Abstract
This study was aimed to develop an electrochemical electrode for the degradation of chemical oxygen demand (COD) of the textile industrial wastewater using a full factorial experimental design. Electrochemical method is noble, effective, and efficient in solving the current environmental pollution challenge. Characterization of the textile industrial wastewater was carried out as per the standard method of APHA. The DC power supply of 15.4 V was used to operate the electrochemical treatment system whereas certain chemicals such as sulphuric acid 1.5 mg/L, nitric acid 6.3 M, and oxalic acid solution 100 g/L were used for surface modification of electrodes. Three factors with three-level such as the reaction time of 12, 16, and 20 min, pH, 4, 6, and 7, and NaCl electrolyte concentrations of 2, 4, and 6 g/L were used. The experimental design of the study was formulated as a 33 which was expected to generate 27 runs but the number of experimental runs was reduced to 20 runs using Design Expert 12. The BOD5, COD, and pH of the textile industrial effluent were found to be 430.00 ± 3.00 mg/L, 1730.00 ± 2.00 mg/L, and pH 6.88 ± 0.20, respectively. The maximum COD removal of 94.1% was recorded at the optimum experimental condition of reaction time 16 min, pH 4, and electrolyte concentration 6 g/L whereas the minimum COD removal value of 65.9% was obtained. The regression analysis of COD removal (R2 0.98) indicated that electrolyte concentration was the dominant factor for the degradation of electrochemical. Generally, electrochemical degradation is a promising treatment technology to be implemented to remediate textile industrial wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The textile industry is a fastly growing manufacturing sector with an incredibly increasing of its demand across the globe [1]. This factory has complex processes with many water-intensive operational units such as sizing, de-sizing, sourcing, bleaching, mercerizing, dyeing, printing, and functional finishing [2]. The commonly used raw materials and synthesized fibers in the factory are cotton, flax, rayon, linen, silk, mohair, wool, polyester, polyamide, nylon, and acrylic [3]. Many chemicals are also consumed in the factory including dyes, salts, etc. [4]. Dyes are organic compounds that serve as a main coloring agent in the textile factory. Normally, dyeing of 1 kg of cotton required about 0.04 kg of dyes, 0.6 kg salts, and 150 L of treated water [5]. Globally, the annual dye production is about 7 × 105 t with 100,000 different types of dyestuffs [6, 7]. Every year, about 5000 t of dyeing stuff is dumped into the nearby environment without proper treatment [8]. Dyes are the major pollutants in textile industrial wastewater which can cause an unpleasant and toxicological effect on living organisms even at low concentrations < 1 ppm [7, 9]. These chemicals have the potential to cause severe public and environmental health problems. Additionally, the public health problems such as reproductive system defect, dysfunction of the kidneys, liver, brain, and nervous system were commonly reported [10]. Generally, textile industrial wastewater is composed of a high concentration of pollutants that has the potential to cause toxicological, mutagenic, and carcinogenic effects.
The high water consumption in a textile industrial production process is another challenge for water sustainability. Recently, high industrial water consumption and pollution have a substantial contribution to water scarcity. Water scarcity which is 1000 m3 per person per year will affect the life of 4.8–5.7 billion by 2050, globally [11,12,13]. Fabrication demanded a water quantity of 30–308 L/kg of the product but the water consumption sometimes can reach up to 933 L/kg [14, 15]. The textile industry is discharging a huge volume of high-strength wastewater which can be rated about 200–350 m3 per ton of finished product [16, 17]. This wastewater is composed of dyes and other chemicals that can contribute to a high concentration of COD (up to 8000 mg/L) [5, 18]. Actually, textile industrial wastewater compositions are significantly influenced by types of fabric produced, chemical types consumed, equipment used in the processes, and the fabric weight [19]. The direct discharge of textile industrial wastewater is restricted by environmental regulatory agencies. The legislations of many industrial effluent discharging standards were restricted unless the effluent is properly treated [20]. The typical nature of the textile industrial wastewater is a recalcitrant, toxic, non-biodegradable, mutagenic, carcinogenic, colorful, and environmentally unacceptable effluent [3].
Many conventional wastewater treatment efforts have been made to remediate textile industrial wastewater through physicochemical treatment methods such as coagulation, flocculation, sedimentation, filtration, and biological methods such as oxidation pond, activated sludge, trickling filter. But, such treatment technologies are not effective to prevent environmental pollution and public health problem [20]. The disadvantages of these technologies are the requirement of large quantities of chemicals, low treatment efficiencies, and a huge volume of sludge production [21, 22]. Sometimes such treatment technologies can also produce toxic secondary byproducts which are other environmental pollution burdens. Furthermore, biological wastewater remediation is very poor since it focused only on the dissolved matter and the biodegradability index is also very low (0.10–0.29) [2, 23]. All these factors have limited the application of these technologies to clean up the high-strength wastewater of the textile industry. Therefore, there is a shift to advanced wastewater treatment technologies.
The application of advanced wastewater treatment technology for the treatment of the textile industrial wastewater was very effective, particularly the most commonly known technologies such as precipitation, membrane separation, ion exchange, reverse osmosis, advanced oxidation processes (Fenton, ozonation, etc.), electrocoagulation, filtration, adsorption, photocatalytic, disinfection, and electrodialysis are very practical [22, 24,25,26]. However, the implementation of these technologies are not evenly distributed across the globe because of certain limitations such as energy-intensive process, expensiveness of the technologies, requiring high operational and capital inputs, needing advanced technologies, low thermal and chemical resistivity, membrane fouling, and requirement of the skilled man powers [27, 28]. However, electrodialysis treatment was effectively removed COD, salt, and color from textile industrial wastewater. But this technology is not applied practically at a large scale [29]. Among other treatment technologies, adsorption is a promising wastewater treatment method [1, 30, 31]. Additionally, it is considered as a flexible, simple to design, environmentally friendly, economically feasible, and efficient treatment technology [32, 33]. However, the practical application of this technology in the water and wastewater treatment sector is limited because commercially activated carbon is very expensive and the effectiveness of locally prepared adsorbent is not completely understood. The low treatment performances, intensive preparation time, cost, regeneration challenge, and adsorption capacity are among the major challenging factor for such technology. Even though many efforts have been made to develop effective, low-cost, and efficient adsorbents across the globe, the studies are still under investigation [26]. Therefore, the current studies are shifting to other treatment technologies such as electrochemical methods, particularly for non-degradable pollutants like textile industrial wastewater. Practically, the removal mechanism of the electrochemical method is the degradation (breakdown) of the pollutants through the oxidation and reduction processes at the points of the electrodes.
The electrochemical method is an advanced oxidation process that is capable of degrading organic materials into CO2 and H2O. This treatment method is a green technology that has recently attracted the attention of the researchers and scientific community. Efficient electrochemical decolorization performance (99%) for methylene blue removal from textile industrial wastewater was reported [34, 35]. Additionally, decolorization of textile wastewater using anodic oxidation with the graphite anode coated with lead dioxide and graphite anodes were found to be 96.2% and 68.3%, respectively [34]. The major advantages of this technology are no sludge generation, simple equipment design, easy operation, advancing reaction by electrons, low cost of construction, and effective mineralization of pollutants. Additionally, increasing the current density will cause the rapid degradation of the pollutants many folds which will be resulted in maximum removal efficiency. However, the anode material, the type of contaminant, reaction time, type, and concentration of electrolytes are the main operational parameters that are significantly affecting the treatment efficiency [34]. Currently, special attention was given to the electrochemical method and many outstanding research outcomes are displayed which are mainly focused on emerging micropollutants particularly the persistent organic pollutants [36,37,38]. Therefore, this study was aimed to develop an electrochemical electrode for the degradation of chemical oxygen demand of the textile industrial wastewater through an optimization approach that was studied by design expert software version 12. The full factorial experimental design of the three factors with three-level of reaction time (12, 16, and 20 min) pH (4, 6, and 7), and concentration of electrolyte (2, 4, and 6 g/L) was well applied. The interaction effect of the three factors of the electrochemical treatment methods was also thoroughly investigated.
2 Materials and Methods
2.1 Wastewater Sample Collection and Characterization
The wastewater sample was collected from Bahir Dar Textile industrial Share Company. Composite sampling techniques were used to collect wastewater samples three times a day which was mixed to get a representative sample in terms of physicochemical compositions. The total generation and discharge of wastewater from the textile industrial process were 400–480 m3/day. A sample container of a polyethylene plastic material was thoroughly washed with detergents and soaked in 10% HNO3. Then, it was repeatedly washed with distilled de-ionized water. The icebox cooling materials were used to transport the sample to Addis Ababa Science and Technology University for analysis purposes. Finally, the wastewater was preserved in a refrigerator at 4 °C until its analysis was performed. The analysis of each wastewater parameter and the corresponding testing method was indicated in Table 1.
2.2 Electrolyte Preparation
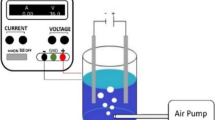
The platinum-iron anode electrode was prepared from waste materials that were damped at Bahir Dar Textile industrial share company whereas the cathode was prepared from a knife handle at ‘torno bet’. A similar size of both the anode and the cathode electrodes was designed and prepared. Accordingly, the dimensions of the electrodes were 50 mm in length, 10 mm in width, and 5 mm in depth. Two treatment systems (duplicated treatments) were used for the degradation of the textile industrial wastewater throughout the study and the overall setup of the study experiment was indicated in Fig. 1. The platinum-iron electrode surface was successively polished using sandpaper on roughing stone using water as a cleaning agent. The alloy substrate surface was cleaned by sandpaper again and again and then treated with 98% sulfuric acid (1.5 mg/L) to remove any oxides. Finally, the prepared electrode was soaked in nitric acid 6.3 M for 5 min and then, it was chemically treated by boiled oxalic acid solution (100 g/L) for 7 min. In the end, it was polished with sandpaper and dried at a temperature of 40 °C. Electrolysis was carried out using the platinum-iron alloy electrode using a sodium chloride solution (2–6 mg/L) which was prepared from an analytical graded chemical in the laboratory. The electrochemical degradation of the textile industrial wastewater which contained a high concentration of chemical oxygen demand was performed in a 250 mL Pyrex glass beaker. This treatment system composed of the platinum-iron electrode which was used as a working electrode while stainless steel served as a cathode electrode. The electrodes are connected to a DC power supply with a model FESTO (15.4 V), while the current measurements readout performed using the digital multimeter.
2.3 Experimental Design
Full factorial experimental was performed throughout the study period with the three selected experimental factors with the three levels as indicated in Table 2. The experimental design of the study was formulated as a 33 which is expected to generate 27 runs but the number of experimental runs reduced to 20 runs using Design Expert 12 but these values were fixed based on previous experimental studies [39]. The predicted COD removal was studied using the quadratic regression model and response surface methodology method. During the experiment period, the initial COD concentration of 1730 mg/L at room temperature was used throughout the experiment. However, the COD removal values were expressed as an independent variable that was evaluated using electrochemical method performance in percentages (%). The level of each factor was designated as lower, middle, and higher with ‘−1’, ‘0’, and ‘+1’, respectively [32, 40].
The COD concentration of textile industrial wastewater was determined using the HANNA digester and COD analyzer according to the standard methods for the examination of water and wastewater. The COD removal percentage was calculated using Eq. 1.
where %R is the COD removal percentage, CODi (mg/L) is the initial COD concentration and CODf (mg/L) is the final COD concentration [41].
The regression analysis was carried out using the quadratic model equation and the response surface methodology was also used to plot the relationship between the dependent and independent variables. In addition to experimental values, the main and interaction effects of the predicted values on the COD removals were well studied. Compared to the linear and cubic regression models, the quadratic regression model was well described as the experimental data. A detailed explanation was given by Eq. 2.
where YCOD is the predicted COD removal, A, B, and C are the contact time, pH, and electrolyte concentration, respectively. But β0 is intercepted, β1, β2, β3, are linear coefficient, β11, β22, β33, are the squared coefficients, β12, β13, β23, are the interaction coefficients. Finally, the electrochemical degradation of chemical oxygen demand of wastewater was tested using ANOVA analysis for the quadratic regression model. In this ANOVA test, the significance of the regression model was checked at a 95% confidence level.
3 Results and Discussion
3.1 Characteristics of Wastewater
The physicochemical properties of textile industrial wastewater were studied and the results of the findings were compared with the Ethiopian industrial effluent discharging limits as shown in Table 3. Total solids of 6437.3 ± 3.0 mg/L and pH 6.9 ± 0.2 were found in the textile industrial wastewater. These high concentrations can be attributed to the chemicals and other raw materials utilized in the textile fabrication process. The pH of the effluent was relatively within the acceptable ranges which was safe to be discharged in this regard. The common pH range of textile industrial wastewater was from 5.5 to 11.8 [19]. However, the concentration of the total solids was beyond the maximum permissible limit of Ethiopian industrial effluent discharging limits of 50 mg/L. Therefore, the effluent has a significant effect on the environment and is urgently required to undergo intensive wastewater treatment technologies. Similarly, the concentration of the total suspended solids was found to be 5224.0 ± 2.0 mg/L. Discharging such high concentrations into water bodies can block the penetration of the sunlight which in turn affects the physicochemical characteristics of the water bodies.
COD and BOD5 are the major indicators of the organic and oxidizable components in textile wastewater. The concentration of these parameters is indicated in Table 3. The COD concentration was found to be 1730.0 ± 2.0 mg/L whereas the BOD5 value of 430.0 ± 3.0 mg/L was recorded. This is clearly showed that the textile industrial wastewater was highly loaded with organic matter which might be associated with the raw materials and chemicals utilized in the production process. The biodegradability index (BI) is the degree of biodegradability of the wastewater which is described in the form of BOD5 to COD ratio. In line with this, the BI of 0.6 refers to the fairly degradable and biological treatment is effective whereas the BI values between 0.3 and 0.6 required additional support like seeding of acclimatized microorganisms are mandatory. However, the BI values below 0.3 are challenging to remediate such wastewater through the biological process. The BI of this study was 0.25 and the wastewater treatment through the biological process is a big challenge. Therefore, shifting toward physicochemical treatment was the right decision. Generally, COD and BOD5 were by far beyond the guideline set by the Ethiopian Ministry of the Environment, Forest, and Climate Change at which the recommended levels of COD and BOD5 of effluents are 60 and 250 mg/L, respectively. This wastewater has great potential to cause environmental pollution which leads to developing appropriate wastewater treatment not to discharge the effluent irresponsible.
3.2 Experimental Results Analysis
The results of COD removal from the textile industrial wastewater were displayed in Table 4. The maximum COD removal of 94.1% was obtained at the optimum experimental condition of the three independent factors which are the reaction time of 16 min, pH 6, and NaCl electrolyte concentration 6 g/L at the given experimental setup. Only a single experiential was found, out of the twenty experiments which resulted in a removal performance of less than 80%. Similarly, twelve experimental runs had results that were associated with more than 90% COD removal efficiencies. This indicated that the three factors with the three levels are equivalently influenced by the removal of the COD. However, the minimum COD removal of 65.9% was obtained at the experimental condition of the reaction time 12 min, pH 4, and NaCl electrolyte concentration 2 g/L. The three experimental factors with their level values were significantly influenced the COD removal. Shifting the values of the three factors from the lower to higher values resulted in increasing COD degradation by 27.2%. Many COD removal values were almost close to each other and the efficiencies were also very high. These high removals were attributed to the change in both the main and interaction effects that enhanced the degradation efficiency. This maximum removal efficiency was very encouraging and could comply with the maximum permissible discharging limits for textile industrial effluent. The initial COD concentration of 1730 mg/L was reduced to 102.1 mg/L at a removal efficiency of 94.1% which is by far below the COD concentration of 250 mg/L, the maximal permissible discharging limit for textile industrial wastewater set by the Ethiopian Ministry of the Environment, Forest, and Climate Change.
3.3 Regression Analysis for COD Removal
The regression model was used to check the impact of the three and four ways of interactions on COD removal. But such interactions were insignificant at a 95% confidence interval. Hence, such expressions were excluded from Eq. 3 since they have no significant impact on COD removal. Only the main and two-way interactions were evaluated for the efficiency of treatment performance. The summary of the significant terms on COD removal was indicated in Eq. 2. This equation can be used to make predictions about the response variable for a given level of each factor. The equation can be also useful for identifying the relative impact of the factors on COD degradation by comparing their corresponding coefficients of the factors. Two important terms were used to evaluate the prediction power of the regression model. The regression coefficient of determination (R2) is the power of explaining the degree of variability made by the model whereas the p-value is used to differentiate statistically significant and non-significant terms in the model equation. The regression model well predicted the 85% of COD removal variation since the R2 of this study was 0.85. The ANOVA test was indicated in Table 5. The p-values for these factors were less than 0.05 at which the ANOVA test was performed (95% confidence interval). All those terms were statistically significant. This depicted the good fitness of the model with the experimental data.
where YCOD is the predicted COD removal (treatment performance in %), A is the contact time, B is the pH and C is electrolyte concentration whereas the two-way interactions of AB, AC, and BC refer to the reaction time with pH, the reaction time with electrolyte concentration and the pH with electrolyte concentration, respectively. The degree of each main and interaction effect on COD removal was determined by the coefficient of the regression term with the positive sign refer synergistic effect whereas the negative sign refers to antagonistic. Based on the value of the coefficient, the regression of the impact on COD removal in the order of C > AC > A > B > AB > BC irrespective of the sign of the coefficient. But the interaction effect between the B and C was inversely proportional to the COD removal. The degree of impact on COD removal was increased by increasing the values of the coefficient of the regression. This regression analysis indicated that the main and two-way interaction effects were directly increasing with the degradation of the COD from textile industrial wastewater whereas a single interaction effect (BC) was suppressed the treatment performance.
The comparison of predicted and the actual value is depicted in Fig. 2. The plot of the predicted value against the actual indicated that the promising association between the two results. The predicted value in the plot helps the degree of relying on the model supporting the accuracy of the model prediction. The plot described a good correlation between the two values. This was approximately indicating the even distribution and good correlation defined by the model. This means that the abnormality in prediction by the model is nearly negligible. External residuals of the experimental operation were used to check whether a run is consistent assuming the chosen model holds.
3.4 The Effect of Each Factor on COD Removal
The removal efficiency of COD removal was increased directly with increasing reaction time indicated in Fig. 3. But, increasing the reaction time resulted in increasing in COD removal which indicated the equilibrium time is beyond 20 min. It was expected that a further increase in the reaction time that improves the degradation of COD removal. Therefore, the reaction time is one of the most important parameters that influence the degradation of COD removal. It is also possible to analyze the effect of the pH on COD removal efficiencies (Fig. 3). When the pH increased from 4.0 to 5.2, the COD removals were increased by 2.38%. But, when the pH of the solution increased from 6.4 to 7.0, the change in COD reduction was insignificant which attributed to the increment of 1.16%. This last section shows the gradual increase in removal efficiency which might be closer to the equilibrium condition. The electrochemical degradation recorded values indicated that pH was insignificantly affected the COD removal during this experimental condition. After this pH point, the production of OH− ions is greater than H+ ions resulting in a shift of pH to the basic region in which COD removal could not favor. In some cases, the interpretation of pH effects on the COD degradation process is a difficult task due to the role of the pH interaction with many components in the degradation system. Increasing electrolyte concentration increased the removal efficiency of COD as indicated in Fig. 2. More than 90% of COD degradation was achieved between 4.2 and 5.2 g/L electrolyte concentration. Increasing NaCl concentration increases the degradation efficiency as the process generates HOCl/OCl− redox which was directly associated with COD removal. Further increase in NaCl concentration of more than 6 g/L has a slight effect on COD removal. In another experiment with no addition of the electrolyte solution, it was checked the degradation of COD and decolorization of the textile industrial wastewater was negligible.
3.5 The Interaction Effects on COD Removal
3.5.1 Reaction Time and pH
Investigation of the interactive effects of the independent factors is the most important power of the full factorial design. The interaction effect of the reaction time and pH on the degradation of chemical oxygen demand was examined and the results of the findings were demonstrated in Fig. 4. The response surface plots and their corresponding contour plots of COD removals were generated, keeping one independent factor as a constant. The values of the two factors were varied gradually within the experimental ranges. This work was carried out at a constant electrolyte concentration of 4 mg/L. The pattern of the interaction was in an increasing manner which resulted in the increasing degradation values of the chemical oxygen demand. The prediction area was varied from green, yellow, and red in the increasing order of the degradation of COD concentration. Increasing the reaction time to 19.97 and pH to 6.95 generate the interaction effect that resulted in degradation of COD removal of 92.95% keeping the electrolyte concentration constant at 4 g/L. The minimum predicted value of COD degradation was 65.9% at the minimum reaction time of 14 min and pH 4.6. Actually, the degradation of COD removal from textile wastewater aimed to reduce the chemical concentration and environmental pollution loads. The maximum predicted and experimental values are in good agreement which reflected the response surface methodology was successfully described the experimental facts.
3.5.2 Reaction Time and Electrolyte Concentration
The interaction effect of the reaction time and electrolyte concentration on degradation of COD from textile industrial wastewater was investigated. The response surface plots of the COD removal performance were examined and the study result was displayed in Fig. 5. This interaction was positively enhanced the degradation performance to a certain extent at the steady-state and then after expected to be constant. Increasing both the reaction time and electrolyte concentration to 12.03 min and 5.97 g/L improved the COD removal efficiency to 94.72% showed in the darkest red region of the graph. Both of the factors, the reaction time and electrolyte concentration have effectively influenced the changes in COD concentration as shown in the curved prediction area of the 3D surface. This gradually changing the factors with increasing of the response variable was clearly observed from both the surface and contour plots. These predicted values were closely similar to the experimentally observed values of COD degradation. This implies that the prediction of the model was in a good fit with the experimental values.
3.5.3 H and Electrolyte Concentration
The interaction effect of the pH and electrolyte concentration on COD degradation was investigated and the trend of the interactions was depicted in Figure 6. When the pH and electrolyte concentration increased, the degradation of COD was increased up to 94 %. This maximum COD removal was achieved at pH 6.95 and the electrolyte concentration of 6 g/L at the constant reaction time of 16 minutes. This interaction was positively enhanced the degradation performance to a certain extent to reach the steady-state after which no further degradation is expected. The individual factor less influenced the reduction in COD concentration from textile industrial wastewater which was the range of 1–2%, whereas the interaction effects of the factors more significant than the individual parameter in the reduction in COD concentration. This indicates that the individual interactions had a less significant effect compared to interaction effects which aggravate the degradation processes of COD removal. It can be seen from the sketch that both the pH and electrolyte concentration affected the COD removal from textile industrial wastewater.
4 Conclusions
The expansion of the textile industry has a significant contribution to the economic development of many nations but the untreated discharge from such a factory is severely impacting freshwater and public health in many places. In line with this, the characteristics of textile industrial wastewater indicated the COD concentration was beyond the maximum permissible discharging limits. Hence, the textile industrial wastewater required intensive treatment efforts to protect the environment and public health. Electrochemical degradation was applied for COD reduction and the corresponding treatment performance of 94.1% was recorded at the optimum experimental condition of reaction time 16 min, pH 4, and electrolyte concentration 6 g/L, whereas the minimum COD removal value of 65.9% was obtained under different experimental condition. This is maximum COD reduction from initial COD concentration of 1.730–102.4 mg/L was recorded. This final COD concentration is below the Ethiopian industrial effluent discharging limit (250 mg/L) and the treated effluent has to be used for various purposes including irrigation. Electrolyte concentration was the most dominant factor in this treatment process that reflected by the regression model. The predicted COD values are nearly the same as the experimental values which imply the model was satisfactorily describing the experimental reality. The main and two-way interaction effects were directly increasing with the degradation of the COD concentration, whereas the single interaction effect of pH and electrolyte concentration suppressed the treatment performance. Generally, the COD electrochemical degradation was encouraging and the modified electrodes were a promising candidate to intervene in textile industrial wastewater pollution.
Data Availability
All data are fully available without restriction.
References
Yang, K.; Liu, Y.; Li, Y.; Cao, Z.; Zhou, C.; Wang, Z.; Zhou, X.; Ali, S.; Xu, X.: Applications and characteristics of Fe-Mn binary oxides for Sb (V) removal in textile wastewater : selective adsorption and the fixed-bed column study. Chemosphere 232, 254–263 (2019). https://doi.org/10.1016/j.chemosphere.2019.05.194
Paździor, K.; Bilińska, L.; Ledakowicz, S.: A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 376, 120597 (2019). https://doi.org/10.1016/j.cej.2018.12.057
Yaseen, D.A.; Scholz, M.: Textile dye wastewater characteristics and constituents of synthetic effluents : a critical review. Springer, Berlin Heidelberg (2018)
Hua, Y.; Xiao, J.; Zhang, Q.; Cui, C.; Wang, C.: Facile synthesis of surface-functionalized magnetic nanocomposites for effectively selective adsorption of cationic dyes. Nanoscale Res. Lett. (2018). https://doi.org/10.1186/s11671-018-2476-7
Azzaz, A.A.; Bengharez, S.J.Z.; Akrout, L.B.H.: Investigations on a dye desorption from modified biomass by using a low-cost eluent: hysteresis and mechanisms exploration. Int. J. Environ. Sci. Technol. 16, 7393–7408 (2019). https://doi.org/10.1007/s13762-018-2171-3
Ma, J.; Jia, Y.; Jing, Y.; Yao, Y.; Sun, J.: Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dye. Pigment 93, 1441–1446 (2012). https://doi.org/10.1016/j.dyepig.2011.08.010
Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A.: Adsorption of methylene blue on low-cost adsorbents: a review. J. Hazard Mater. 177, 70–80 (2010). https://doi.org/10.1016/j.jhazmat.2009.12.047
Liyana, A.; Hanis, N.; Hairom, H.; Yong, L.; Yin, C.; Khairul, M.; Wahab, A.: Industrial textile wastewater treatment via membrane photocatalytic reactor (MPR) in the presence of ZnO-PEG nanoparticles and tight ultra fi ltration. J. Water Process Eng. 31, 100872 (2019). https://doi.org/10.1016/j.jwpe.2019.100872
Sivarajasekar, N.; Baskar, R.: Adsorption of basic red 9 on activated waste Gossypium hirsutum seeds: process modeling, analysis and optimization using statistical design. J. Ind. Eng. Chem. 20, 2699–2709 (2014). https://doi.org/10.1016/j.jiec.2013.10.058
Adegoke, K.A.; Solomon, O.: Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 12, 8–24 (2015). https://doi.org/10.1016/j.wri.2015.09.002
UN-Water: Water for a sustainable world, The United Nations World Water Development Report 2015 Report WATER. Paris, (2015)
Mekonnen, M.M.; Hoekstra, Y.A.: Four billion people facing severe water scarcity. Am. Assoc. Adv. Sci. 2, 1–7 (2016). https://doi.org/10.1016/j.acra.2014.09.014
Pintilie, L.; Torres, C.M.; Teodosiu, C.; Castells, F.: Urban wastewater reclamation for industrial reuse: An LCA case study. J. Clean. Prod. 139, 1–14 (2016). https://doi.org/10.1016/j.jclepro.2016.07.209
Blus, K.; Foszpa, M.; Gmurek, M.; Bili, L.: Catalytic ozonation of textile wastewater as a polishing step after industrial scale electrocoagulation. J. Environ. Manag. (2020). https://doi.org/10.1016/j.jenvman.2020.110502
Pathak, A.K.; Kothari, R.; Tyagi, V.V.; Anand, S.: Integrated approach for textile industry wastewater for efficient hydrogen production and treatment through solar PV electrolysis. Int. J. Hydrog. Energy. 45, 25768–25782 (2020). https://doi.org/10.1016/j.ijhydene.2020.03.079
Mahdi, M.; Shirazi, A.; Bazgir, S.; Meshkani, F.: A novel dual-layer, gas-assisted electrospun, nano fi brous SAN4-HIPS membrane for industrial textile wastewater treatment by direct contact membrane distillation (DCMD). J. Water Process Eng. 36, 101315 (2020). https://doi.org/10.1016/j.jwpe.2020.101315
Sen, S.K.; Patra, P.; Das, C.R.; Raut, S.; Raut, S.: Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: an impact on its use in irrigation of wheat crop. Water Resour. Ind. 21, 100106 (2019). https://doi.org/10.1016/j.wri.2019.100106
Bouazizi, A.; Saja, S.; Achiou, B.; Ouammou, M.; Calvo, J.I.; Aaddane, A.; Younssi, S.A.: Applied clay science elaboration and characterization of a new fl at ceramic MF membrane made from natural moroccan bentonite: application to treatment of industrial wastewater. Appl. Clay Sci. (2016). https://doi.org/10.1016/j.clay.2016.05.009
Yaseen, D.A.; Scholz, M.: Textile dye wastewater characteristics and constituents of synthetic effluents : a critical review. Springer, Berlin Heidelberg (2019)
Kaur, P.; Kushwaha, J.P.; Sangal, V.K.: Transformation products and degradation pathway of textile industry wastewater pollutants in Electro-Fenton process. Chemosphere 207, 690–698 (2018). https://doi.org/10.1016/j.chemosphere.2018.05.114
Lidiya, M.; Gopalakrishnan, A.; Aravindakumar, C.T.: Low-cost multilayered green fiber for the treatment of textile industry waste water. J. Hazard. Mater. 365, 297–305 (2019). https://doi.org/10.1016/j.jhazmat.2018.11.014
Núñez, J.; Yeber, M.; Cisternas, N.; Thibaut, R.; Medina, P.; Carrasco, C.: Application of electrocoagulation for the efficient pollutants removal to reuse the treated wastewater in the dyeing process of the textile industry. J. Hazard. Mater. 371, 705–711 (2019). https://doi.org/10.1016/j.jhazmat.2019.03.030
Fito, J.; Hulle, S.W.H.V.: Wastewater reclamation and reuse potentials in agriculture : towards environmental sustainability. Environ. Dev. Sustain. (2020). https://doi.org/10.1007/s10668-020-00732-y
Tebeje, A.; Worku, Z.; Nkambule, T.T.I.; Fito, J.: Adsorption of chemical oxygen demand from textile industrial wastewater through locally prepared bentonite adsorbent. Int. J. Environ. Sci. Technol. (2021). https://doi.org/10.1007/s13762-021-03230-4
Ayanpeju, K.; Giwa, A.A.; Motunrayo, J.: Optimization studies for decolourization of textile wastewater using a sawdust-based adsorbent. Chem. Data Collect. 27, 100400 (2020). https://doi.org/10.1016/j.cdc.2020.100400
Fito, J.; Abrham, S.; Angassa, K.: Adsorption of methylene blue from textile industrial wastewater onto activated carbon of parthenium hysterophorus. Int. J. Environ. Res. (2020). https://doi.org/10.1007/s41742-020-00273-2
Khadijah, S.; Ha, M.; Othman, D.; Sheng, Z.; Riduan, M.; Kamilah, N.; Ahmad, A.; Rahman, M.A.; Jaafar, J.; Hamimah, S.; Abdul, S.; Harun, Z.: Novel hydroxyapatite-based bio-ceramic hollow fi ber membrane derived from waste cow bone for textile wastewater treatment. Chem. Eng. J. (2020). https://doi.org/10.1016/j.cej.2019.122396
Fito, J.; Said, H.; Feleke, S.; Worku, A.: Fluoride removal from aqueous solution onto activated carbon of Catha edulis through the adsorption treatment technology. Environ. Syst. Res. 8, 1–10 (2019). https://doi.org/10.1186/s40068-019-0153-1
La, R.; Gzara, L.; Hadj, R.; Ha, A.: Treatment of textile wastewater by a hybrid ultra fi ltration / electrodialysis process. Chem. Eng. Process. Process Intensif. 132, 105–113 (2018). https://doi.org/10.1016/j.cep.2018.08.010
Bedada, D.; Angassa, K.; Tiruneh, A.; Kloos, H.; Fito, J.: Chromium removal from tannery wastewater through activated carbon produced from Parthenium hysterophorus weed. Energy Ecol. Environ. 5, 184–195 (2020). https://doi.org/10.1007/s40974-020-00160-8
Salazar, R.; Gallardo-arriaza, J.; Vidal, J.; Rivera-vera, C.; Toledo-neira, C.; Sandoval, M.A.; Cornejo-ponce, L.; Thiam, A.: Treatment of industrial textile wastewater by the solar photoelectro-Fenton process: in fl uence of solar radiation and applied current. Sol. Energy. 190, 82–91 (2019). https://doi.org/10.1016/j.solener.2019.07.072
Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y.: Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 202, 266–276 (2017). https://doi.org/10.1016/j.matchemphys.2017.09.028
Singh, K.; Lataye, D.H.; Wasewar, K.L.: Removal of fluoride from aqueous solution by using bael (Aegle marmelos) shell activated carbon: kinetic, equilibrium and thermodynamic study. J. Fluor. Chem. 194, 23–32 (2017). https://doi.org/10.1016/j.jfluchem.2016.12.009
Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A.: Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab. J. Chem. 13, 6847–6864 (2020). https://doi.org/10.1016/j.arabjc.2020.06.038
Zou, R.; Angelidaki, I.; Jin, B.; Zhang, Y.: Feasibility and applicability of the scaling-up of bio-electro-Fenton system for textile wastewater treatment. Environ. Int. 134, 105352 (2020). https://doi.org/10.1016/j.envint.2019.105352
Kamaraj, R.; Vasudevan, S.: Facile one-pot electrosynthesis of Al(OH)3-kinetics and equilibrium modeling for adsorption of 2,4,5-trichlorophenoxyacetic acid from aqueous solution. New J. Chem. 40, 2249–2258 (2016). https://doi.org/10.1039/c5nj02407b
Vasudevan, S.; Kamaraj, R.; Pandiarajan, A.; Vasudevan, S.: Facile one-pot electrosynthesis of zinc hydroxide for the adsorption of hazardous 2-(2-methyl-4-chlorophenoxy) propionic acid (MCPP) from water and its modelling studies. J. Environ. Chem. Eng. 6, 2017–2026 (2018). https://doi.org/10.1016/j.jece.2018.03.011
Pandiarajan, A.; Kamaraj, R.; Vasudevan, S.: Enhanced removal of cephalosporin based antibiotics (CBA) from water by one-pot electrosynthesized Mg(OH)2: a combined theoretical and experimental study to pilot scale. New J. Chem. 41, 4518–4530 (2017). https://doi.org/10.1039/c6nj04075f
Hegazy, A.K.; Abdel-Ghani, N.T.; El-Chaghaby, G.A.: Adsorption of phenol onto activated carbon from Rhazya stricta: determination of the optimal experimental parameters using factorial design. Appl. Water Sci. 4, 273–281 (2014). https://doi.org/10.1007/s13201-013-0143-9
Tayebee, R.; Mazruy, V.: ARTICLE ORIGINAL acid-thermal activated nanobentonite as an economic industrial adsorbent for malachite green from aqueous solutions: optimization, isotherm and thermodynamic studies. J. Water Environ. Nanotechnol. 3, 40–50 (2018). https://doi.org/10.22090/jwent.2018.01.004
APHA: Standard methods for the examination of water and wastewater. American Public Health Association; American Water Works Association; Water Environment Federation, Washington, DC (1998)
Acknowledgements
We would like to thank the Ethiopian Road Authority (ERA) for the research fund and Addis Ababa Science and Technology University (AASTU) for the research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Yehuala, G., Worku, Z., Angassa, K. et al. Electrochemical Degradation of Chemical Oxygen Demand in the Textile Industrial Wastewater Through the Modified Electrodes. Arab J Sci Eng 47, 5911–5922 (2022). https://doi.org/10.1007/s13369-021-05776-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05776-4