrous agricultural biomass as a potential source for bioconversion to vanillic acid. Int J Polym Sci 2014:1–8. https://doi.org/10.1155/2014/509035
Zhang YHP (2008) Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J Microbiol Biotechnol 35:367–375
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod Biorefin 6:465–482
Yu Z, Jameel H, Chang H, Park S (2011) The effect of delignification of forest biomass on enzymatic hydrolysis. Bioresour Technol 102:9083–9089
Liu Z, Padmanabhan S, Cheng K, Schwyter P, Pauly M, Bell AT, Prausnitz JM (2013) Aqueous-ammonia delignification of miscanthus followed by enzymatic hydrolysis to sugars. Bioresour Technol 135:23–29
Yoo CG, Nghiem NP, Hicks KB, Kim TH (2013) Maximum production of fermentable sugars from barley straw using optimized soaking in aqueous ammonia (SAA) pretreatment. Appl Biochem Biotechnol 169:2430–2441
Chaudhary G, Singh LK, Ghosh S (2012) Alkaline pretreatment methods followed by acid hydrolysis of Saccharum spontaneum for bioethanol production. Bioresour Technol 124:111–118
Kim TH, Lee YY (2005) Pretreatment of corn stover by soaking in aqueous ammonia. Appl Biochem Biotechnol 124:1119–1132
Sun R, Lawther JM, Banks WB (1996) Fractional and structural characterization of wheat straw hemicelluloses. Carbohydr Polym 29:325–331
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Holladay JE, White JF, Bozell JJ, Johnson D (2007) Top value-added chemicals from biomass. Volume II—results of screening for potential candidates from biorefinery lignin. PNNL-16983, Pacific Norwest National Laboratory, Richland. http://www.pnl.gov/publications/abstracts.asp?report=230923. Accessed 26 Sept 2014
William OSD, Payam M, Christopher MF (2011) Value-adding to cellulosic ethanol: lignin polymers. Ind Crop Prod 33:259–276
Franco C, Gianluca C, Andrea N, Mattia G, Valentina C, Alessandro P, Lucia B (2014) Lignin as co-product of second-generation bioethanol production from lignocellulosic biomass. Energy Procedia 45:52–60
Norgren M, Edlund H (2014) Lignin: recent advances and emerging applications. Curr Opin Colloid Interface Sci 19:409–416
Jin W, Tolba R, Wen J, Li K, Chen A (2013) Efficient extraction of lignin from black liquor via a novel membrane-assisted electrochemical approach. Electrochim Acta 107:611–618
Zaied M, Bellakhal N (2009) Electrocoagulation treatment of black liquor from paper industry. J Hazard Mater 163:995–1000
Soares Rodrigues CI, Jackson JJ, Montross MD (2016) A molar basis comparison of calcium hydroxide, sodium hydroxide and potassium hydroxide on the pretreatment of switchgrass and miscanthus under high solids conditions. Ind Crop Prod 92:165–173
Xu J, Cheng JJ, Sharma-Shivappa RR, Burns JC (2010) Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy Fuel 24:2113–2119
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass. Technical report NREL/TP-510-42618. National Renewable Energy Laboratory, Colorado
Sun RC, Fang JM, Tomkinson J, Bolton J (1999) Physiochemical and structural characterization of alkali soluble lignins from oil palm trunk and empty fruit bunch fibers. J Agric Food Chem 47:2930–2936
Sherman SR, Goodell JJ, Milliken CE, Morris JA, Gorensek MB (2012) A new process developed for separation of lignin from ammonium hydroxide pretreatment solutions. Environ Prog Sustain Energy 31:130–138
Zhao X, Zhang L, Liu D (2008) Comparative study on chemical pretreatment methods for improving enzymatic digestibility of crofton weed stem. Bioresour Technol 99:3729–3736
Kim S, Park JM, Seo JW, Kim CH (2012) Sequential acid-/alkali-pretreatment of empty fruit bunch fiber. Bioresour Technol 109:229–233
Li MF, Fan YM, Sun RC, Xu F (2010) Characterization of extracted lignin of bamboo (Neosinocalamus affinis) pretreated with sodium hydroxide/urea at low temperature. BioResources 5:1762–1778
Hatfield R, Fukushima RS (2005) Can lignin be accurately measured? Crop Sci 45:832–839
Shi H, Fatehi P, Xiao H, Ni Y (2011) A combined acidification/PEO flocculation process to improve the lignin removal from the pre-hydrolysis liquor of kraft-based dissolving pulp production process. Bioresour Technol 102:5177–5182
Kim TH, Gupta R, Lee YY (2010) Pretreatment of biomass by aqueous ammonia for bioethanol production. In: Mielenz JR (ed) Biofuels: methods and protocols. Humana Press, New York, pp 79–91
Kim SB, Lee SJ, Lee JH, Jung YR, Thapa LP, Um Y, Park C, Kim SW, Kim JS (2013) Pretreatment of rice straw with combined process using dilute sulfuric acid and aqueous ammonia. Biotechnol Biofuels 6:109
Kim TH, Kim JS, Sunwoo C, Lee YY (2003) Pretreatment of corn stover by aqueous ammonia. Bioresour Technol 90:39–47
Jung YH, Kim IJ, Han JI, Choi IG, Kim KH (2011) Aqueous ammonia pretreatment of oil palm empty fruit bunches for ethanol production. Bioresour Technol 102:9806–9809
Lisperguer J, Perez P, Urizar S (2009) Structure and thermal properties of lignins: characterization by infrared spectroscopy and differential scanning calorimetry. J Chil Chem Soc 54:460–463
Zhang J, Deng H, Lin L, Sun Y, Pan C, Liu S (2010) Isolation and characterization of wheat straw lignin with a formic acid process. Bioresour Technol 101:2311–2316
Xu F, Sun JX, Sun RC, Fowler P, Baird MS (2006) Comparative study of organosolv lignins from wheat straw. Ind Crop Prod 23:180–193
Mao JZ, Zhang LM, Xu F (2012) Fractional and structural characterization of alkaline lignins from Carex meyeriana Kunth. Cellul Chem Technol 46:193–205
Sun R, Xiao B, Lawther JM (1998) Fractional and structural characterization of ball-milled and enzyme lignins from wheat straw. J Appl Polym Sci 68:1633–1641
Gupta R, Lee YY (2010) Investigation of biomass degradation mechanism in pretreatment of switchgrass by aqueous ammonia and sodium hydroxide. Bioresour Technol 101:8185–8191
Garcia A, Toledano A, Andres MA, Labidi J (2010) Study of the antioxidant capacity of Miscanthus sinensis lignins. Process Biochem 45:935–940
Zhang C, Li B, Pang F, Kang Y (2014) Recycled aqueous ammonia expansion (RAAE) pretreatment of sorghum stalks at low temperature and long residence time. Chem Eng Process Technol 2:1025–1030
Trajano HL, Engle NL, Foston M, Ragauskas AJ, Tschaplinski TJ, Wyman CE (2013) The fate of lignin during hydrothermal pretreatment. Biotechnol Biofuels 6:110
Funding
Our deepest appreciation to Universiti Kebangsaan Malaysia (UKM) and Tunku Abdul Rahman University College for the financial support under the grant scheme with project code DIP-2012-018, GUP-2018-105 and UC/I/G2016-00013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, P., Hassan, O., Yue, C.S. et al. Lignin extraction from oil palm empty fruit bunch fiber (OPEFBF) via different alkaline treatments. Biomass Conv. Bioref. 10, 125–138 (2020). https://doi.org/10.1007/s13399-019-00413-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00413-5