Abstract
Quantitative and qualitative analyze of toxic and nutritional elements existing in different kinds of black tea (Camellia Sinensis) leaves available in Pakistan was conducted with calibration free/laser-induced breakdown spectroscopy (CF/LIBS). A Q-switched Nd:YAG laser employed to create micro-plasma on the tea sample surfaces. The quantitative analysis accomplished by studying the spectral emissions of plasma using a dedicated spectrometer. Iron, magnesium, sodium, potassium, calcium, aluminum, and zinc were measured as essential nutrients, while chromium, copper, manganese, nickel, and lead as toxic metals. In order to get precise and reliable analysis, the LIBS setup was optimized by performing various parametric dependence studies. The Boltzmann plot method was adopted for calculation the plasma temperature its average values are between ~ 7500 and 9000 K for all kinds of tea leaves. To find out the electron number density (Ne), the Stark broadened line of atmospheric hydrogen pea (Hα) was chosen and was found between 1.13 × 1017 cm−3 and 1.61 × 1017 cm−3. The laser-generated micro-plasma was optically thin, and the effect of self-absorption in spectral emission lines was found negligible. The LIBS results were verified by means of inductively coupled plasma/atomic emission spectroscopy (ICP/AES). The findings obtained using LIBS and ICP techniques displayed an excellent agreement which exhibit that LIBS is very good to for spectro-chemical analysis of food products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tea (Camellia Sinensis), a fragrant drink usually ready via pouring hot water upon the cured grass of the tea leaf, an evergreen bush present in most of Asian countries [1]. Tea leaves contains several essentials which are highly beneficial to human health in addition to other nonessential heavy metals [2]. Since Camellia Sinensis is the most common beverage consumed worldwide, therefore its qualitative and quantitative contents have great impact significance for the human health. Particularly the consumption of tea has also some healthy benefits to humans like prevention from various diseases like cardiovascular disease, cancer, and decrease of cholesterol level in blood [3,4,5]. Based on such perception, chemical content of tea leaves could be categorized into requisite and unessential or toxic elements. The excess of tea containing nonessential toxic metals are of great concern due to their resultant hazardous health related issues. Furthermore, the previously carried out investigations predict that whatever is consumed as food might cause the disturbance in metabolism system if its nutrients levels do not lie within the permissible range. Excess and insufficiency of metals (like Zn and Fe) may cause severe health consequences [6]. Several factors, like tea processing methodology, place of origin and the storage means have effect on the worth of tea leaves that prompts uneven costs between them. Thus, some lawbreakers utilize the unique tea instead of an original tea leaves or take substandards tea leaf in place of the first-class tea leaves to attain better financial profits. Thus, a quick, precise, and trustworthy method are needed to ensure the tea quality sold in the market. The standard techniques employed for food quality testing among inductively coupled plasma/ mass spectrometry (ICP/MS), inductively coupled plasma/optical emission spectrometry (ICP/OES), atomic absorption spectrophotometry, and neutron activation analysis and; yet, such approaches have an intrinsic limitations particularly in their specimen preparations that demands prolonged and laborious pre-treatment strategies and utilization of acid digestions under elevated temperatures, as well as the utilization and dumping of environmental harmful chemicals [7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Among the existing elemental analytical procedures, LIBS provides attractively characteristics, like in situ multi-elemental disclosure, quick responding for online study, no or extremely little sample treatment necessities, and elevated spatial resolving power to map the sample surface.
In this experiment, (CF-LIBS) technique was utilized effectively to compute contents of essentials and toxins which exist in mainly general utilized black tea leaves available in Pakistan. Five diverse varieties of black tea leaves (local as well as branded) were considered for small, heavy, and abundant nutritional species. The understanding achieved during the current work established that LIBS is an influential technique and can be employed easily to test the quality of food products.
2 Methods and Materials
2.1 LIBS Experimental Setting
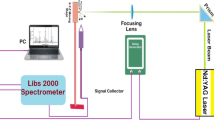
The Setting arrangement in this study is depicted in Fig. 1. In short, it comprises of a laser, a specimen controller, and a high resolving power spectrometer (LIBS-2000 + , Ocean Optics, USA) together with a detection system. A Q-switched Nd-YAG laser @ 532 nm utilized as origin of excitation with a pulse duration of 5 ns and repetition rate (10 Hz). The energy of incident laser pulse was adjusted using the built-in electronics (OOILIBS + , Ocean Optics) and measures via a calibrated energy meter. To prevent the absorption of laser photons by the LIBS generated plasma plume at highest pulsed energy and achieving the best signal-to-noise ratio of (S-to-N), the ideal pulse energy of 100 mJ/pulse was selected. A focusing lens of focal length 10cm was used to focus the pulsed incoming laser beam on the surface of test tea sample. The plasma emissions were acquired using a tiny lens coupled with an optical fiber positioned perpendicularly (90 0) to the plasma plume and transferred to LIBS spectrometer using a 10 µm slit width.
The LIBS2000 + detector was outfitted with five HR(2000) of high-resolving power tiny spectrometers all with 2048 element linear charge-coupled device (CCD) array having an optical resolution (~ 0.06 nm) that covered the spectral range ~ 200–700 nm. The time span connecting the laser pulse and the aperture of detector was adjusted and set to ~ 3 µs. To get best S/N ratio, all the spectra were recorded taking the average of 20 such spectra with similar experimental conditions. The electronic noise was subtracted from the considered spectra.
2.2 Samples Preparation for LIBS Study
In the current study, five commonly consumed black tea leaves of various qualities (covering from low-priced to costly) were collected from food stores located in Rawalpindi city of Pakistan. The collected samples were analyzed for heavy metals and other essentials using LIBS setup. For ethical reasons the product’s names of samples are kept secrets and as an alternative, the specimens are marked as S1 (local open market), S2 (international brand), S3 (international brand), S4 (international brand), and S5 (local branded of good quality). For LIBS analysis, the samples were first fine grounded using agate mortar for certain time duration to get fine and uniform powder and then pelletized via putting ~ 1.2-gm of each powdered specimen in a cylindrical shaped dye and pressurized under a load of ten tons for ½ hour time duration. Luckily, the pallets were pretty compact that was adequate to withstand the power of laser beam with no addition of any binder. The pelletized samples were then mounted on a motorized sample holder for laser ablation.
2.3 Samples Preparation for ICP-AES Analysis
To analyze the tea specimens using ICP-spectroscopy, about 0.01 gm of grounded powder of every tea samples weighted using an analytical balance were treated with 5 milliliter of nitric acid (HNO3) and warmed at ~ 60 °C for proper metallic dissolution in acid. The solution was placed for cooling down and reheated the solution after mixing 40 milliliter of deionized (D-I) water to ~ 45 °C for 2 h. After cooling the solution, the non dissolvable remaining fragments were detached and then shacked the solution thoroughly. The solution form was then tested using ICP-AES spectrometer (Optima 2100-DV; Dual-view, PerkinElmer).
3 Results
The laser-produced plasma of various black tea leaves samples was generated by focusing 100 mJ pulse−1 laser beam of a pulsed Nd-YAG laser upon the tea pallets. The representative discharge spectrum of target#1 is depicted in Fig. 2 that covered the spectral section from 230 to 700 nm.
The emission spectrum contained diverse intense and weak transition peaks of the elements which exist in the Tea specimen. In general, the intense emission peaks affiliated to main species, whereas the weak peaks are due to minor elements. The greater part of spectral peaks lies of the plasma spectra beside with the atmospheric hydrogen line (Hα) at 656.26nm. The other atmospheric peaks were not detected in our spectra as majority of them lie in the NIR region of the spectra which is beyond the spectral range of our present spectrometer. From the LIBS spectrum, the typical emission lines of heavy metals such as Cr, Cu, Mn, Ni, and Pb beside the other species such as Ca, Fe, K, Mg, Na, Si, Zn, and Al were identified.
3.1 Selection of experimental parameters
To execute reliable LIBS by means of the calibration-free method various experimental parameters like laser energy, acquirement of gate span and the effect of space between lens and test sample upon the signal intensity were idealized.
In case of LIBS, the time span between the laser ignition and spectra recording is important as it plays a significant part in generating precise neutral transition peaks with minimum plasma expansion. At the premature state of plasma creation, the continuum discharges are predominated and weak spectral peaks of minor metals could not be recognized. To reduce the impacts due to continuum and to obtain enhanced signal-to-noise ratio (S/N) ratio, the data at different gate delays were recorded by in the 0.1 to 3.5 μs. The maximum signal development was obtained at gate delay of 3.0 μs.
The pulsed laser beam energy is a critical parameter to be idealized in the LIBS technique. The consequence of laser pulse energy on the spectral peaks intensity of Ca at 422.67 nm was analyzed. It was observed that the intensity of LIBS signal enhances in a linear manner versus the laser pulse energy in the studied range (20 to 100) mJ/pulse. Though, by elevated focusing laser energy to catch additional LIBS intensity will yield optically thick plasma having vigorous background continuum, thereby making the study more complex. The energy of laser pulse beam at 100 mJ-pulse−1 was selected for the current study. The beam energy of 100 mJ-pulse−1 was found adequate to get the requisite LIBS technique and was utilized during the experimentation.
Similarly; the effect of space between lens and specimen (SLS) on peak strength was also idealized for precise and reproducible LIBS spectra. The space between convex lens and specimen was idealized by investigated the impact on the characteristics of spectra. The specimens positioned at the focal point of the convex lens, the acquired signal magnitude of the test elements was fluctuating forcefully, signifying an unstable plasma generation. The finest positioning was detected to be when the sample was positioned at a distance less than the focal length of the lens (d˂f), i.e., distance of lens to specimen (DLTS) at 9 cm, the discharge peaks attained then very stable peak heights. The electron temperature and the density of electron were calculated at this positioning and tested for LTE conditions. To correct the spectral responding, the spectrometer was improved by means of calibrating intensity against typical intensity calibrated lamp employing the typical procedure. The normal Hg-Ar lamp was utilized for the calibration of wavelength. The discharge spectra of tea leaves samples are acquired for the spectral analysis at above-mentioned selected specifications.
3.2 Plasma parameters and their determinations
To verify the LTE state of laser created plasma, the information regarding the electron temperature (Te) and electron density (Ne) are important. For the determination of Te, the scheme of Boltzmann plot is employed frequently which is fundamentally a linear shape of Boltzmann distribution whereas the Ne is estimated by the Stark broadening profile of an isolated emission peak of a specific specie. The selection of emission peaks for the approximation of electron temperature is incredibly important. The selected peaks must be optically thin, with no self-absorption and the plasma plume has to also satisfy the condition of local thermodynamic equilibrium (LTE). The Boltzmann relation, the integrated peak intensity of an emission peak is given as [12, 13] ,
whereas F is known as experimental factor, \({C}_{n}^{z}\) provides the number density of neutral elements, Aki called the transitional probability, gk gives the degeneracy aspect of the higher level k, Ek provides the energy of higher level, k stands for the Boltzmann constant, Te provides the excitation temperature, and \({U}_{s}({T}_{e})\) stands for partition function. We can restate the above relation as,
A straight line was acquired by plotting \(\mathrm{ln}\left(\frac{{I}^{\mathrm{ki}}{\lambda }^{\mathrm{ki}}}{{\mathrm{hc}A}_{\mathrm{k }}{g}_{\mathrm{k}}}\right)\) versus Ek of the observed emission peaks thus providing a slope (− 1∕kT) with an intercept \({q}_{s}=\mathrm{ln}\frac{{FC}_{n}^{z}}{{U}_{s}({T}_{e})}\). Then the plasma temperature can be determined using the slope obtained from straight-line relation while the element’s abundance is associated to the intercept [14,15,16]. Since the amount of iron peaks was present in all the emission spectra therefore well-isolated and optically thin peaks of neutral and ionized iron were utilized to evaluation the temperature of electron. The spectral data of the chosen peaks were obtained from atomic National Institute of Standards and Technology (NIST) data base as existing in Table 1.
Figure 3 shows a typical Boltzmann plot of the S1 employing Fe-I peaks. From the slope of the Boltzmann plot, the electron temperature was deduced to be ~ 7500 K. Correspondingly, we also measured Te using Fe-II lines providing an outcomes of ~ 7200 K. The measured electron temperature contained an uncertainty of less than ± 10-% error, which in general owing to uncertainty in transition probabilities and in measuring the intensities of spectral peaks [15,16,17,18,19,20].
Generally the electron density (Ne) is estimated through the measuring the Stark broadening of efficiently resolved and isolated peak from the LIBS discharge spectra. We selected the Stark broadened peak shape of Hα peak at 656.28 nm to compute the full width at half Maximum (FWHM) by the Lorentzian fitting of the investigated data as presented in Fig. 4. To estimate the electron density, we employed the FWHM of hydrogen Hα spectral line at wavelength of ~ 656.28 nm as,
The estimated electron density was found = 1.13 × 1017 cm−3.
In similar way, we estimated the electron densities for different chosen types of tea leaves samples in the range (1.13 –1.62) × 1017 cm−3.
Furthermore, we also measured Ne from the Stark broadened of an lonely Fe-I at 538.3 nm via the Lorentzian fitting to the experimental facts point as below[16],
where \({\mathrm{\Delta \lambda }}_{1/2}^{\mathrm{s}}\) stands for the Lorentzian FWHM of the detected spectral peak, ωs stands for the Stark broadening variable and to some degree is a function of wavelength and temperature[17]. Also, Nr is called reference electron density [13, 18, 20].
The estimated Ne was found as 1.4 × 1017 cm−3. Remarkably, both the number densities estimated (via Hα line and Fe line) were in good conformity. One of the critical situations to be fulfilled by the Boltzmann plot method is that the plasma has to follow LTE criteria.
Further, to verify LTE, the minimum electron density to be achieved by the McWhirter criteria [13, 19, 20] as,
where Te (eV) stands for plasma temperature, ΔE in (eV) is the net energy difference between the upper and lower states being involved in the transition. In the current case, Hα peak was utilized to define the electron number density. By putting the value ΔE (eV) for H-alpha peak (1.889 eV) and calculated plasma temperature (eV), gives Ne to be ~7.52×1014 cm-3. The Ne was greatly larger than the limit thus imply the justification of LTE criteria.
While estimating the plasma parameters, it is too imperative to test that the plume is optically thin. Linearity of the Boltzmann scheme is one means to ensure whether the plasma is thin optically as evident from Fig. 5. The second way is based on comparison of the integrated intensity between the ratio of two peaks observed experimentally and theoretically as below:
If the chosen two peaks have a unified higher state the term in the exponential part is neglected and the theoretical integrated intensity ratio could be calculated by simply by means of the spectroscopic constants. The ratio of neutral emission of Ca-(I)/Ca-(I) = 428.94 nm/429.90 nm was 1.22 where the quotient of their corresponding transition probabilities was observed to be 1.29. Likewise, the ratio of Mg-I at 517.26 nm/Mg-I at 518.36 nm was found to be 2.90 while; the ratio of their related transition probabilities was 2.98. Likewise, the ratio of K-I at 691.1 nm and K-I at 693.8 nm was 1, while, the ratio of their transition probability was about 0.7. Such agreement among the intensities’ ratios and their transition probabilities shows clearly that our LIBS produced plasma is not optically thick and there is no chance of self-absorption to occur.
4 Discussion
4.1 Quantification and content determination of tea leaves by CF-LIBS and ICP- AES methods
After calculation of the plasma parameters and fulfilling the LTE condition, one of the significant tasks is to carry out quantitative determination of chemical contents from the experimental LIBS data.
The quantitative analysis of all the tea leaves samples was accomplished using calibration free CF-LIBS approach [20]. This approach depends upon the determination of precise outcomes of plasma parameters, i.e., (Te) and (Ne). In (OL) CF-LIBS the Boltzmann relation is engaged to evaluate the number of neutral elements in the plasma as:
where \({I}^{ki}\) is the integrated peak intensity, \({C}_{n}^{z}\) gives the contents of atomic species inside the plasma, F is the experimental factor and is correlated to the mass ablated (and can be measured via normalization of elemental abundance), \({A}_{ki}\) gives the transition probability, gk stands for statistical weight, Ek stands for the energy of higher state, k is the Boltzmann constant, and Te is the electron temperature, and Us(Te) is the partition function. Equation (8) is used to estimate the amount of atomic particles \(({C}_{n}^{z})\) and the quantity of ions \({(C}_{n}^{z+1}\)) is measured by Saha–Boltzmann relation, linking the concentration in the two successive charged levels Z and Z + 1 of a specific specie[13, 20].
Equation (9) provides the ratio of abundances of two charged levels Z + 1 and Z of the similar specie \(\frac{{C}_{n}^{z+1}}{{C}_{n}^{z}}\), from here the concentration of non-neutral particles \(({C}_{n}^{z+1})\) can be determined via placing the value of \({C}_{n}^{z}\) attained using Eq. (7). The total quantification of specie in the test sample is the addition of the contributions of the neutral and the charged species as,
Amounts of all the elements found in all of the tea leaves samples were evaluated utilizing this procedure. Following approximating the quantities of nutritional as well as trace heavy metals in all the chosen brands of tea leaves, the outcomes of CF-LIBS were too re-verified. For this purpose, the chemical composition of identical samples (black tea leaves) was performed through a more typical method, i.e., inductively coupled plasma-atomic emission spectroscopy (ICP-AES). It is remarkable to point out that both the outcomes (LIBS and ICP-AES) were in exceptional agreement which reconfirms the precision of the current LIBS results. The chemical contents of each the chosen black tea leaves determined by CF-LIBS and ICP-AES schemes were revealed in Fig. 5a, b and tabulated as in Table 2.
From the Fig. 5a, b, it is obvious that each of considered tea samples were rich of essential species in order like potassium, and then by sodium, iron, calcium, magnesium, zinc, and silicon correspondingly, which are extremely required in human body system for fine fitness. The amount of manganese and magnesium was almost the equal in all the chosen samples. The amount of potassium (K) varies from 28.91 to ~ 29.97%. The maximum quantity of potassium was observed in S2 even as its lower abundance was found in S4. K plays a valuable role in protein and carbohydrates metabolism of the body. The quantity of iron (Fe) is ranging between ~ 18.81 and ~ 20.90%. Iron is crucial to mainly life forms and to usual human physiology. It is a critical ingredient of proteins in relation to oxygen transfers from the lungs to the human’s tissues. Moreover, Fe is vital to regulate cell growth. The concentration of magnesium (Mg) in black tea leaves are ranging from 8.68 to 10.87%. Mg performs central function in ~ 300 metabolic processes including production of nucleic acid (DNA, RNA), creation of cellular energy as well as protein production. It is critical for enough brain energy and soft transmission through the main nervous system. As far as calcium (Ca) is related, its concentration observed from ~ 15.90 to 17.91%. Ca is a chief mineral for both teeth as well as bone. It also enhances the resistant protection scheme of body. The amount of sodium (Na) in black tea leaves is varying from 19.81 ~ 20.92%. Potassium and sodium keep normal the fluid stability within and in outer surface of the cells. K if in use along with the sodium stops the increase in blood pressure (B.P.) level. As far as the silicon is concerned, it was found in the scale from 0.002 to 0.004% in the selected tea specimens. Additionally, Zn was found in the range from 0.79 to 1% in the samples analyzed.
A glimpse at the Fig. 5a, b indicates the presence of toxic metals other than nutritionals. The observed outcomes revealed that the studied black tea leaves samples were polluted with varying amounts of heavy metals. Their elevated amounts may be due to some contaminations during handling, processing and the growth polluted environment and other factors. Heavy metals are enzyme toxins that agitate the resistant taxonomy; therefore, they required to be removed through some treatment.
The toxic lead (Pb) metal was observed in variable quantity in all the tea leaves samples. The highest amount (0.0008%) of Pb is observed in S1, whereas least concentration (0.0004%) of lead was found to be in S4. Yet in all of the selected tea samples, lead was detected within the maximum permissible limit of Pb contents in herbs (0.001%). The amount of manganese ranges from (0.00008 to 0.0002%) in the considered tea samples, which are outside the permitted boundary of Mn (0.00001%) [21].
The results presented in Fig. 5a, b, revealed the existence of hazardous Ni (0.004%) in S1 only which is somehow above the maximum permissible level (2.0 E−6%) [22]. The toxic chromium and copper were found in the range from (0.005 to 0.013), and (0.002 to 0.004) in the studied tea samples.
The variation of elemental contents from sample to sample may be due to the differences in mineral composition of soil in which the plants were cultivated, botanical structure, processing procedure, place of origin as well as the storage means. Other factors responsible for such variations may be the climate conditions, quality of irrigation water, use of fertilizers, and preferential absorbability of tea plants.
5 Conclusions
In this study, calibration free-laser induced breakdown spectroscopy was applied to quantify the essential and toxic elements in five different categories of tea leaves samples available in Pakistan. Each of the analyzed specimens were found rich of essential nutrient species like potassium, sodium, iron, calcium, magnesium, zinc, and silicon. Interestingly, the quantity of Mn and Mg was almost the equal in all the selected samples. The toxic Ni was found in Silicone sample S1 only. This study proved that the sample # 1 (open marketplace tea leaves locally available) contained some toxins in larger amount. The issue of self-absorption was taken into consideration and observed insignificant. In addition, to reconfirm the analytical determined contents using (OL) CF-LIBS, a standard analytical tool like ICP-AES was also applied. It was remarkable to notice that our LIBS results were in excellent accord with that achieved from standard method like ICP-AES. This in-depth study proved that LIBS appears to be a highly effective and significant technique for the food product analysis and to scrutinize the nutritional and toxic metals present in the foodstuff.
References
Martin, L.: Tea: the Drink that Changed the World. Tuttle Publishing, Clarendon (2011)
Saud, S.; Oud, A.: Heavy metal contents in tea and herb leaves. Pakistan J. Biol. Sci. 6, 202–208 (2003)
Qin, F.; Chen, W.: Lead and copper levels in tea samples marketed in Beijing, China. Bull. Environ. Contam. Toxicol. 79, 247–250 (2007)
Siddiqui, I.A.; Raisuddin, S.; Shukla, Y.: Protective effects of black tea extract on testosterone induced oxidative damage in prostate. Cancer Lett. 227, 125–132 (2005)
Fujita, H.; Yamagami, T.: Antihypercholesterolemic effect of Chinese black tea extract in human subjects with borderline hypercholesterolemia. Nutr. Res. 28, 450–456 (2008)
Takács, S.; Tatár, A.: Trace elements in the environment and in human organs: I. Methods and results. Environ. Res. 42, 312–320 (1987)
Hussain, I.; Khan, F.; Iqbal, Y.; Khalil, S.: Investigation of heavy metals in commercial tea brands. J. Chem. Soc. Pak. 28, 246–251 (2006)
Csikkel-Szolnoki, A.; Kiss, S.; Veres, S.: Elemental analysis of tea leaves by atomic spectroscopic methods. Magnes. Res. 7, 73–77 (1994)
Tokalıoğlu, Ş: Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 134, 2504–2508 (2012)
Ahmad, S.; Chaudhary, M.; Mannan, A.; Qureshi, I.: Determination of toxic elements in tea, leaves by instrumental neutron activation analysis. J. Radioanal. Chem. 78, 375–383 (1983)
Hussain, T.; Gondal, M.A.: Detection of toxic metals in waste water from dairy products plant using laser induced breakdown spectroscopy. Bullet. Env. Contam. Toxic. 80, 561–565 (2008)
Aldakheel, R.K.; Gondal, M.A.; Nasr, M.M.; Almessiere, M.A.; Idris, N.: Spectral analysis of Miracle Moringa tree leaves using X-ray photoelectron, laser induced breakdown and inductively coupled plasma-optical emission spectroscopic techniques. Talanta 217, 121062 (2020)
Rehan, I.; Gondal, M.; Rehan, K.: Determination of lead content in drilling fueled soil using laser induced spectral analysis and its cross validation using ICP/OES method. Talanta 182, 443–449 (2018)
Tognoni, E.; Cristoforetti, G.; Legnaioli, S.; Palleschi, V.; Salvetti, A.; Müller, M.; Panne, U.; Gornushkin, I.: A numerical study of expected accuracy and precision in calibration-free laser-induced breakdown spectroscopy in the assumption of ideal analytical plasma. Spectrochim. Acta, Part B 62, 1287–1302 (2007)
Rehan, I.; Gondal, M.; Rehan, K.; Sultana, S.; Dastageer, M.; Al-Adel, F.: LIBS for the detection of lead in ready to use henna paste and nutrients in fresh henna leaves and cultivated soils. Talanta 199, 203–211 (2019)
Gondal, M.A.; Dastageer, A.; Maslehuddin, M.; Alnehmi, A.J.; Al-Amoudi, O.S.B.: Detection of sulfur in the reinforced concrete structures using a dual pulsed LIBS system. Opt. Laser Technol. 44, 566–571 (2012)
Rehan, I.; Gondal, M.; Almessiere, M.; Dakheel, R.; Rehan, K.; Sultana, S.; Dastageer, M.: Nutritional and toxic elemental analysis of dry fruits using laser induced breakdown spectroscopy (LIBS) and inductively coupled plasma atomic emission spectrometry (ICP-AES). Saudi J. Biol. Sci. 28, 408–416 (2020)
Cirisan, M.; Cvejić, M.; Gavrilović, M.; Jovićević, S.; Konjević, N.; Hermann, J.: Stark broadening measurement of Al II lines in a laser-induced plasma. J. Quant. Spectrosc. Rad. Transf. 133, 652–662 (2014)
Gondal, M.A.; Nasr, M.M.; Ahmed, Z.; Yamani, Z.H.: Determination of trace elements in volcanic rock samples collected from cenozoic lava eruption sites using LIBS. J. Env. Sci. Health Part A 44, 528–535 (2009)
Ciucci, A.; Corsi, M.; Palleschi, V.; Rastelli, S.; Salvetti, A.; Tognoni, E.: New procedure for quantitative elemental analysis by laser-induced plasma spectroscopy. Appl. Spectrosc. 53, 960–964 (1999)
Wenlock, R.; Buss, D.; Dixon, E.: Trace nutrients: 2.* Manganese in British food. Br. J. Nutr. 41, 253–261 (1979)
Iqbal, Y.; Khalil, S.; Khan, F.; Hussain, I.: Investigation of heavy metals in commercial tea brands. J. Chem. Soc. Pak. 28, 246 (2011)
Acknowledgements
The authors are thankful to King Fahd University of Petroleum and Minerals to support this work and KACRAE under fellowship scheme.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rehan, I., Gondal, M.A., Aldakheel, R.K. et al. Determination of Nutritional and Toxic Metals in Black Tea Leaves Using Calibration Free LIBS and ICP: AES Technique. Arab J Sci Eng 47, 7531–7539 (2022). https://doi.org/10.1007/s13369-021-06233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-06233-y