Abstract
This study presents a comparison of combustion performance, losses, and efficiency at steady-state and transient conditions for different biomass types in a residential boiler. The types of biomass used were Ø6 and Ø8 mm wood pellets, Ø6 mm sugarcane bagasse pellets, Ø6 mm sunflower husk pellets, and Brazil nut shells. The comparison in the development of the temperature in the combustion chamber during the start-up revealed that the Ø6 mm wood pellets ignite and propagate faster than the rest of the biomass fuels due to their smaller size compared with Ø8 mm wood pellets and lower ash content compared with the rest of the biomass sorts. Thermal power output and efficiencies under steady-state and transient conditions were calculated by the direct method, i.e., by measuring the heat recovery by the water boiler, and the indirect method, i.e., by measuring the heat losses. By using the indirect method, the availability of the flue gas thermal power during the start-up was seen more in detail than when the other method was applied. When comparing both methods as tools for boiler efficiency evaluation for different fuel types, the discrepancy of the resulting efficiencies between is larger when there are higher amounts of chemical losses in the boiler. Therefore, this method shows good agreement also for bagasse pellets but is, without modification, proposed not to be valid for fuels emitting higher amount of carbon monoxide (CO). Boiler efficiencies reached class 3 boilers according to EN 303-5 (>74.8 %) for all biomass sorts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of biomass as a renewable energy source contributes to minimize dependence on fossil fuels and to mitigate greenhouse gas emissions since biomass is considered to be carbon dioxide (CO2) neutral [1]. In isolated houses or villages, where the extension of the electrical grid is unfeasible, the use of locally available biomass could be a viable alternative for power generation [1]. Agricultural residues coming from the harvest or from a nearby food industry are among the biomass sorts locally available. In food industries, the energy demand of the process is usually covered by the generated biomass residues such as sugarcane bagasse, sunflower husks, and Brazil nut shells [2–4]. However, the energy potential of these residues is often larger than the actual energy need in the process [2–4], and therefore, these residues could potentially be used outside the industries in small-scale heat and power plants [3, 4].
Sugarcane bagasse is the fibrous residue which originates from the cane milling in the sugar-ethanol industry. It has high moisture content (50 %) and is used directly for heat and power generation to the sugar-ethanol process. Along with improvements inside the sugar-ethanol industry, surplus of bagasse is expected [2]. This fact represents a potential to propose other alternatives to efficiently use the surplus bagasse in small-scale modern conversion technologies. For example, bagasse has been pelletized and tested in a small-scale gasification unit [3] and in a residential pellet boiler [4] demonstrating similar combustion behavior as commercial wood pellets under steady-state.
Sunflower husk is another residue from food industry, which has called for attention by some studies, in which its potential as fuel in the residential heating sector has been explored. Cardozo et al. [4], Verma et al. [5], and Smith [6] have used pellets of sunflower husk in residential boilers of different types and sizes. Smith [6] presented a combustion efficiency of about 62 % when burning sunflower husk pellets. Cardozo et al. [4] and Verma et al. [5] showed that higher pollutant emissions are found during the combustion of these pellets compared to wood pellets in two different types of pellet combustors under steady-state conditions.
A third type of agro-industrial residue is the Brazil nut shells. In research, they have been used to determine a kinetic model [7] and tested in a residential boiler [4] showing that it could be feasible to use this residue in small-scale combustion units. However, under steady-state conditions, higher emission levels and lower temperature in the combustion chamber were found in comparison to wood pellets.
The use of agricultural residues in small combustion units mostly designed for wood pellets has an increased interest among researchers. This is mainly due to the need to find alternative fuels to provide fuel-flexible combustion pellet technologies to spread the market overseas. Apple and olive pomace, reed canary grass, straw, and spent coffee grounds are examples of residues used in residential pellet boilers or stoves [4–12]. Verma et al. [5] used a 40-kWth pellet boiler and found boiler efficiencies in the range of 88–91 % when burning pellets of apple pomace, peat, sunflower husk, reed canary grass, straw, and pectin, respectively. Gonzales et al. [8] employed a mural boiler of 11.6 kWth and found boiler efficiencies in the range of 89.7–91.6 % when using olive kernels and pellets of forest, tomato, and cardoon residues. These authors estimated the heat losses as basis for the boiler efficiencies reported in their studies. Diaz et al. [9] used four types of biomass pellets and found boiler efficiencies of up to 77 % by applying the direct method, i.e., by measuring the heat absorbed by the boiler water. They also presented heat losses in the range of 1.4–3.7 % of the thermal power input. Miranda et al. [10] calculated the efficiency of a 5.8-kWth pellet stove by using, for example, the ratio between emitted carbon dioxide (CO2) and this CO2 added to the carbon monoxide (CO) emission. They found efficiencies over 70 % when using different blends of olive pomace and Pyrenean oak in the form of pellets. The same analysis of efficiencies was performed by Arranz et al. [11] using wood, fruit tree, and Pyrenean oak pellets. Limousy et al. [12] compared combustion parameters of pellets made of pure spent coffee grounds (SCG), blends of SCG and pine, and pure pine in a commercial residential boiler. They evaluated the combustion efficiency based on thermal, chemical, and due to combustible constituents in the residue ash losses (indirect method) and found that the highest efficiency (92 %) corresponds to the blend (50/50 %) pellets and the lowest (86 %) was found for pure SCG pellets. They also presented boiler efficiencies of 84 and 64 %, respectively, for the fuels mentioned, but these efficiencies were calculated based on the direct method. Fournel et al. [13] found efficiencies between 63 and 75 % (direct method) using wood and four dedicated energy crops in a 29-kW boiler. The SS-EN 303-5 standard [14] proposes the direct method to estimate the boiler efficiency when using solid fuels. On the other hand, the SS-EN 14785 standard [15] proposes the indirect method to calculate the efficiency of residential heating appliances fired by wood pellets. The type of method to be used for efficiency estimation is relevant in case not only steady-state should be reflected in the evaluation. Pellet combustion technologies work under intermittent conditions due to automatic ash removal cycles at certain time intervals, in which the combustion temporarily stops. This will affect the accumulated emission levels, the overall efficiency, and fuel consumption among other parameters. Besides that, standard and evaluation methods are based on wood pellet use; however, when introducing other types of biomass, these methods could give different results.
Persson et al. [16] validated a mathematical model for wood pellet boilers and stoves to calculate both the energy balance and the CO emission under transient conditions. They suggested that improvements such as increasing the time of tests are needed in experimental measurements of pellet consumption and CO concentration in the flue gas due to the large uncertainties found in their study. The transient conditions are of large importance in case the combustion unit should be combined with a prime mover and not only providing hot water to an accumulator tank. The delays in start-up, the available energy of the flue gas, and the intermittent operation of the burner are some of the parameters to consider in this aspect. Thiers et al. [17] presented a test bench to characterize steady-state and transient conditions of a micro-scale combined heat and power (CHP) plant fuelled on wood pellets. The start-up, normal operation, and shutdown were identified to develop a more accurate numerical model.
As explored above, there are several studies which include efficiencies, emission levels, and other combustion performance parameters of different types of biomass pellets; however, these mainly report steady-state conditions. A few studies have reported the transient conditions using wood pellets [16, 17]. To develop the small-scale combustion technology for CHP application and with other fuels than wood pellets, there is a need to study the combustion of these alternative fuels at transient conditions and over time at steady-state. The objective of the research study presented herein is thus to address alternative fuels for small-scale combustion at both steady-state and transient conditions. The research study brings up several of these new issues, such as:
-
The thermal availability, temperature development and CO emission levels in flue gas, energy losses and efficiencies under both steady-state and transient conditions highlighting the differences and similarities between wood pellets and agricultural residues
-
Evaluation of the direct or indirect method, respectively, as tools for boiler efficiency calculation depending on which fuel is being used
The data herein can be used for developing energy system models using agricultural residues and to perform more accurate estimations of annual emission levels as a consequence of using other fuels than wood. The data is also important for integration of a prime mover, for example a Stirling engine, for CHP configuration.
2 Methodology
2.1 Raw material
Five biomass sorts were used during the experiments: Ø6 mm sugarcane bagasse (B) pellets, Ø6 mm sunflower husk (SFH) pellets, Ø6 mm Brazil nut (BN) shells, Ø6 and Ø8 mm wood (W) pellets. Sunflower husks with origin in Bolivia and sugarcane bagasse with origin in Colombia were pelletized in the Energy Technology Centre (ETC) in Piteå, Sweden [18]. Parameters of the pelletizing process are explained in [19, 20]. Brazil nut shells with origin in Bolivia were reduced up to a uniform size. The wood pellets were standard pellets found in the Swedish market [21].
Table 1 [4] shows the chemical composition, heating values, and physical characteristics of the different biomass sorts. Chemical composition and heating values were analyzed by the accredited laboratory Bränslelaboratoriet Umeå AB, in Sweden [22]. The list of methods and relative uncertainties are given with the results reported by the laboratory [22]. Mean physical characteristics of each piece (i.e., single pellet or shell) were determined of 100 random samples. The average bulk density was obtained by measuring the mass of three samples with a normal laboratory balance (±0.001 g) and the volume using a graduated cylinder (±1 mL). The moisture content was measured according to ÖNORM G 1074. Table 1 includes expanded uncertainties with a coverage factor of 2 determined according to the National Institute of Standards and Technology (NIST) [23].
The highest heating value is attributed to the SFH and the lowest value to bagasse. However, the amount of heat recovered during combustion may vary due to differences in fuel composition and physical characteristics as shown in a previous study regarding the emission levels, operational variables, and conversion efficiencies [4].
2.2 Description of setup and procedure
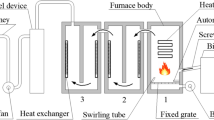
A scheme of the experimental setup is shown in Fig. 1 [4]. A commercial overfed wood pellet burner of 20 kWth, designed for 8 mm wood pellets and manufactured by Värmebaronen, Kristianstad in Sweden [24], was connected to a boiler CTC 1100 (previously used with an oil burner) which provides hot water. The different biomass types were fed to the system through a screw feeder. The burner has an automatic start-up process and a light indicator to inform when the flame is established. During the start-up phase, the burner fan first starts to draw air in at maximum speed during 20 s, followed by the screw feeder dispensing the pellets or shells for 12 s, and immediately the igniter starts to heat up the fuel (the light indicator flashes). The fan runs periodically at low speed until the light indicator flash is constant. This time of ignition was recorded for all fuels, i.e., the time for the establishment of the flame. After the start-up phase, the motor-driven screw feeder was controlled through an external sequence timer. This timer was used for setting a start-stop cycle (on–off cycle) of the motor. The start-stop working cycle is defined as sequence of feeding in this work. The sequence of feeding for each biomass type was chosen based on the combustion properties and reactivity levels of the fuels discussed in [4]. The sequence time was of 9 s (3 s on and 6 s off) for wood and bagasse pellets, while for BN shells, it was 11 s (3 s on and 8 s off) and for SFH pellets it was 8 s (2 s on and 6 s off). The air supply was kept the same for all the biomass fuels, only the fuel-feeding rate was changed. A calibrated air meter could not be used since the air admission was considerably reduced; therefore, the excess air ratio was estimated based on the flue gas composition as described in [4].
Scheme of the experimental setup used for the experiments [4]
To estimate the instantaneous fuel consumption, a cold condition test series was set up in which the mass of pellets (or shells) that fall onto the grate was determined per each start-up (x ig) and sequence of feeding (x s). A scale with a resolution of ±0.0001 kg was used, and 60 repetitions were performed per fuel. The details about the calculation of the consumption are found in the next section. The instantaneous fuel consumption was compared with measurements of the initial and final mass of the pellets (or shells) in the hopper and feeder, before and after each test. In this case, a scale with resolution of ±0.01 kg was used. The difference between the two methods was ±5 %. A suggested method by Persson et al. [16] is to consider only the mass of the pellets’ store. However, due to the continuous vibration during the feeding, accurate measurement when placing a scale under the pellet store is not possible to achieve. Higher accuracy is suggested by applying the method proposed in this study.
Temperatures T 1 and T 2, shown in Fig. 1, correspond to the flue gases before and after the heat exchanger of the boiler, respectively. T 1 was measured 25 cm above the burner and is considered to be the temperature in the combustion chamber. T 2 was measured in the base of the chimney, and it is considered as the flue gas exhaust temperature. T wi and T wo were the temperatures measured of the water entering and exiting the boiler, respectively. A Coriolis flow meter was located in the water inlet; the uncertainty of the measurement was of ±0.1 % of the measured value. Temperature data was recorded every 3 s using type K thermocouples with Ø1 mm size and an expanded uncertainty of ±3.4 °C with a coverage factor of 2.
The flue gas compounds were continuously monitored using a non-dispersive infrared analyzer for CO and CO2 (Uras 10P) and a magnetic pressure analyzer for O2 (Magnos 6G). Before and after each test, the instruments were calibrated with zero and span gases with an uncertainty of ±2 % vol. The data of the flue gas compounds was recorded every 3 s during every test. The tests were performed during 3 h for the wood pellets and 2 h for the other fuels. Each test was duplicated.
After finalizing each test, the bottom ashes were collected and weighted. Two samples of collected ashes per fuel were analyzed to determine the amount of unburned carbon by the laboratory Bränslelaboratoriet Umeå AB, in Sweden [22]. Soot values were measured using a Bacharach True Spot smoke test kit [25], an internationally recognized instrument to determine smoke density. For all the tests and fuels, the soot values were below 3 in the scale of this instrument which indicates a fair combustion.
The mean concentration and standard deviation of flue gas compounds and temperatures were calculated for steady-state normal conditions and according to NIST [23]. In the case of Ø6 and Ø8 mm wood pellets, the last 1 h of the test was considered to calculate average data. The last 30 min was considered as steady-state for the bagasse and SFH pellets.
2.3 Thermal power analysis
The thermal power output of the flue gas is evaluated by means of the direct and indirect methods proposed by the authors of this study and based on SS-EN 303-5:2012 [14] and SS-EN 14785:2006 [15], respectively. The direct method considers that the total thermal power output (P o1-i ) is equal to the absorbed power of the water (P w-i ). The indirect method takes into account the thermal losses due to hot flue gases in the chimney, chemical losses, and unburned carbon in ash in order to calculate the thermal power output (P o2-i ). Both thermal power outputs were calculated for the data collected every 3 s (denoted by -i) to show the transient and steady-state conditions.
2.3.1 Instantaneous fuel consumption and thermal input power
The fuel consumption at any operation time (t i) (i.e., every 3 s) is calculated by following the next steps.
By considering that before the ignition, x ig amount of pellets or shells that fall onto the grate and the time of ignition is t ig, the consumption of pellets at the time of ignition is:
The consumption of pellets at any operation time (t i) is considered as the addition of the pellets that falls before the ignition (x ig) and the amount of pellets (x i ) that is onto the grate per any operation time (Eq. 2). According to Eq. (3), x i is the total amount of pellets that falls onto the grate for every sequence time (x s /t s ) multiplied by the time of operation after the ignition.
Where:
The thermal power input (P fuel-i ) is calculated using Eq. (4) which is based on the consumption of pellets/shells calculated above for transient conditions and steady-state. The LHV for the biomass is corrected for the moisture content according to Table 1.
2.3.2 Direct method
The thermal power of the hot flue gas (i.e., thermal power output) (P o1-i ) is considered equal to the thermal power absorbed by the water (P w-i ) at any operation time (t i) as indicated in Eq. (5). The thermal power output (P o1-i ) is calculated according to SS-EN 303-5:2012 [14], European standard, based on the measured (every 3 s) water temperatures in the inlet and outlet (Twi-i ) and (T wo-i ), respectively, the water flow rate (\( {\overset{.}{m}}_{\mathrm{w}} \)), and water heat capacity (Cpw-i ) at the average temperature of the two temperatures mentioned previously.
2.3.3 Indirect method
The thermal power output (P o2-i ) is based on SS-EN 14785:2006 [15] where the thermal power of the hot flue gas is considered as thermal power input of the fuel (P fuel-i ) minus the thermal losses (P t-i ), the chemical losses (P ch-i ), and the heat losses due to unburned carbon in ash (P r-i ) as expressed in Eq. (6).
The thermal losses due to hot flue gas in the chimney (P t-i ) at any operation time (t i) are evaluated with Eq. (7) [15].
The chemical losses due to the CO emission (P ch-i ) are calculated using Eq. (8) [15].
It can be observed that chemical losses due to unburned hydrocarbons are not included in the standard [13].
The heat losses due to unburned carbon in ash are found with the following expression [15]:
2.3.4 Thermal efficiency
Thermal efficiency (ε) is evaluated using Eq. (10) at any operation time, t i, for both methods.
where j = 1 and 2 (direct respective indirect methods).
3 Results and discussion
3.1 Influence of fuel type on temperature development in the combustion chamber and CO emission levels during start-up
The temperature in the combustion chamber as a function of the time is shown in Fig. 2 for the different biomass sorts. This figure highlights the start-up process which is relevant for the comparison in the use of the different fuels and a later integration with a Stirling engine. To describe the differences among the time of ignition or establishment of the flame, four tests were performed per fuel. Average time of ignition is 276 s (±32 s) for Ø6 mm wood pellets which is the fastest; 308 s (±15 s) for Ø8 mm wood pellets; 326 s (±48 s) for B pellets; 392 s (±56 s) for SFH pellets; and 426 s (±68 s) for BN shells. The shells ignite after the longest time in comparison to the rest of the residues. The time of propagation of the hot flue gases up to the place of thermocouple 1 after the ignition is different among the fuels. The propagation time is illustrated in Fig. 2. For the Ø6-mm wood pellets, the propagation is faster than for the other fuels, meanwhile BN shells show the slowest propagation. The delay of propagation for the BN shells, SFH, bagasse, and Ø8 mm wood pellets with Ø6 mm wood pellets as zero point is 5.2, 3.8, 2.8, and 1.5 min, respectively. It seems that both a larger size of the fuel (compare Ø6 and Ø8 mm wood pellets) and a higher ash content result in longer ignition time and propagation delay. As observed, the total time of propagation and ignition is the longest for the BN shells. Previous studies [4, 7] showed that the reactivity of the BN shells was inferior which is suggested to delay the time of ignition and propagation and to reduce the temperature. The shell form affects the delay by reducing the transport of heat and mass during combustion: the biomass molecules are stronger bound in the shell forming a hard surface compared to pellets, which are formed by fine compressed particles easily reacting.
SFH and Ø8 mm wood pellets reach the highest temperature over time in comparison to the other biomass fuels (Fig. 2). SFH pellets have a lower moisture content which contributes to increasing the adiabatic flame temperature and therefore also to increase the combustion chamber temperature. The higher combustion temperature for SFH is also suggested to be due to the high reactivity with high burnout ratio [4, 26]. Comparing Ø6 and Ø8 mm wood pellets, less mass of Ø6 mm pellets falls onto the grate (compare Table 2) and therefore the ignition and propagation is faster than the other.
Figure 3a, b shows the CO emission levels as a function of the time for the different fuels used. Higher levels of CO are measured during the start-up for all fuels. While Ø8 mm wood and bagasse pellets reach steady CO level after some time, the Ø6-mm wood pellets continue to give fluctuating CO emissions. In the case of the BN shells and SFH pellets, the CO levels clearly fluctuate along time at much higher values than those of Ø6 mm wood pellets. Diaz et al. [9] also measured high concentration of CO during start-up of the combustion of four sorts of biomass pellets. The high levels of CO during the start-up are mainly suggested to be due to the larger amount of pellets onto the grate and a rapid decrease of oxygen in the start-up stage [9]. The oxidation of CO to CO2 is also dependent on higher temperature levels [27], and during the start-up, the temperatures are still increasing. A previous study by the authors [4] showed mean values of CO emission levels under steady-state conditions for these biomass sorts where the effect of properties and operational variables are explained in detail. Figure 3a, b clearly indicates that the combustion loss due to CO is highly fluctuating for certain fuels, and the combustion temperature seems to have less relevance to this fluctuating behavior when comparing with Fig. 2.
In another previous study by the authors [4], slightly higher NO emission levels were found when using bagasse pellets in comparison to wood pellets but higher levels when using BN shells and SFH pellets. Higher SO2 emission levels were also found for the agro-residues in comparison to wood pellets. Therefore, the impact of fuel type on emission levels, conversion efficiency of fuel compounds, and operating variables was seen in that previous study where it was noticed that the emission levels of CO fulfill the requirements of the EN 303-5 standard for a manual feeding and the burner was able to satisfactory works with the different fuels. Further analysis of particulate matter, volatile organic compounds (VOC), and HCl compounds is required.
3.2 Influence of fuel type on heat losses under steady-state and transient conditions
The heat losses evaluated in this study are the thermal losses due to hot flue gases leaving the chimney (P t), the chemical losses due to CO emission (P ch), and heat losses due to unburned carbon in ash (P r). Figure 4 shows the relative value of thermal losses related to the thermal power input (P fuel) as function of time and biomass sort. In this figure, during the start-up, the thermal losses fluctuate more for all the fuels but for the SFH pellets. This difference might be driven by the moisture content of the fuel. SFH pellets have significantly lower moisture content compared to the other fuel sorts, why the temperature fluctuation due to the vaporization of the water in the fuel is less pronounced during the establishment of the combustion process. Once the steady-state is reached, the thermal losses drop below 12 % of the power input except for the SFH pellets. In this case, the thermal losses are superior due to the higher amount of gases in the chimney at higher temperature. As seen in Table 1, the SFH pellets have both higher density and lower moisture content than the rest of the fuels, why more mass is introduced in the boiler and consequently more combustion gases are produced. Lower moisture content also promotes higher combustion temperature. Overall, the recovery of heat by the water from the hot flue gases has a delay due to the gradual heat transfer to the surface of the heat exchanger. The next section explains the efficiency in the heat transfer.
The relative value of the chemical losses to the thermal power input as function of time and biomass sort is presented in Fig. 5a for the Ø8-mm wood, Ø6-mm wood, and bagasse pellets and in Fig. 5b for the BN shells and SFH pellets. During the start-up, higher chemical losses are revealed, which is related to the higher release of CO shown in Fig. 3a, b. The lowest chemical losses are found when using bagasse pellets. Here, it is suggested that the lower density of this fuel and high reactivity contribute to keep the CO emission at low levels during the start-up providing enough residence time, oxygen, and temperatures of the glow bed. BN shells and SFH pellets as indicated in Fig. 5b present much higher chemical losses which fluctuate considerably in time in comparison to the other fuels. The main reason for the higher chemical losses in the BN shells and SFH pellets combustion is suggested to be due to the high ash content of these fuels which affect the transport of oxygen to the active char surfaces. This causes an unevenly distributed temperature profile [28], which in turn affects the oxidation rate of CO to CO2 and higher amount of CO is able to be released. A better distribution of the air inside the grate and a longer grate may contribute to reduce the CO release and therefore to reduce the chemical losses especially in the case of high ash content biomass.
Table 2 presents the average heat losses as a function of biomass sort under steady-state conditions. The lowest thermal loss (P t) is found for the Ø6-mm wood pellets, and the highest was found for the SFH pellets. SFH pellet combustion gives the highest chemical losses (P ch) due to the highest release of CO emission [4]. The higher heat losses due to unburned carbon in ash (P r) are found for the BN shells, which are directly related to the amount of unburned carbon in ash. Thermogravimetric analysis of BN shells has shown that this fuel gives higher amount of char after the pyrolysis step in comparison to both wood and bagasse [19]. This suggests that BN shells are more likely to leave a larger portion of unburned carbon in the char. As seen in Fig. 2, the combustion temperature is lower for BN shells, thus leading also to lower char conversion rate and thereby larger amount of unburned carbon is found in the residual ash. Due to the reduce amount of flue gas and lower temperatures during the combustion of the BN shells, the steady-state in 1-h test could not be reached and therefore thermal losses under steady-state are not presented in Table 2.
3.3 Thermal power output and efficiency evaluation under steady-state and transient conditions
The thermal power input, P fuel, and thermal power outputs, P o1 and P o2, as function of time are shown in Fig. 6a–e for each biomass sort. The fuel power input increases rapidly to thereafter smoothen out as shown in these figures. Also, the thermal power available in the flue gas determined by the indirect method, P o2, shows a rapid increase, with more stable development for Ø6 mm wood and bagasse pellets than for SFH and Ø8 mm wood pellets and BN shells. If the flue gas is used for a prime mover, for example a Stirling engine, it is expected that the Ø6-mm wood and bagasse pellets would give a more stable operation during start-up than the other fuels. For all the fuels, the difference between the direct and indirect method to estimate the thermal power output is manifested in the start-up phase of the boiler. While thermal power, P o2, is almost immediately available in the flue gas, there is a time delay until thermal power is available in the water line, P o1. This delay is suggested to be due to the cold surface of the water heat exchanger in the boiler during the start-up and the time it takes to heat it. Therefore, the indirect method is more suitable to determine how much thermal energy the flue gases carry in the start-up phase.
However, once the water line has reached steady temperature, the thermal power determined by the direct method is in the magnitude of the one determined with the indirect method (Fig. 6a–c, d) but for the BN shells and SFH pellets. The thermal response of the heat exchanger is lower than the change of available heat in the flue gas, with a possibility to momentary store heat in mass of the exchanger, which could explain the higher thermal power available in the water line. In the case of the BN shells and SFH pellet combustion, the thermal power output P o2 (Fig. 6d, e) is variable in time due to the disparity in CO concentration. The fluctuation of P o2 is mostly dependent on the fuel. The delay in the recovery of the heat by the water is higher when using BN shells in comparison to the rest of the fuels which is suggested to be due to the reduced amount of flue gas and lower temperatures during the combustion as mentioned before. This would highly affect integration of a prime mover, for example a Stirling engine in the system. While the thermal availability is high for wood and bagasse pellets, the electrical efficiency based on fuel input for such an engine would also be higher in comparison to both Brazil nut shells and SFH pellets, which both show lower thermal availability in comparison to the fuel input. However, the higher combustion temperature of SFH pellets may compensate this inefficiency, since higher temperature would lead to higher Stirling engine power within its operational limits [29].
The thermal efficiency (ε 1) shown in Fig. 7a–e determined by the direct method follows the same tendency as the thermal power output P o1, i.e., during the start-up phase, the water of the boiler absorbs little amount of heat from flue gases. After the start-up period, ε 1 becomes higher and more stable. The thermal efficiency determined by the indirect method (ε 2), shown in Fig. 7a–e, fluctuates during the start-up for all biomass sorts and continue to vary in time for the BN shells and SFH pellets similar to the behavior of the thermal power output P o2 shown in Fig. 6d, e. It is however observed that the Ø6-mm pellets of both wood and bagasse give better efficiency stability of ε 2 than the Ø8-mm wood pellets during start-up, but the Ø8-mm wood pellets give the fastest thermal response in the water line, ε 1. This is suggested to be due to the temperature development as observed in Fig. 2, where the Ø8-mm wood pellets reach higher temperature region fastest among the fuels. The direct method provides, but for the BN shells, slightly higher efficiency under steady-state conditions in all the cases which is suggested to be due to the momentary heat storage capacity of the heat exchanger. The thermal power available in the flue gas during the start-up period is transferred to the heat exchanger mass and thus stored. BN shells and SFH pellets present lower and irregular efficiencies than the other fuels over time, and BN shells give an unreasonable long start-up time before the heat in the flue gas is available in the water line. Figure 7 clearly manifests that the two methods for predicting the boiler efficiency give very distinct results when the chemical losses are high and fluctuating with time (compare Fig. 5). While ε 1 and ε 2 approach each other after the start-up of wood respective bagasse pellets, the discrepancy of the two efficiency values still is large for BN shells and SHF pellets. Specifically for BN shells, with the low combustion temperature and high CO emission, it is highly probable that there also are unburned hydrocarbons, UHC, which are not accounted for in the standard based on the indirect method (i.e., SS-EN 14785:2006 standard). This standard for estimating the residential boiler efficiency is suggested not to be valid for fuels showing higher emission of CO during combustion, without introducing a compensating loss accounting for unburned hydrocarbons. However, except from wood pellets, the standard shows good agreement with the direct method also for bagasse pellets. The indirect method to calculate efficiencies ignores some thermal losses due to convection heat from the boiler surface and therefore is higher especially in the start-up phase, and it is expected to keep higher values after longer time of operation when CO emission levels are low.
Table 3 shows average values of thermal power outputs and efficiencies under steady-state conditions as function of the biomass sort. Temperatures of the water flow in the inlet and outlet of the boiler are included in Table 3 as well. The temperature of the water in the inlet is raised since it is recycled in a closed circuit where a plate heat exchanger cold down the water. Higher values of thermal power outputs and efficiencies are determined by using the direct method for the Ø6-mm wood, bagasse, and SFH pellets, which is also observed in Figs. 6 and 7. Both types of wood pellets show higher thermal power outputs and efficiencies under steady-state conditions than the other fuels. Even though there is a notable reduction in the bagasse power input, shown in Table 2, high efficiencies are found by applying both methods. In the case of the SFH shells, by applying the direct method, high thermal output and efficiency are found during the steady-state. This is suggested to be due to the high temperature measured in the flue gas (Fig. 2), resulting in good heat transfer to the water line. On the other side, the efficiency determined by the indirect method is lower due to the high and fluctuating losses measured, i.e., the thermal and chemical losses shown in Figs. 4 and 5b. The thermal losses are considerably high since the full potential of the heat in the flue gas of the SFH pellets could not be absorbed in the water line. The chemical losses are directly related to the CO emission (Fig. 3) which depends strongly on the properties of the fuel as shown in a previous study [4]. The thermal efficiencies determined by the direct method have provided information of the capability of heat recovery of the boiler itself. On the other side, by applying the indirect method, the thermal efficiency during the start-up phase has been shown without taking the thermal response time of the heat exchanger into consideration. This information is valuable if the burner is, for example, integrated with a Stirling engine where the heat transfer will differ compared to that of the water line in a common residential boiler.
The high ash content of the biomass fuels could cause operational problems in longer runs due to a rapid accumulation of ash onto the grate as it was seen using SFH pellets. But when using bagasse, no evident problems were observed; therefore, it is expected that the efficiency of the boiler will be kept similar to the use of wood pellets. Chlorine content in the biomass fuels could also represent an issue after a longer time of operation due to the chlorine dioxide formed during combustion may attack the metallic surface of the boiler [8], which therefore could reduce the efficiency in the heat transfer process; this aspect requires further analysis when using agricultural residues.
Efficiencies according to EN 303-5 standard [30] should be a minimum of 54.8 % for class 1, 64.8 % for class 2, and 74.8 % for class 3 boilers. The efficiency according to this standard should be evaluated based on the heat recovery by the water. Therefore, the boiler used in this study, whatever the fuel is, reached the class 3 limit indicated by EN 303-5.
Verma et al. [5] presented boiler efficiencies of about 89 % for SFH pellets with 11.8 % of moisture content and 2.78 % of ash (wt%) using a horizontally fed pellet burner in a residential boiler. They determined the thermal efficiency using the Siegert formula which only takes into account the thermal losses. In this study, if only the thermal losses are considered when using SFH pellets, the efficiency reaches 87 %, which is close to the data presented by Verma et al. [5].
4 Conclusions
A comparison of the thermal power availability has been performed among Ø6 and Ø8 mm wood pellets, Ø6 mm sugarcane bagasse pellets, Ø6 mm sunflower husk pellets, and Brazil nut shells (L × W × H = 11.5 × 2.0 × 7.4 mm). The temperature development in the combustion chamber during start-up was diagrammed. Direct and indirect methods to evaluate the thermal power output and efficiencies have been applied under steady-state and transient conditions. Heat losses and efficiencies have been compared among the fuels under steady-state and transient conditions.
The following conclusions were drawn from this study:
-
The ignition time or establishment of the flame was different depending on the fuel type; larger particles and higher ash content resulted in longer ignition time which was the case of the Brazil nut shells and sunflower husk pellets. Higher temperatures in the combustion chamber were observed for the Ø8-mm wood and sunflower husk pellets but did not result in highest efficiencies recorded due to high thermal losses of both fuels and high chemical losses in the case of the SFH pellets.
-
The fuel type influences the level of thermal and chemical losses. Higher percentage of chemical losses was found for the Brazil nut shells and sunflower husk pellets mainly due to the higher CO release, which was also fluctuating over time. It was found that the combustion chamber temperature is of less relevance for this fluctuation of CO emission over time.
-
Thermal power availability of flue gases and efficiencies varied according to the biomass sort. The lowest thermal power output and efficiencies were recorded for the Brazil nut shells mainly due to low temperatures in the combustion chamber and high amount of unburned carbon in ash.
-
While the indirect method of determining the efficiency gives instant information about the heat available in the flue gas, the direct method with thermal response delay in the heat exchanger generally shows higher efficiency levels at steady-state.
-
When comparing the direct and indirect method as tools for boiler efficiency evaluation, the discrepancy of the resulting efficiencies between the two methods is larger when there are higher amounts of chemical losses in the boiler and there is a lower temperature of the hot flue gases in the heat exchanger. The indirect method is showing good agreement also for bagasse pellets in addition to wood pellets but is, without modification, proposed not to be valid for fuels with high emission level of CO.
The boiler efficiencies found when using the different fuels but the BN shells showed slight difference among them which refers that the boiler was able to absorb efficiently the heat available from each fuel. High efficiencies of heat recovery and combustion are found for bagasse pellets showing a great advantage in the use of this fuel in residential boilers. Modifications in the ash content by blending Brazil nut shells and sunflower husk pellets could provide improvements to reduce CO emission levels and therefore chemical losses.
Abbreviations
- Ba:
-
Bottom ash collected referred to the mass value of the test fuel (wb) (% of mass)
- B:
-
Bagasse
- BN:
-
Brazil nuts
- Ca:
-
Calculated
- CHP:
-
Combined heat and power
- db:
-
Dry basis
- SFH:
-
Sunflower husks
- UHC:
-
Unburned hydrocarbons
- W:
-
Wood
- wb:
-
Wet basis
- C:
-
Carbon content of the test fuel (wb) and in residue ash (% of mass)
- CO:
-
Carbon monoxide content of the dry flue gas (% of volume)
- CO2 :
-
Carbon dioxide content of the dry flue gas (% of volume)
- Cp:
-
Specific heat (kJ/kg K)
- H:
-
Hydrogen content of the test fuel (wb) (% of mass)
- LHV:
-
Lower heating value (MJ/kg)
- M :
-
Moisture content of the test fuel (% of mass)
- \( \overset{.}{m} \) :
-
Mass flow (kg/s)
- P :
-
Thermal power (kW)
- x :
-
Mass of fuel (kg)
- t :
-
Time (s)
- T :
-
Temperature (°C, K)
- u :
-
Unburned carbon in ash, referred as to mass of ash (% of mass)
- Ø :
-
Diameter (mm)
- ε :
-
Thermal efficiency (%)
- -i :
-
Calculated every 3 s
- 1:
-
Flue gas inside the combustion chamber
- 2:
-
Flue gas in the chimney
- ch:
-
Chemical losses
- i :
-
Any operation time
- ig:
-
Ignition
- md:
-
Dry flue gas in standard conditions
- mH2O:
-
Water vapor in standard conditions
- o1:
-
Output 1: direct method
- o2:
-
Output 2: indirect method
- r :
-
Residue ash
- s :
-
Sequence
- t :
-
Thermal loss
- th:
-
Thermal energy
- w :
-
Water
- wi:
-
Water inlet
- wo:
-
Water outlet
References
Demirbas AH, Demirbas I (2007) Importance of rural bioenergy for developing countries. Energy Convers Manag 48:2386–2398
Ferreira-Leitâo V, Fortes LM, Ferrara MA, Lima A, Correa Molinari B, Bon E (2010) Biomass residues in Brazil: availability and potential uses. Waste Biomass Valoriz 1:65–76
Erlich C, Fransson T (2011) Downdraft gasification of pellets made of wood, palm-oil residues respective bagasse: experimental study. Appl Energy 88:899–908
Cardozo E, Erlich C, Alejo L, Fransson T (2014) Combustion of agricultural residues: an experimental study for small-scale applications. Fuel 115:778–787
Verma VK, Bram S, Delattin F, Laha P, Vandendael I, Hubin A, De Ruyck J (2012) Agro-pellets for domestic heating boiler: standard laboratory and real like performance. Appl Energy 90:17–23
Smith G, Lindley J (1988) An evaluation of two sunflower residue fuels. Biomass 17:215–224
Bonelli PR, Della Rocca PA, Cerrella EG, Cukierman AL (2001) Effect of pyrolysis temperature on composition, surface properties and thermal degradation rates of Brazil nut shells. Bioresour Technol 76:15–22
Gonzales JF, Gonzales-Garcia C, Ramiro A, Gonzales J, Sabio E, Gañan J, Rodriguez M (2004) Combustion optimization of biomass residue pellets of domestic heating with a mural boiler. Biomass Bioenergy 27:145–154
Dias J, Costa M, Azevedo J (2004) Test of a small domestic boiler using different pellets. Biomass Bioenergy 27:531–539
Miranda T, Arranz JI, Montero I, Román S, Rojas CV, Nogales S (2012) Characterization and combustion of olive pomace and forest residue pellets. Fuel Process Technol 103:91–96
Arranz JJ, Miranda MT, Montero I, Sepúlveda FJ, Rojas CV (2015) Characterization and combustion behaviour of commercial and experimental wood pellets in south west Europe. Fuel 142:199–207
Limousy L, Jeguirim M, Dutournié P, Kraiem N, Lajili M, Said R (2013) Gaseous products and particulate matter emissions of biomass residential boiler fired with spent coffee grounds pellets. Fuel 107:323–329
Fournel S, Palacios JH, Morissette R, Villeneuve J, Godbout S, Heitz M, Savoie P (2015) Influence of biomass properties on technical and environmental performance of a multi-fuel boiler during on-farm combustion of energy crops. Appl Energy 141:247–259
SS-EN 303-5.2012. Heating boilers—part 5: heating boilers for solid fuels, manually and automatically stoked, nominal heat output of up to 500 kW—terminology, requirements, testing and marking
SS-EN 14785:2006. Residential space heating appliances fired by wood pellets—requirements and tests methods
Persson T, Fiedler F, Nordlander S, Bales C, Paavilainen J (2009) Validation of a dynamic model for wood pellet boilers and stoves. Appl Energy 86:645–656
Thiers S, Aoun B, Peuportier B (2010) Experimental characterization, modeling and simulation of a wood pellet micro-combined heat and power unit used as a heat source for a residential building. Energy Build 42:896–903
Energy Technology Centre, Piteå, Sweden; www.etcpitea.se
Erlich C (2009) Comparative study of residue pellets from sugar cane and palm-oil industries with commercial wood pellets, applied in downdraft gasification. Doctoral Thesis, Dept of Energy Technology, Royal Institute of Technology, Sweden
Cardozo E. Pelletizing study of agricultural residues—parameters and energy consumption. Internal report EKV 05/11. Department of Energy Technology, Royal Institute of Technology, Sweden; 2011
Neova, Hudiksvall, Sweden; http://neova.se.sv
Bränslelaboratoriet, Umeå, Sweden; http://www.branslelaboratoriet.se
Taylor BN, Kuyatt CE (1994) Guidelines for evaluating and expressing the uncertainty of NIST measurement results. NIST Technical Note 1297
Värmebaronen, Kristianstad, Sweden; http://www.varmebaronen.com
Bacharach, Inc.; http://www.bacharach-inc.com
Zabaniotou A, Kantarelis E, Theodoropoulos D (2008) Sunflower shells utilization for energetic purposes in an integrated approach of energy crops: laboratory study pyrolysis and kinetics. Bioresour Technol 99:3174–3181
Farizan N (2013) Combustion of gasified biomass: experimental investigation on laminar flame speed, lean blowoff limit and emission levels. Doctoral Thesis, Department of Energy Technology, Royal Institute of Technology, Sweden
SP Technical Research Institute of Sweden, Rönnbäck M, Johansson M, Claesson F (2008) ERA-Net Evaluation of technology status for small-scale combustion of pellets from new ash rich biomasses-combustion tests in residential burners. Energy Technology, SP Report, 31, ISBN 9789185829484
Nishiyama A, Shimojima H, Ishikawa A, Yoshinori I, Kambara S, Moritomi H, Mori S (2007) Fuel emissions properties of Stirling engine operated with wood powder. Fuel 86:2333–2342
Oravainen H, VTT Energy. Testing methods and emission requirements for small boilers (<300 kW) in Europe. Motiva’s Publication B3/2000. [Internet]. [Accessed 05/13]. Available from: http://www.vtwoodsmoke.org/pdf/euBoileremission.pdf
Acknowledgments
Swedish International Development Agency (SIDA), Heat and Power Technology Division of the Energy Technology Department in the Royal Institute of Technology (KTH), Sweden, and Universidad Mayor de San Simon, Bolivia are acknowledged for the financial support of this work. Thanks for the technical support of Stellan Hedberg, Christer Blomqvist, Leif Petterson, and Göran Arntyr.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardozo, E., Erlich, C., Alejo, L. et al. Comparison of the thermal power availability of different agricultural residues using a residential boiler. Biomass Conv. Bioref. 6, 435–447 (2016). https://doi.org/10.1007/s13399-016-0200-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-016-0200-3