Abstract
Several wellbore instabilities issues arise when drilling into any reactive shale formation. These problems affect the drilling activity seriously which eventually results in non-productive time events. This article primarily focuses on the reduction in filtration loss volume and increased wellbore stability in shale by making it hydrophobic in nature upon its contact with industrial polymers classified as biopolymers, synthetic polymer, and modified polymer. The comparison is performed to highlight the advantages of ecofriendly biopolymers over other classes of polymeric systems. The result of the study reveals significant reduction in filtrate loss volume with the use of biopolymers. Their polymeric chain entangled with the hydrogen bonding in xanthan gum and decreased the loss of filtrate volume. Moreover, biopolymers also formed thin, smooth, and denser mud cake. On the other hand, the presence of monovalent cations in the base mud system reduced the performance of modified polymer and caused them to dehydrate. These mud systems were also tested on Ghazij shale obtained from the western part of Pakistan. The cutting disintegration test after 24 h revealed the efficacy of biopolymers with 100% and shale cutting recovery of 95%. The encapsulation of the shale cutting was the reason behind the higher recovery percentage. This result was also validated by the linear dynamic swell meter test. In addition, the contact angle measurement revealed that biopolymers were capable of increasing the hydrophobicity of shale formation, preventing the migration of water molecules and increasing the overall shale stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Literature Review
Drilling fluids play a major role in the execution of a successful well drilling venture. These fluids are particularly used for the purpose of maintaining an overbalance over the exposed part of the formation during drilling, removal of cuttings from the bottom hole and carrying them to the surface, forming a thin layer across the formation to avoid further penetration of fluid into the formation, lubrication and cooling the drill bit, minimizing the frictional force between the drill string and the borehole, preventing fluid seepages into thief zones, and providing assistance in well logging operations. In order to accomplish all these tasks, different chemical additives along with a base component such as water, oil, or gas, are mixed together to design a particular drilling fluid [1,2,3,4]. Drilling fluids are classified into oil-based mud (OBM), water-based drilling mud (WBDF), and inverted emulsion fluid [5, 6].
These fluids are used to provide wellbore stability during any drilling activity [7]. The most important application for these drilling fluids is to maintain the well integrity; particularly in shale formation [7]. During a drilling activity, 90% of the wellbore instability issues are mainly associated with the shale rock [8,9,10]. These formations can be drilling using OBM systems because of preeminence in terms of maintaining shale integrity, stability at elevated temperature conditions, and providing effective lubrication [8, 11]. However, their extreme cost and environmental concerns are the major problems responsible for their restriction [8, 12, 13]. Hence, WBDF are fundamentally used to drill 75% of shale formations [14]. In general, WBDF are inexpensive and environmentally friendly [14, 15]. However, poor rheological properties, clay swelling [16], and high loss of drilling fluid are some of the common problems faced with this system [14, 17].
Various additives are added to improve the characteristics of WBDF. These additives are primarily functioning as shale inhibitors, helping in avoiding any hydration, disintegration and swelling problems during drilling operations [18]. The ultimate objective of these additives is to improve and sustain the hydrophobic behavior of clay surfaces, which will eventually inhibit the migration of water molecules in the nanoplatelet of clay mineral [18, 19]. The most commonly used shale inhibitor used in the industry is potassium chloride (KCl) [18, 20]. Nevertheless, higher concentration of this salt ultimately results in dephasing of the mud system into two separate entities; sediments and liquid [20, 21]. Therefore, the application of polymers, nanoparticles, natural products, and surfactants gained popularity [5, 8, 18, 22,23,24,25]. The effect of polymeric material on the mitigation of shale swelling is the primary goal of this article. Four different polymeric materials classified as biopolymers, synthetic polymer, and modified polymers are focused in this article. Several studies are performed to investigate the performances of these polymeric materials individually; however, a comparative study as performed in this article was found scarce in the literature.
Two biopolymers namely, pure-bore and sodium alginate, are used as the filtration control additives and shale swelling inhibitors in this study. Pure-bore is known for its environmental-friendly and non-toxic nature. It is a polysaccharide material with a surface activity [26]. This polymer is primarily used to improve the inhibition characteristics of drilling fluid [26], by sealing the micropores present in a formation. Similar behavior is also shown by sodium alginate. This polymer is a natural polymer extracted from brown algae [27]. It is formed by the combination of two different monomers of α-1-guluronic acid and β-Mannuronic acid [27, 28], and is classified as linear polysaccharide polymer that belongs to the family of carbohydrate [28]. Moreover, a synthetic polymer partially hydrolyzed polyacrylamide (PHPA) is also used in this article. This is an anionic synthetic polymer that mainly comprises of repeating units of acrylamide and acrylic acid in its chemical structure [29, 30]. This polymer is essentially used as a shale inhibitor and as a flocculants [29]. In addition, a polymer belonging to modified natural polymers naming polyanionic cellulose (PAC) was also used in this study. This is a high molecular weight polymer, formed by the monomers of β-d-glucopyranose units [31]. This polymer has outstanding shale stabilization and suspending properties.
With continuous escalation in environmental constraints, the use of biodegradable ingredients in drilling fluid gain significant prominence. The study in this article provides a substitution for modified and synthetic polymers in the form of biopolymers. These polymers are not only ecologically friendly but also outperformed other polymeric substance in terms of shale inhibition and decrease in filtrate loss volume.
In the present study, biopolymers like pure-bore and sodium alginate were tested against the already used modified and synthetic polymers in the industry in terms of filtration and shale stability. This effectiveness of biopolymers was tested through a series of experimentations that includes LPLT filter press, pH measurement, and shale cutting dispersion test, contact angle measurement on shale coated with polymer mud and linear dynamic swell meter test. This extensive work on filtration and Pakistan shale stability characteristics has never been reported in the literature. Therefore, this study can prove to be of significance for oil and gas sectors, which are drilling in western area of Pakistan and are encountering Ghazij shale. Furthermore, this formation is extremely common and is one of the problematic formations encounters in western part of Pakistan; hence, it is the focus of this study.
1.1 Shale Formation and its Characteristics
1.1.1 Lithology of Ghazij Shale
Ghazij Formation was obtained from the western part of Pakistan near the Suleiman and Kohat-Potwar areas of the upper and middle section of Indus basin with the help of a drilling operator company. The deposition in Ghazij formation can be traced back to the late Paleocene to the early Eocene periods on the geological time scale [32]. Figure 1 shows the location of the Ghazij formation in Pakistan [33]. It is composed of mainly fine to medium grained calcareous sandstone along with certain varying percentages of shale, impure clay stone and mudstone. Ghazij formation is considered to be a possible source rock due to its high TOC [34].
Location of Ghazij Formation of Pakistan [29]
1.1.2 Cation Exchange Capacity (CEC) of Ghazij Shale
The cation exchange capacity of shale sample reflects the hydration tendency of shale sample upon its contact with water [35]. The CEC test was performed in accordance with API-RP-131 [36]. The amount of exchangeable ions in the shale is determined using this test, which directly correlates with the reactivity of the shale sample [19]. This test is primarily a qualitative test; hence, the uncertainties would be part of the testing procedure. The CEC value for Ghazij shale was 10 meq/100gm.
1.1.3 Scanning Electron Microscope (SEM)
It is extremely important to observe the shape, microstructure and arrangement of minerals in shale formation [35]. This information helps in understanding the hydration capacity of a mineral [35]. Scanning electron microscope (SEM) image of the shale sample before and after contact with the fluid is shown in Fig. 2. Flaky clay minerals are tightly packed as it can be observed in the shale sample. These clay minerals upon contact with water would expand, which eventually result in higher swelling rate of the shale sample.
1.1.4 Contact Angle of Ghazij Shale Pellet upon interaction with Water
Changes in the wetting characteristics of shale formation are counted as an imperative approach in the shale stability [35]. For the determination of contact angle, the powder Ghazij shale was first pressed in hydraulic compactor at a pressure of 8000 psi to form a shale pellet. Next, with the help of syringe, a water droplet was placed on the surface of the shale pellet. The test was conducted at room temperature and ambient pressure. Figure 3 shows the contact angle (CA) measuring setup formed during the testing. The CA angle was measured using image processing software called ImageJ. This software comprises a built in option called angle tool that is shown in Fig. 4. Measuring the angle is a two-step process with this option. In the first step, the angle tool based on two lines is placed on the droplet. In the next step angle between the two lines is calculated and displaced by the ImageJ software. This procedure for measuring the contact angle was conducted thrice, and the average values are reported in the manuscript. Similar technique was implemented for the uncertainty measurements. Figure 4 shows the contact angle θ = 48.504° that was established by the water on the solid surface. The hydrophilic nature of the shale formation was proved with θ < 90°. This clearly indicates the presence of stronger affinity of Ghazij shale toward water which will be present in the drilling fluid.
Experimentation setup for the determination of contact angle in this study [37]
1.2 Physical Appearance of Ghazij Shale
The Ghazij shale was obtained from the depth of 7790–8290 ft. The physical appearance of the formation is shown in Fig. 5. The formation is predominantly light greenish in color [34, 38] and can be classified as middle Ghazij shale formation [38]. Sticking and swelling are the most common behavior associated with this formation [38]. This clearly indicates the presence of considerable concentration of hydratable clays [38].
1.3 Mineral wt% in Shale Formation
The mineralogy of the shale formation under the study was provided by the drilling operator working in Pakistan. The sample of shale comprises of 23% clay mineral, 48% silicates, 10% iron ores, 9% feldspar and 10% carbonates. The clay mineral concentration was dominated by Smectite mineral with 12 wt%. Remaining 11% was divided among kaolinite, illite and chlorite. The higher wt% of Smectite also indicates higher CEC of the sample. Similar minerals were also observed by Intizar Hussain in his work [39]
2 Methodology
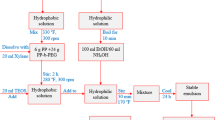
Figure 6 shows the complete experimental framework followed during this research. A total of five samples were prepared during this study. 350 cc, i.e., equivalent to 1 laboratory barrel, was prepared for each sample, and was tested in the laboratory. During the testing and preparation of the mud API recommended standard 13B-1 was methodologically followed. The base mud system comprises of 320 mL of water, 0.30 g soda ash (pH stabilizer), 16 g potassium chloride (inhibitor), 0.75 g xanthan gum (viscosifiers), and 75 g barite (weighing agent). The chemicals were obtained from a service company operating in Pakistan. Next, the polymeric mud systems were formulated. These systems comprised of four different polymers obtained from Sigma-Aldrich and a service company working in Pakistan. Xanthan gum in the base mud system was replaced by these polymers to investigate the effect of filtration and inhibition of shale. The shale sample during this work was collected from the western part of Pakistan with the help of a service provider. The shale cuttings were gathered from the depth of 7790–8290 ft. Part of the cutting was converted into the powder form through the help of Mortar Grinder, which was, then, eventually transformed into the shale pellet for further testing.
Once the mud samples were prepared, they were tested in Low pressure low temperature (LPLT) filtration at 100 psi for a standard period of 30 min. The permeability of each sample was calculated using the Darcy law and methodology was presented in Fattah [40]. Furthermore, these mud samples were kept at ambient condition for 24 h, in order to observe the stability of each sample. Some of the shale cuttings were directly added into these mud systems and were recovered after 24 h. This test was conducted with the intention of determining the recovery of the samples. Moreover, some of the shale samples were transmuted into uniform size pellets. These tablets were added into the mud system for a period of 24 h in order to observe the surface modification. Contact angle on these coated samples after 24 and 48 h, respectively, were determined so that the nature of the change in surface morphology can be observed for these polymeric systems. In addition, Ghazij shale and these polymeric systems were also experimentally investigated in linear dynamic swell-meter (LDSM) with hydraulic compactor (Model 150-80-230 V). The testing continued until equilibrium state was achieved, and no further swelling was observed. The standard time for this test is 18 h; however, during this experimental analysis, the testing prolong until stability was achieved.
3 Results and Discussion
3.1 Low Pressure Low Temperature (LPLT) Filter Press
The impact of polymers on fluid loss volume is shown in Fig. 7. The test was performed by putting the mud samples in the API Filter Press. Each mud was tested for standard thirty minutes against a differential pressure of 100psi with values being noted after every five minutes. During the initial period, all samples demonstrated high filtrate loss volume owing to the lack of formation of filter cake [41]. As the experiment continued for 30 min, the filtrate volume decreased by deposition of solid particles as filter cake [41].The drilling mud with Sodium Alginate showed the least filtrate loss with just 5.6 mL lost at the end of the duration. This was followed by Pure Bore 6.2 mL, PHPA 7.5 mL, and finally PAC-L 11.3 mL. The highest filtrate loss of volume was shown by non-polymer system with a filtrate loss of 13.7 mL. Sodium alginate comprises of profuse polar bonds in the form of carboxyl and hydroxyl. These bonds entangled with the hydrogen bonds of xanthan gum, which eventually increases the viscosity of the suspension and decreases the filtrate loss volume [42]. PAC-L showed the highest filtrate loss volume out of all the polymeric systems. PAC-L is particularly sensitive to the presence of monovalent cations (K +) [43]. Potassium ions induced the dehydration of PAC-L system which eventually decreased the viscosity of the fluid and increases the fluid loss [43].
3.2 Mud Cake Texture and Shape
Figure 8 shows the slickness and texture of the mud cake formed during the API filter press test. Based on the physical investigation of the mud presented in Fig. 8, it was observed that the mud cake formed by sodium alginate and Pure-bore demonstrates slickness and smooth texture, an important characteristic required in the wellbore. The smooth surfaces formed by these polymeric materials indicate that all the pores spacing present in the filter paper were properly sealed and no aggregation and accretion were observed for these polymers. These polymers efficiently preserve their polymeric chain structures, thus forming a compacted and impenetrable filter cake, which considerably reduces the filtration loss [42].
The filter cake texture prepared by PAC-L and PHPA is rough and irregular. This clearly indicates higher friction and presence of drag forces for these systems in the wellbore [44]. The system with no polymer shows a thick mud cake, this clearly specifies the potential pipe sticking problem with this mud type.
3.3 Permeability and Mud Cake Thickness
The measurement of permeability of filter cake formed during the API LPLT filter press test followed the principle of Darcy’s law [40]. A relationship is formulated between the accumulative loss of filtrate volume collected during the experiment and time [45]. The method is primarily based on measuring the thickness of the mud cake ε (mm), \({Q}_{f}\) the fluid loss volume in mL. μ is the viscosity of filtrate loss volume in cP. Equation (1) [44], based on the principle of Darcy’s Law used to calculate the permeability of each mud cake formed during the experimentation.
Figure 9a, b shows the mud cake thickness and permeability of all the polymeric systems of the study. Among all polymeric systems, PAC-L showed the highest mud cake thickness as shown in Fig. 9a. This clearly justified the higher filter loss volume in Fig. 7. PAC-L is particularly sensitive to the presence of monovalent cations (K+) [43]. Potassium ions induce the dehydration of PAC-L system which eventually decreases the viscosity of the fluid and increases the fluid loss [43]. The least mud cake thickness was formed by sodium alginate. The wide-ranging size distribution of sodium alginate and xanthan gum suspension interconnects with other another and results in the formation of compacted and thin filter cake.
Figure 9b shows the permeability of the filtration cake obtained for each sample using a ruler. The results clearly demonstrate a significant decrease in permeability as the polymers are added in the mud systems. The permeability of all the systems followed a similar trend as it was in filtrate loss volume \(K_{{\text{PAC - L}}} > K_{{{\text{PHPA}}}} > K_{{{\text{Pure}} - {\text{bore}}}} > K_{{\text{Sodium Alginate}}}\). Sodium alginate shows the least mud cake thickness and lower permeability than any of the other polymeric materials. Almost five times reduction in permeability was observed in all the samples.
3.4 Potential of Hydrogen (pH) for All Systems
The pH for all the polymeric systems was measured using the Plastic Hydrion vivid pH test paper. Potential of hydrogen (pH) is the most essential parameter responsible for influencing the characteristics of the mud and its performances [13]. From the experimentation, it was observed that the pH for all the systems was in alkaline range. Each system demonstrated a pH in range of 9–10 on the pH scale. The alkalinity of the mud was greatly affected in pure-bore and sodium alginate systems. The pH paper color was darker comparatively to other systems. These systems comprise of hydroxyl group that are responsible for the strong alkaline nature.
3.5 Stability Test for all Polymeric Mud Systems
The stability of the drilling fluid is another crucial phenomenon that evaluates the efficacy of drilling fluid while drilling at different conditions [46]. All the mud samples were kept under normal room temperature and pressure condition for the purpose of investigating the stability of the mud. Figure 10 shows the stability of all the samples after 24 h. It was observed that after 24 h only pure-bore mud sample demonstrated stability in terms of not dephasing into two distinct entities. This polymer in aqueous medium tends to form supramolecular 3-D network of spiral structure components, which are linked through Van der Waal and hydrogen bonding [47]. Moreover, this particle also comprises of carboxylate groups in its structure, which is responsible for the stability of this mud system.
All the remaining mud systems degraded in their stability after 24 h. PAC-L is an anionic polymer [43], the presence of potassium ion in the base mud system dehydrated PAC-L, which eventually broke the mud system into two separate phases, therefore, the stability of this mud decreased substantially. Sodium alginate also demonstrated poor stability performance after 24 h. The exchange reactions clotting of mud occurred breaking the mud into two discrete units. The hemostasis or the denser part settled at the bottom, while the liquid phase having lower density remained on the top [48]. On the other hand, the PHPA mud did not split into two phases; rather it formed a gel type structure. The small fine gaps in PHPA beaker as visible in Fig. 10 shows the degradation of the fluid with time.
3.6 Disintegration of Shale Drill Cutting
Figure 11 shows the drill cutting recovery test conducted for each of the four polymeric systems. Twenty samples of shale were added in each drilling fluid system as shown in Table 1 and were kept submerged for 24 h. The recovery of the shale samples after 24 h depends on the reaction between the fluid and the shale. Small size drill cutting was preferred for this particular test as these would establish closer contact with drilling fluid and the additive present [43]. All cuttings were recovered from the pure-bore samples, and the recovery was 100% for this mud system. The percentage dropped to 95% for both sodium alginate and PHPA, while it drastically reduced to 75% for PAC-L.
The test clearly demonstrated the sensitivity of Ghazij shale to PAC-L drilling fluid sample. 25% of drill cutting samples disintegrated into the PAC-L mud. The clay particles in this mud system are hydrated; therefore, they easily break down in smaller fragments. Moreover, the presence of potassium ion in the mud system induces the dehydration mechanism of PAC-L, which ultimately reduces its performance [43]. On the other hand, the pH for PHPA and sodium alginate drilling fluid systems was in alkaline range, hence, when shale samples were placed in them, these polymers adsorbed its negatively charge surface of the shale. This mechanism prevented the decomposition of the shale cutting and it resulted in higher shale cutting recovery. Therefore, it can be concluded that bio-polymers are effective in preventing shale disintegration.
3.7 Shale Pellet Immersion Test
In this test, Ghazij shale cuttings were first dried in oven for a period of two hours in order to remove any moisture content associated with them. Next, those dried cuttings were placed in mortar grinder to form shale cutting powder. 15 g of powder shale was placed in the cell of hydraulic compactor at 8000 psi for a period of 30 min as shown in Fig. 12 (1–2). A cylindrical form of shale pellet was formed as shown in Fig. 12 (3), which was used for further testing.
Four pellets of Ghazij shale samples were prepared and each was placed in a separate polymeric mud system. Figure 13a, b shows the top and side view of the pellets that were recovered after 24 h from the drilling mud systems. No substantial changes were observed in any of the system when the samples were observed from the top. Slight roughness at the boundaries of all the samples was detected, whereas none of the noticeably erosion effect was spotted. However, cracks at the middle of the samples and minimum erosion effect were noticed after the interaction of samples with drilling fluid.
3.8 Contact Angle Determination
The instability of any shale sample is directly related to the physicochemical characteristic of drilling fluid and the shale [49]. The alteration in wettability of shale sample from the hydrophilic nature (water-loving) to hydrophobic nature (water-repellent) is counted as a smart practice [49]. This approach provides the desired stability to any shale formation. Figure 14 shows the variation in the wetting characteristics of water in terms of contact angle when in contact with different surfaces [50]. If the contact angle θ > 90, then the surface shows negligible wetting behavior. The surface affinity to water starts to increase as θ decreases [50]. These surfaces are called hydrophilic surfaces.
Contact angle effect on the physiochemical characteristic [50]
Figure 15a–h shows the contact angles on the surfaces of Ghazij shale formation by four polymeric mud samples after 24 and 48 h, respectively, obtained using ImageJ software. Figure 15a and b shows CA measurement on shale sample coated with PAC-L drilling fluid after 24 and 48 h, respectively. In both the cases, the θ = 0° clearly indicating toward the strong wetting characteristics of shale sample after its contact with PAC-L. When CA for PHPA coated sample was recorded as shown in Fig. 15c and d, a slight increase in wetting characteristics was observed at 48 h. A decrease in CA was noted from θ = 48.629° to θ = 45.427° after 48 h. On the other hand, biopolymers (sodium alginate and pure-bore) both exhibit some hydrophobic nature after 48 h. An increase in CA was recorded in both the polymeric systems at 48 h, as observed from Fig. 15e till Fig. 15h. Table 2 also shows the uncertainty associated with the placement of angle in the ImageJ software. This uncertainty arises as result of placement of angle on to the water droplet. The table reports uncertainties at both 24 and 48 h of experimentation. This modification from hydrophilic nature to hydrophobic nature can be reflected as a pivotal approach in obtaining shale stability.
Figure 16 demonstrates the possible mechanism of biopolymers to inhibit shale swelling. These biopolymers strongly attached to the surface of the shale and encapsulating the shale nanoplatelet [51, 52]
3.9 Swelling Analysis of Polymeric Mud Systems
The influence of each polymeric material on the swelling rate of Ghazij shale was recorded using linear dynamic swell-meter. Figure 17 shows that it demonstrates maximum swelling rate in presence of distilled water. While comparing the swelling rates of polymeric additives, it was observed that PAC-L system displays the highest swelling rate after 24 h of testing. The dehydration of polymeric additive in the presence of potassium ion was the main reason for its failure [43]. Almost similar swelling characteristics were observed with PHPA and biopolymers systems. These systems adhere to the negative surface of shale sample and form a gel like structure, which prevents the migration of hydrogen ion from the water into the shale sample [52, 53]. This mechanism eventually assists in decreasing the swelling behavior of Ghazij shale.
4 Conclusion
In the current study, a complete set of experimentations was carried out to investigate the filtration loss and wellbore stability in Ghazij shale using four different polymeric mud systems. Two out of the four systems are classified as biopolymers (sodium alginate and pure-bore), one is categorized under the umbrella of modified polymers (PAC-L), and one is a synthetic polymer (PHPA).
Based on the results of the study, following conclusions were drawn:
-
1.
All the polymeric mud systems demonstrate alkaline behavior with pH > 8.
-
2.
During the LPLT API filter press test, it was observed that the loss of filtrate volume was lower in biopolymers. These polymers entangled with the hydrogen bonding, present in xanthan gum, eventually increased the viscosity of the fluid and helped in reducing the filtrate loss. The existence of K + ions in the base mud system, however, dehydrated the PAC-L system, which ultimately reduced its performance.
-
3.
The physical appearances of the filter cake formed by the biopolymers mud systems were thin, smooth and tough in nature.
-
4.
The immersion test showed 100% and 95% cutting recovery after 24 h from the two biopolymers mud, respectively. These muds encapsulated the drill cutting resulting in prevention of drill cutting from crumbling into finer fragments.
-
5.
No significant texture and shape changes were observed in any of the shale pellet boundaries placed in the mud system for 24 h.
-
6.
The biopolymers increased the hydrophobicity in Ghazij shale after 48 h which prominently changed its stability.
-
7.
The linear dynamic swell meter results showed a significant decrease in the swelling behavior of Ghazij shale after its contact with biopolymers. Almost threefold reduction in swelling was observed in bio-polymeric mud systems.
Abbreviations
- NPT:
-
Non-productive time
- PAC:
-
Polyanionic cellulose
- WBDF:
-
Water-based drilling fluids
- OBDF:
-
Oil-based drilling fluid
- PHPA:
-
Partially hydrolyzed poly acrylamide
- CMC:
-
Carboxymethyl cellulose
- CEC:
-
Cation exchange capacity
- SEM:
-
Scanning electron microscope
- θ :
-
Contact angle
- LPLT:
-
Low pressure low temperature
- API:
-
American petroleum institute
- pH:
-
Potential of hydrogen
- K:
-
Darcy
- K+ :
-
Potassium ion
- LDSM:
-
Linear dynamic swell meter
- CA:
-
Contact angle
References
Apaleke, A.; Al-Majed, A.; Hossain, M.E.: Drilling Fluid: State of The Art and Future Trend (2012)
Hossain, M.; Al-Majed, A.: Fundamentals of Sustainable Drilling Engineering, pp. 1–754 (2015)
Caenn, R., et al.: Composition and Properties of Drilling and Completion Fluids (2011)
Leusheva, E.; Morenov, V.; Moradi, S.: Effect of carbonate additives on dynamic filtration index of drilling mud. Int. J. Eng. Trans. B 33, 934–939 (2020)
Elkatatny, S.K.M.S.; Alakbari, F.; Mahmoud, M.: Optimizing the rheological properties of water-based drilling fluid using clays and nanoparticles for drilling horizontal and multi-lateral wells. Appl. Rheol. 28(4), 43606 (2018)
Lalji, S.M.; Ali, S.I.; Ahmed, R., et al.: Influence of graphene oxide on salt-polymer mud rheology and Pakistan shale swelling inhibition behavior. Arab. J. Geosci. 15, 612 (2022)
Díaz-Pérez, A., et al.: Roegiers, The role of water/clay interaction in the shale characterization. J. Petrol. Sci. Eng. 58(1–2), 83–98 (2007)
Mobeen Murtaza, M.S.K., and Mohamed Mahmoud, Application of a Novel and Sustainable Silicate Solution as an Alternative to Sodium Silicate for Clay Swelling Inhibition. ACS Omega, 2020. 5(28): p. 17405–17415.
She, H., et al.: Determination of the hydration damage instability period in a shale borehole wall and its application to a fuling shale gas reservoir in China. Geofluids 2019, 3016563 (2019)
Lalji, S.M.; Ali, S.I.; Ahmed, R., et al.: Comparative performance analysis of different swelling kinetic models for the evaluation of shale swelling. J Petrol. Explor. Prod. Technol. 12, 1237–1249 (2022)
Abduo, M.I., et al.: Elhossieny, comparative study of using water-based mud containing multiwall carbon nanotubes versus oil-based mud in HPHT fields. Egypt. J. Petrol. 25(4), 459–464 (2016)
Joel, O.F.D., et al.: Effect of KCL on rheological properties of shale contaminated water-based mud (WBM). Global J. Res. Eng. Chem. Eng 12(1), 11–18 (2012)
Lalji, S.M.; Ali, S.I.; Haneef, J., et al.: Changes in Pakistan crude oil properties contaminated by water-based drilling fluids with varying KCL concentrations. Chem. Pap 76, 4189–4201 (2022)
Adnan Aftab, M.A., et al.: Environmental friendliness and high performance of multifunctional tween 80/ZnO-nanoparticles-added water-based drilling fluid: an experimental approach. ACS Sustain. Chem. Eng. 8(30), 11224–11243 (2020)
Lalji, S.M.; Ali, S.I.; Sohail, H., et al.: Combine effect of graphene oxide, pure-bore and sodium alginate on rheological and filtration properties and cutting carrying capacity of water-based drilling fluid. Chem. Pap 76, 6461–6473 (2022)
Khan, R.A., et al.: Ionic liquids as clay stabilizer additive in fracturing fluid. Fuel 334(1), 126154 (2023)
Al-Ansari, A.P.C.; Abahussain, A.; Abuhamed, A.M.; Pino, R.; El Bialy, M.; Mohamed, H.; Lopez, C, Reservoir drill-in fluid minimizes fluid invasion and mitigates differential stuck pipe with improved production test results. In: SPE Middle East Oil Gas Show Conference (2017)
Mobeen Murtaza, H.M.A.; Zhou, X.; Al-Shehri, D.; Mahmoud, M.; Kamal, M.S.: Okra mucilage as environment friendly and non-toxic shale swelling inhibitor in water based drilling fluids. Fuel 320, 123868 (2022)
Lalji, S.M.; Khan, M.A.; Haneef, J., et al.: Nano-particles adapted drilling fluids for the swelling inhibition for the Northern region clay formation of Pakistan. Appl. Nanosci. 13, 503 (2021)
Aftab, A., et al.: Nanoparticles based drilling muds a solution to drill elevated temperature wells: a review. Renew. Sustain. Energy Rev. 76, 1301–1313 (2017)
Chang, W.-Z., et al.: Ageing and collapse of bentonite gels—effects of Li, Na, K and Cs ions. Rheol. Acta. 53, 109–122 (2014)
Wajheeuddin, M.H., et al.: Development of an environmentally-friendly water-based mud system using natural materials. Arab. J. Sci. Eng. 43(5), 2501–2513 (2018)
Aftab, A., et al.: Influence of tailor-made TiO2/API bentonite nanocomposite on drilling mud performance: towards enhanced drilling operations. Appl. Clay Sci. 199, 105862 (2020)
Abdo, J.; Zaier, R.; Hassan, E., et al.: ZnO–clay nanocomposites for enhance drilling at HTHP conditions. Surf. Interface Anal. 10–11, 970–974 (2014)
Ahmad, H.M., et al.: Influence of hydrophobically modified polymer and titania nanoparticles on shale hydration and swelling properties. Energy Fuels 34(12), 16456–16468 (2020)
Xianyu Yang, Z.S., et al.: Environmental-friendly salt water mud with nano-SiO2 in horizontal drilling for shale gas. J. Petrol. Sci. Eng. 156, 408–418 (2017)
Mohanty, U.S., et al.: Physicochemical characterization of zirconia nanoparticle-based sodium alginate polymer suspension for enhanced oil recovery. Energy Fuels 35(23), 19389–19398 (2021)
Tamilisai, R.; Palanisamy, P.N.; Selvasekarapandian, S., et al.: Sodium alginate incorporated with magnesium nitrate as a novel solid biopolymer electrolyte for magnesiumion batteries. J. Mater. Sci. Mater. Electron. 32, 22270–22285 (2021)
Ryen Caenn, G.V.C.: Drilling fluids: state of the art. J. Petrol. Sci. Eng. 14(3–4), 221–230 (1996)
Lam, C.; Martin, P.J.; Jefferis, S.A.: Rheological properties of PHPA polymer support fluids. J. Mater. Civ. Eng. 27(11), 04015021 (2015)
Siqueira, G.; Bras, J.; Dufresne, A.: Cellulosic bionanocomposites: a review of preparation, properties, and applications. Polymers 2(4), 728–765 (2010)
Waheed, A.; Wells, N.A.: Changes in paleocurrents during the development of an obliquely convergent plate boundary (Sulaiman fold-belt, southwestern Himalayas, west-central Pakistan). Sed. Geol. 67(3), 237–261 (1990)
Khan, I.H.; Clyde, W.C.: Lower paleogene tectonostratigraphy of Balochistan: evidence for time-transgressive late paleocene-early eocene uplift. Geosciences 3(3), 466–501 (2013)
Amjad, M.R., et al.: Exploring the untapped gas potential of Ghazij shale in Pirkoh area, Pakistan: integrated approach of Attribute analysis and maturity modeling. Turk. J. Earth Sci. 31, 1–19 (2022)
Xiangjun Liu, W.Z., et al.: Experimental study on hydration damage mechanism of shale from the longmaxi formation in southern Sichuan Basin, China. Petroleum 2(1), 54–60 (2016)
Alsaba, M.; Al Marshad, A.; Abbas, A., et al.: Laboratory evaluation to assess the effectiveness of inhibitive nano-water-based drilling fluids for Zubair shale formation. J. Petrol. Explor. Prod. Technol. 10, 419–428 (2020)
Siddiqui, M.A.Q.; Chen, X.; Iglauer, S.; Roshan, H.: A multiscale study on shale wettability: spontaneous imbibition versus contact angle. Water Resour. Res. 55, 5012–5032 (2019)
Khan, I.H.: Paleoenvironmental and tectonic reconstruction of the lower Eocene Ghazij Formation and related units, Balochistan, Pakistan: Implication for India -Asia collision. University of New Hampshire, Durham (2006)
Khan, I.H.: Paleoenvironmental and tectonic reconstruction of the lower Eocene Ghazij Formation and related units, Balochistan, Pakistan: Implication for India -Asia collision, p. 221. University of New Hampshire, Durham (2006)
Fattah, K.A.: Investigation of mud density and weighting materials effect on drilling fluid filter cake properties and formation damage. J. Afr. Earth Sci. 117, 345–357 (2016)
Hanyi Zhong, X.G.: Minimizing the filtration loss of water-based drilling fluid with sustainable basil seed powder. Petroleum 8(1), 39–52 (2022)
Wei, Z., et al.: Sodium alginate as an eco-friendly rheology modifier and salt-tolerant fluid loss additive in water-based drilling fluids. RSC Adv. 12(46), 29852–29864 (2022)
Mohamed Khodja, J.P.C.; Bergaya, F.; Fourar, K.; Khodja, M.; Cohaut, N.; Benmounah, A.: Shale problems and water-based drilling fluid optimisation in the Hassi Messaoud Algerian oil field. Appl. Clay Sci. 49(4), 383–393 (2010)
Agwu, O.E., et al.: Rice husk and saw dust as filter loss control agents for water-based muds. Heliyon 5, 7 (2019)
Soares, Y.C.F., et al.: Influence of oxidation degree of graphene oxide on the shear rheology of poly(ethylene glycol) suspensions. Fluids 5(2), 41 (2020)
Xia, S., et al.: Application of polysaccharide biopolymer in petroleum recovery. Polymers 12(9), 1860 (2020)
Ismail, A.R.; Mohd, N.M.N.A.; Basir, N.F., et al.: Improvement of rheological and filtration characteristics of water-based drilling fluids using naturally derived henna leaf and hibiscus leaf extracts. J. Petrol. Explor. Prod. Technol. 10, 3541–3556 (2020)
Lee, K.Y.: Alginate: properties and biomedical applications. Prog. Polym. Sci. 37(1), 106–126 (2012)
Moslemizadeh, A.S.R.S.: A natural dye in water-based drilling fluids: swelling inhibitive characteristic and side effects. Petroleum 3, 355–366 (2016)
Gurram Giridhar, R.K.N.R.; Manepalli, G.A.: Contact angle measurement techniques for nanomaterials. In: Thermal and Rheological Measurement Techniques for Nanomaterials Characterization, pp. 173–195. Elsevier (2017). https://doi.org/10.1016/B978-0-323-46139-9.00008-6
Xinliang Li, G.J., et al.: Poly-L-arginine as a High-performance and biodegradable shale inhibitor in water-based drilling fluids for stabilizing wellbore. ACS Sustain. Chem. Eng. 8(4), 1899–1907 (2020)
Saleh, T.A.: Surface-modified biopolymer as an environment-friendly shale inhibitor and swelling control agent. J. Mol. Liq. 342, 117275 (2021)
Ahmed, H.M., et al.: Polymeric and low molecular weight shale inhibitors: a review. Fuel 251, 187–217 (2019)
Acknowledgements
The authors would like to thank Almas Ashraf, Assistant Professor in the Department of Humanities, NED University of Engineering and Technology, for improving the quality of the manuscript. Moreover, the authors are also grateful to Ms. Anum Imran and Ms. Shahzana Zulfiqar for helping in software parts.
Funding
There is no funding associated with this work.
Author information
Authors and Affiliations
Contributions
SM Conceptualization; Formal analysis; Investigation; Methodology; Resources; Visualization; Roles/Writing -original draft; Writing—review and editing; Testing; Supervisor; Software. SIA Conceptualization; Methodology; Formal analysis; Investigation; Resources; Writing—original draft; Testing; Supervisor; Software. MA Testing; Investigation; Methodology; Formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors that are part of this study, the corresponding author states that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lalji, S.M., Ali, S.I. & Asad, M. Experimental Effect of Biopolymers, Synthetic and Modified Polymers on Western Pakistan Shale (GHAZIJ) Stability. Arab J Sci Eng 48, 16639–16653 (2023). https://doi.org/10.1007/s13369-023-07737-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-07737-5