Abstract
An increasing share of power production from sun and wind energy in Europe led to an increasing interest in novel energy storage technologies. The production of hydrogen from electricity via electrolysis enables the conversion of electrical energy into chemical energy, which can be stored with high energy density, if further process steps are applied. The Fischer-Tropsch process is well-known for the production of diesel fuel from different fuel types. Within the present work, results of an experimental campaign with a laboratory-scale Fischer-Tropsch plant are illustrated. The described experimental campaign was executed to determine the performance of a diesel fuel production from biomass. Furthermore, the investigation included the integration of hydrogen from wind power promoting a combined power-to-gas and biomass-to-liquid process. As a result, the investigated process is aiming at the storage of wind energy by the use of a chemical process enabling high energy density. Therefore, extensive measurement data was collected illustrating the influence of load changes on the operated laboratory-scale Fischer-Tropsch plant. The experimental campaign showed that an increased gas stream feed, enabled by the addition of hydrogen from wind power, leads to an increased output of Fischer-Tropsch products. Furthermore, the executed experimental campaign proved the suitability of different catalysts with respect to fluctuating load changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The latest agreements in Paris within the framework of the United Nations Framework Convention on Climate Change (UNFCCC) aim on a full replacement of energy sources based on fossil carbon until the end of the present century [1]. Energy policy in Europe has been focusing at the development of innovative energy technologies as new high performance low-carbon technologies. Nowadays, an innovation strategy and the market implementation of innovative technology is foreseen to be carried out worldwide [2]. As a part of this development, increasing production capacities for electricity generation from sun and wind energy were installed lately. Therefore, the future demand for energy storage capacities became a part of the agenda. Power-to-Gas (PtG) and Power-to-Liquid (PtL) technologies are discussed as promising options enabling energy storage with high energy density. Due to the early stage of technology development, the results of their economic assessment vary in a wide range. Different studies investigated investment costs and economic aspects of different Power-to-Gas (PtG) processes. These studies showed that mainly the necessary electrolysis stacks are responsible for high investment costs of PtG plants. The reported total investment costs vary in the range of 1–2 million EUR per MW electrical input for plants with a capacity of 30 to 100 MW [3,4,5,6,7]. The cost of synthetic natural gas (SNG) generated by such PtG plants was calculated 150 EUR/MWh assuming 3000 full load operation hours per year. If full load electrical surplus power is available only 1200 operation hours per year, the production costs can increase up to 300 EUR/MWh [8, 9]. Similar results were reported by another author [10]. A strategic overview between biological methanation and catalytic PtG results is given by [7] illustrating even higher production costs. The main reason for this is reported due to high hydrogen production costs contributing between 100 and 200 EUR/MWh. These reports illustrate important pre-conditions for the investigated “wind-diesel” concept within the present work. Besides, the current market price of diesel determines an important benchmark for the ongoing process development. The present work is focused on important technological aspects for the scale-up of the investigated process. The results achieved are used for an evaluation of a commercial plant based on the described economic pre-conditions for large-scale plants. First results indicate that production costs below 100 EUR/MWh for Fischer-Tropsch diesel could be reached.
The Fischer-Tropsch process is one option for the production of liquid fuels. Since 2005, the Fischer-Tropsch process is investigated in Güssing, Austria, by the use of a laboratory-scale plant. Executed research activities focused on the production of diesel fuel from woody biomass. Carried out investigations so far thereby have focused on the determination of ideal process conditions allowing a maximum output of valuable products. The results of several experimental campaigns were reported by different authors [11,12,13,14].
The rising interest in energy storage capacity nowadays led to the following research questions within the present work:
Which efficiencies can be reached by applying the Fischer-Tropsch process as a technological pathway for the chemical storage of wind energy?
Which influence does the addition of hydrogen to the conventional Fischer-Tropsch process have on the process performance?
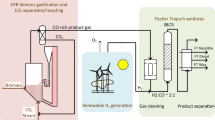
Fig. 1 illustrates a simplified flow chart of the investigated process idea for diesel production from biomass and wind power by the use of the Fischer-Tropsch process (FT synthesis).
Woody biomass is used as feedstock and gasified by the use of a dual fluidized bed steam gasification process. The product gas achieved is cooled by heat exchangers to about 40 °C and cleaned with several gas cleaning steps. The applied gas cleaning strategy aims at the removal of dust, tar, water and all further substances which could harm subsequent equipment as well as the used catalyst. Afterwards, the product gas is compressed to around 20 bar and carbon dioxide (CO2) is removed from the processed product gas.
The removed carbon dioxide (CO2) is fed to the gasification reactor to enhance the reverse water-gas-shift reaction (RWGS) as follows.
By the use of the reverse water-gas-shift reaction, additional carbon monoxide (CO) can be supplied to the Fischer-Tropsch reactions. Besides, hydrogen (H2) is produced from wind energy by the use of an electrolysis cell. It can be seen, that both gas streams are mixed and supplied to the Fischer-Tropsch (FT) synthesis. The overall process concept aims at the supply of a synthesis gas with a hydrogen (H2) to carbon monoxide (CO) ratio of 2:1 to favor good process conditions for the subsequent FT synthesis. The addition of hydrogen and the removal of carbon dioxide can contribute to an optimization of the overall process. The described process idea was used to design and execute an experimental campaign in Güssing, Austria, to determine the suitability of the Fischer-Tropsch process for the purpose of energy storage.
Table 1 shows a typical product gas composition of the used gasification process before the addition of hydrogen (H2) and before the removal of carbon dioxide (CO2). During the experimental campaign, real gas from the power plant Güssing according to the shown composition was used. Besides, hydrogen from compressed gas bottles was mixed with the product gas to simulate additional hydrogen supply from an electrolysis process. Overall, the carried out investigations included a literature data review, several experimental campaigns and the validation of measured data enabling an experimental optimization of the overall process.
2 Experimental and methodology
Between September 2014 and September 2016, five experimental campaigns were carried out. All campaigns were operated with product gas provided by the combined heat and power plant Güssing (8 MWth) shown in Fig. 2 (left). Fig. 2 (right) shows a picture of the laboratory-scale Fischer-Tropsch plant which was used to convert the provided gas into Fischer-Tropsch products.
Combined heat and power plant Güssing (left) and laboratory-scale Fischer-Tropsch plant (right) [13]
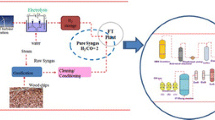
In Fig. 3, a simplified process flow chart is illustrating the experimental setup. Before the gas stream is fed to the experimental setup, the provided gas already passed a particle filter and a rapeseed methyl ester (RME) scrubber within the commercial power plant. Inside the laboratory-scale Fischer-Tropsch plant, Fischer-Tropsch synthesis is executed. The ingoing gas is cleaned and cooled to 10 °C within a second RME scrubber to remove remaining water and tar components. Subsequently, the gas stream passes an activated carbon bed to remove sulphur, aromatics and remaining tar components before the gas is compressed to about 20 bar. Within the next process steps, the syngas stream passes a zinc oxide (ZnO) and two copper oxide beds (CuO) to protect the cobalt-based catalyst within the Fischer-Tropsch reactor from deactivation. The overall applied gas cleaning strategy mainly aims at the removal of sulphur, chlorine and tar. The Fischer-Tropsch synthesis itself is processed within a slurry reactor containing about 2 kg of catalyst. The products achieved are finally separated by the use of liquid separators to reach a gasoline/naphta (C5–C9), diesel (C10–C19), wax (C20–C60) and water fraction. A detailed description of the laboratory-scale plant can be found in literature [15]. Table 2 gives an overview of main operation parameters during the executed campaigns. During the investigations, following main process conditions have been analyzed: constant load vs. fluctuating load and catalyst type.
Simplified process flow chart of laboratory-scale Fischer-Tropsch plant [5]
The experimental campaign was aiming at the investigating of important aspects of the “wind-diesel” concept. Therefore, the experimental investigation included different types of commercially available cobalt-aluminum oxide (Co, Al2O3) catalysts. As commercial catalysts have been used, a detailed characterization of the catalyst has not been carried out within the present work. Furthermore, the experimental campaigns were carried out at a constant base load in comparison with fluctuating load changes representing fluctuating wind power occurrence. Within the present work, expected load changes were simulated by feeding a fluctuating gas volume flow into a laboratory-scale Fischer-Tropsch plant. During the experimental campaigns, the fluctuating gas volume flow contained a mixture of syngas as well as added hydrogen from compressed gas bottles. Fig. 4 shows such a fluctuation gas volume flow during a load change experiment.
Gas volume flow entering laboratory-scale Fischer-Tropsch plant during load change experiment (cf. gas analysis Fig. 3)
The gas volume flow entering the laboratory-scale plant and the resulting product mass flows were measured during each experiment. Moreover, the gas composition of the incoming gas flow and the outgoing off-gas flow were measured by the use of a gas analyzer specified as “Perkin Elmer–Clarus 500” and “ABB EL3020” (cf. gas analysis Fig. 3). The liquid separators of the laboratory-scale plant were used to separate water (H2O), a gasoline/naphta (C5–C9), a diesel (C10–C19) and a wax fraction (C20–C60) as final products of the laboratory-scale plant. The composition of the achieved products was also analyzed by the use of a “Perkin Elmer–Clarus 500.” During the described load change tests, additional hydrogen was added to the process maintaining the optimal H2/CO ratio around 2:1. Each single experiment was monitored by the use of a process control system logging important measurement data.
The data achieved was validated by the use of a process simulation model allowing the calculation of mean values reflecting the mass and energy balance for each experiment. As a result, performance indicating key figures such as product distribution (α) and carbon monoxide conversion (X CO) were calculated to determine the performance of the observed process. The main Fischer-Tropsch reaction can be understood as exothermic polymerization reaction as follows [16]:
The product distribution can be described by the use of the Anderson-Schulz-Flory distribution as follows [17]:
Furthermore, the carbon monoxide conversion has been defined according to the following equation:
The carbon monoxide conversion represents an important performance indicating key figure with respect to the overall process efficiency. The described equations have been used for the evaluation of the executed experimental campaigns by the use of process simulation software.
3 Results and discussion
As a result of the experimental campaigns, extensive measurement data has been achieved. Several experimental points have been selected to evaluate the efficiency of the operated process. Fig. 5 shows the simulation model, which was used for an analysis of the experimental data achieved with the described experimental setup (cf. Fig. 4).
The described simulation model has been used to calculate validated mass and energy balances for single operation points. Table 3 shows the calculated results of the used simulation model. As an outcome of the Fischer-Tropsch reactions, a water, a gasoline/naphta, a diesel, and a wax fraction were generated as final products of the laboratory-scale plant.
Shown values represent validated measurement data of five experimental points. As it can be seen, the complex configuration led to varying process parameters. At the same time, the experimental team tried to keep main process parameters as constant as possible, whereas catalyst type and load type have been intentionally changed. Furthermore, all experimental points show quite similar results with respect to the performance indicating key figures. The CO conversion (X CO) has been measured between 20 and 40%. The product distribution (α) was measured between 0.89 and 0.93. Besides, the results show that catalyst B showed a better performance than catalyst A and C.
As it can be seen, a higher gas flow feed leads to an increased product output in case of catalyst B. Carried out load change experiments indicated, that product distribution (α) and CO conversion (X CO) are affected by higher gas volume flows. Besides, catalyst C showed an unsatisfactory process performance with respect to CO-conversion rate (X CO). Additional, increasing abrasion of the catalyst caused by a fluctuating volume flow was observed during the experimental campaign. The observed attrition indicated that a catalyst loss between 3 and 4 wt.-% per year is expected during base load operation. Whereas, the catalyst loss during load change operation is expected to increase up to 10–15 wt.-% per year.
The main goal of the overall process idea was the conversion of the provided syngas into liquid products. Therefore, the off-gas stream measurement gives a good indication about the process efficiency. Fig. 6 shows the development of the off-gas composition during a single experimental phase. The hydrogen content (H2) and the carbon monoxide content (CO) in the off-gas stream is lower than in the gas feed (cf. Table 1) due to the maintained Fischer-Tropsch reactions.
Furthermore, Fig. 7 shows the development of the process temperature inside the slurry reactor. The temperature development during base load operation is compared with load change operation. As a result, the good heat transfer capabilities of a slurry reactor can be identified. This aspect represents an important result for the investigated process concept. It leads to the conclusion that the production of diesel supported by fluctuating wind energy can be realized without the addition of a cost-intensive gas buffer for hydrogen from electrolysis.
4 Conclusion and outlook
Major points of the present investigations were to find out which process efficiencies can be reached and which influence can be achieved by the addition of hydrogen. The results of the present work show that around 0.2 kg/h Fischer-Tropsch products can be produced from around 1 Nm3/h syngas by the use of the described laboratory-scale plant. It has to be mentioned that the present laboratory-scale plant was operated as single-through process. If the off-gas is recycled, the production can be increased significantly. Therefore, the presented data contains valuable information for further scale-up steps.
Furthermore, the experimental campaign showed that the addition of hydrogen can be used to increase the output of the investigated process. The fluctuating addition of hydrogen (H2) from wind power increased the output of the Fischer-Tropsch plant in the case of catalyst B. This means that the proposed process could be used for energy storage purposes. Besides, it can be summarized that the laboratory-scale plant showed good operational behavior during the experimental campaigns. Catalyst B showed slightly better results than catalyst A and significantly better results than catalyst C. The results indicate once more the importance of the used catalyst type. Future campaigns should focus on an improvement of the catalyst performance and process integration as well as economic aspects of large-scale applications. Assuming a scenario of relevant times with a surplus of wind power in the grid, the economy of such a process seems attractive. Although there is still a need for further research to optimize the synthesis process, the technology is about to be ready for the demonstration at an industrial scale.
Abbreviations
- BtL :
-
Biomass to liquid
- DVGW:
-
German Association for Gas and Water (Deutscher Verein des Gas- and Wasserfachs)
- FTsynthesis:
-
Fischer-Tropsch synthesis
- HP reactor:
-
Heat pipe reactor
- PtG:
-
Power-to-Gas
- PtL:
-
Power-to-Liquid
- RME:
-
Rapeseed methyl ester
- RWGS:
-
Reverse water-gas-shift reaction
- SNG:
-
Synthetic natural gas
- SSD:
-
Soft shut down
- UNFCCC:
-
United Nations Framework Convention on Climate Change
- α :
-
Anderson-Schulz-Flory product distribution [−]
- C5–C9 :
-
Gasoline/naphta fraction [kg/h]
- C10–C19 :
-
Diesel fraction [kg/h]
- C20–C60 :
-
Wax fraction [kg/h]
- H2/CO ratio:
-
Ratio between hydrogen and carbon monoxide [−]
- n :
-
Variable number for polymerization reaction [−]
- n.CO.out:
-
Carbon monoxide entering slurry reactor [mol/h]
- n.CO.in:
-
Carbon monoxide exiting slurry reactor [mol/h]
- m :
-
Chain length number [−]
- W m :
-
Fraction share with specific chain length [−]
- X CO :
-
Carbon monoxide conversion [%]
References
http://unfccc.int (read on October 17th 2016)
http://europa.eu/legislation_summaries/energy/european_energy_policy/ (read on Oct. 17th 2016)
Sterner M (2009) Bioenergy and renewable power methane in integrated 100% renewable energy systems: limiting global warming by transforming energy systems. Kassel Univ, Press
Aicher T, Gonzalez MI, Schaub G, Götz M (2014) Betrachtungen des Gesamtsystems im Hinblick auf Dynamik und Prozessintegration. in: Energiewasserpraxis 65, 51–55
Mayer J, Jakuttis M, Binder S, Hornung A (2014) Energetische und wirtschaftliche Betrachtung einer dezentralen Methanolsynthese in: Proceedings 13.Symposium Energieinnovation Graz
Götz M, Lefebvre J, Mörs F, McDaniel KA, Graf F, Bajohr S, Kolb T (2015) Renewable Power-to-Gas: a technological and economic review. Renew Energy 85:1371–1390. https://doi.org/10.1016/j.renene.2015.07.066
Graf F, Krajete A, Schmack U (2014) Techno-ökonomische Studie zur biologischen Methanisierung bei Power-to-Gas-Konzepten. DVGW Forschungsbericht
Götz M, Graf F, Koch A, Lefebvre J, Bajohr S, Kolb T (2015) Coupling of biomass based processes with PtG, methanation technologies, process concepts, and economics in: Proceedings World Gas Conference, Paris
Graf F, Henel M, Tichler R, Schaaf T (2014) Technoökonomische Studie von Power-to-Gas-Konzepten. DVGW Forschungsbericht
Kaim-Albers N, Holtrup F, Tornic S (2015) Energie für Deutschland 2015 - Fakten, Perspektiven und Positionen im globalen Kontext. World Energy Council - Weltenergierat Berlin
Sauciuc A, Abosteif Z, Weber G, Potetz A, Rauch R, Hofbauer H, Schaub G, Dumitrescu L (2011) Influence of pressure on the performance of biomass based Fischer-Tropsch synthesis, in: Hofbauer H. (Ed.): Proceedings of the International Conference on Polygeneration Strategies (ICPS11), Vienna
Rauch R, Kiennemann A, Sauciuc A (2013) Fischer-Tropsch synthesis to biofuels (BtL Process), in: the role of catalysis for the sustainable production of bio-fuels and bio-chemicals, Triantafyllidis K. (Ed.), Amsterdam
Maier L (2014) Production of synthetic iso-paraffinic kerosene from wood, Master thesis, FH Burgenland, Pinkafeld
Fürnsinn S (2007) Outwitting the dilemma of scale: cost and energy efficient scale-down of the Fischer-Tropsch fuel production from biomass, PhD thesis, TU Wien
Müller S (2013) Hydrogen from biomass for industry—industrial application of hydrogen production based on dual fluid gasification, PhD thesis, TU Wien
Faajii PC, Hamelinck CN, Van Hardeveld MRM (2002) Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 23:129–152. https://doi.org/10.1016/S0961-9534(02)00037-5
Patzlaff J, Liu Y, Graffmann C, Gaube J (1999) Studies on product distributions of iron and cobalt catalyzed Fischer-Tropsch synthesis. Appl Catal A: Gen 186:109–119. https://doi.org/10.1016/S0926-860X(99)00167-2
Funding
The authors acknowledge financial support by the Austrian government through the “Klima- und Energiefonds” financed project Winddiesel_klienIF within the “e!Mission.at” funding scheme. The project Winddiesel_klienIF is executed in cooperation with Energie Burgenland AG, Bilfinger SE, Güssing Energy Technologies GmbH, REPOTEC GmbH & Co KG, Energy & Chemical Engineering GmbH, and the Institute of Chemical Engineering from TU WIEN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, S., Groß, P., Rauch, R. et al. Production of diesel from biomass and wind power – Energy storage by the use of the Fischer-Tropsch process. Biomass Conv. Bioref. 8, 275–282 (2018). https://doi.org/10.1007/s13399-017-0287-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-017-0287-1