Abstract
In this study, four compounds of 2-(phenylthio)-ethyl benzoate derivatives were synthesized and evaluated for their antitumor, antibacterial, and antifungal activities. These compounds were characterized and evaluated for their cytotoxic activity in MCF-7-human carcinoma cells that showed a significant decrease for compounds 2a–2d in the average of G2-M phase as 8.13 ± 1.4, 10.66 ± 1.5, 14 ± 2.2, and 3.66 ± 0.8%, respectively, compared with untreated cells (21 ± 2%; p < 0.05). The data suggest that 2d compound could have an anticancer potential in the G2-M phase arrest of MCF-7 cells ultimately leading to necrosis. The compounds were tested for their antibacterial activity against Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Proteus vulgaris, Enterococcus faecium, Pseudomonas aeruginosa, MRSA, and Candida albicans and showed antimicrobial potential with MIC value average (3.125–6.25 mg/ml), compared with ampicillin (0.001–3.125 mg/ml). When the MIC of the four compounds was compared with known references, we found that the 2a compound has the same MIC as fluconazole (1.56 mg/ml), inhibitor of Candida growth. Moreover, 2a, 2b,, and 2d have the same MIC as ampicillin (3.125 mg/ml) for the inhibition of S. aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is considered the second leading cause of death, after heart disease. The incidence of different carcinomas is estimated to be about 10 million worldwide, and half of these incidences are in developed͑ countries [1, 2]. Five decades of systemic drug discovery and development have led to a respectable accumulation of useful and important chemotherapeutic agents [3,4,5], and several important achievements in the curing and management of human cancer [6, 7]. The scientific literature shows numerous epidemiological reports that support significant differences in the occurrence of carcinoma between oriental and occidental populations [8, 9].
Since decades, chemotherapy was the first option for cancer treatment has faced dramatic problems. Moreover, the lack of selectivity is the drawback of conventional anticancer agents, that damage not only malignant but also normal body, and blood cells have compelled scientists to develop selective and targeted medications [10]. Another reported drawback of cancer chemotherapy is the appearance of drug-resistant cancer cells [11]. This has increased the interest of researchers to develop new anticancer agents from natural or synthetic origins [12].
Drugs having antimicrobial effects are prepared to inhibit microorganisms without any bad effects on the human body [13]. Antibiotics are valuable weapons for fighting bacterial strains, and their preparation has a strong relationship with the healthy state of concerned patients. Nowadays, the health values involve limitations due to natural selection, and the resistance of the bacterial strain against the available drugs becomes a critical issue. Therefore, developing medicinal preparations derived from synthetic or natural sources can play a crucial role in preventing and curing disorders among people [14]. An antifungal medication is a medical preparation that selectively acts to get rid of fungal pathogens from the infected tissues with minimal side effects for the target [15]. Compared with bacterial diseases, fungal diseases are more difficult to treat. Topical and oral treatments used for long duration are partially effective in managing these fungal infections. Several of these infections can become chronic, while in some cases the disease is difficult to reoccur [16]. The most available and widespread classes of mycoses are usually related to fungal infections of the skin, affecting > 20–25% population of the world [17].
Benzoic acid is a naturally occurring compound present in plants, some animals’ tissues, and microorganisms. Various types of benzoic acid derivatives including benzoyl peroxide, benzaldehyde, benzyl alcohol, parabens, and alkyl esters, are commonly utilized as antifungal and antibacterial preservatives and as flavoring agents in pharmaceutical, hygiene, cosmetic, and food products. As a result of their widespread use, production, and occurrence, these molecules are broadly distributed in the environment and found in air, soil, and water. Consequently, human exposure to them can be lengthy, common, and high [18].
The incidence of adverse effects of benzoic acid and benzoic acid derivatives are less than 1% in the general population, but positive challenges in patients with chronic urticaria vary from 4 to 44% [19].
Benzoic acid (C7H6O2) is a simple aromatic carboxylic acid, a crystalline solid, colorless, and odorless, with a sweetish and astringent taste. In many countries, the interest in phenols, polyphenols, and acid esters has increased, since several types of these compounds exert a wide variety of biological effects, particularly controlling inflammation, anti-atherogenic, inhibition of bacterial strains, and antiviral and anticancer activities [20]. Many phenolic compounds are biologically active such as amoxicillin with broad-spectrum antibacterial potential; eugenol (naturally extracted oil) has antifungal activity [21], and combretastatin A-4 (CA-4) has potent anticancer activity (Fig. 1) [5]. The current study aims to synthesize new synthetic agents containing the phenol moiety and evaluate their antimicrobial and antitumor activities.
2 Materials and Methods
2.1 Chemicals and Instruments
The chemicals used in the experiments are 2-thiophenyl ethanol, ortho-hydroxybenzoic acid, benzoic acid, 3-hydroxy benzoic acid, and para-hydroxyl benzoic acid were purchased from Aldrich, Germany. The chemicals purchased from Alfa Aesar (USA) were ethyl acetate, NaHCO3, brine, Na2SO4, cyclohexane, ethyl acetate, Na2HPO4\NaH2PO4 (disodium phosphate\monosodium phosphate), NaCl, methanol, and Na2CO3. All the used chemicals were of analytical grade. The melting range of each compound was measured by a Gallenkamp melting point apparatus (SG96/07/705, UK), while IR was detected by an infrared spectrophotometer (Nicolet Is5–Id3) at An-Najah National University. The 1H-NMR and 13C-NMR spectral analysis were determined by a Bruker 500 MHz-Avance III at the University of Jordan.
2.2 Cancer Cell Line
The cytotoxicity was carried out by two cell lines, MCF-7 and MCF-10A. Actually, MCF-7 is a known breast cancer cell line, characterized by the overexpression of a receptor related to estrogen. In contrast, MCF-10A is not a tumorigenic epithelial breast cell line. The MCF-7 case was achieved by RPMI-1640 media (Germany, Sigma) mixed with 10% fetal bovine serum (Germany, Sigma), 1% l-glutamine (France, Sigma), 1% streptomycin and 1% penicillin (USA, Sigma), and a pH of 7.2 was controlled using Dulbecco’s phosphate-buffered saline (DPBS) (USA, Sigma). The cells were grown in an atmosphere containing humidity, and 95% air and 5% CO2 at 37 °C in an ESCO incubator adapted for cell culture. Moreover, the construction of the MCF-10A case was carried out in DMEM media (Sigma, Germany) that was nourished with 10% fetal bovine serum, 1% l-glutamine, 1% streptomycin, and 1% penicillin, and the pH was kept at 7.2 using Dulbecco’s phosphate-buffered saline (DPBS). The growth of cells was observed in an atmosphere with humidity and containing 95% air and 5% CO2 at 37 °C in an ESCO incubator adapted for cell culture [22].
2.3 Flow Cytometry Analysis
After the culture application, MCF-7 and MCF-10A cells were collected and controlled to 106/ml in staining buffer (in saline containing 1% bovine albumin; Israel, Biological Industries). For viability as well as for apoptosis reporting, propidium iodide (PI)-fragmented DNA was stained and phosphatidylserine was stained by Annexin V-conjugated to FITC (R&D Systems, Minneapolis, MN) which were utilized depending on the manufacturer’s information. Apoptosis is usually defined as annexin-V (+) but propidium iodide (−). Viable cells were defined as annexin-V (−) but propidium iodide (−). Every experimental part involved setup, unstained control, IgG isotype control with FMO control. To analyze the cell cycle using DNA, content quantitation was applied using propidium iodide. The MCF-7 and MCF-10A cells were stabilized in cold 70% ethanol for nearly 30 min at 4 °C. They were then washed by 2× in PBS and spun at 2000 rpm, and the supernatant was discarded once finished. To ensure that only DNA was stained, the cells were treated with the ribonuclease enzyme (50 μl of 100 μg/ml RNase), stained with 5 μl of 50 μg propidium iodide/100 ml, and analysis were done using a flow cytometer (Immune fluorometry systems, Becton–Dickinson LSR II, Mountain View, CA) [23].
2.4 Antimicrobial Method
Eight harmful for human microbial strains were selected for antibacterial and antifungal activities of the synthesized compounds, and they were supplied by the American Type Culture Collection (ATCC); Klebsiella pneumonia (ATCC13883), Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 9027), Proteus vulgaris (ATCC 8427), Escherichia coli (ATCC 25922), and Enterococcus faecium (ATCC 700221) in addition to the clinical isolates of MRSA (methicillin-resistant Staphylococcus aureus). The compounds were examined also against the growth of Candida albicans to assess the fungus inhibition ability of the compounds. Furthermore, the antimicrobial effect of the synthesized molecules in our study was reported using the broth microdilution process [24, 25]. Fifty milligrams of every compound was fully dispersed in 50 ml dimethyl sulfoxide to establish a final concentration of 1 mg/ml. Filter sterilization was carried out on the resulting solution to serially micro-dilute it by two- and sixfold under a sterilized nutrient broth. The dilution processes were carried out using aseptic conditions in the available 96-well dishes. Inside the micro-wells, which were selected to examine the antibacterial ability of the tested compounds, the concentration ranged from 1.53 to 25 mg/ml. The same conditions existed inside the micro-wells of the compounds assigned to examine the antifungal effect where the concentration also ranged from 1.53 to 25 mg/ml. Besides, there was a micro-well plate, holding number 11, which included compounds that were free of nutrient broth and which was adopted as a positive control for the growth of microbes. Moreover, micro-well holding number 12 included compounds free of nutrient broth that was kept away from the others and was not inoculated with any of the examined microbes. Each of the bacterial pathogen and C. albicans samples were examined in duplicate in this assay. All inoculated microplates were incubated at 35 °C, and plates inoculated with the examined bacterial strains were incubated for 18 h, and plates inoculated with examined C. albicans were incubated for 48 h. The lowest value of the assessed concentration of the synthesized molecules at which there was no visible microbial growth in the micro-well was also recorded and was defined as the minimal inhibitory concentration (MIC) of the tested compound [25]. This micro-well was considered as a negative reported control of bacterial growth. The micro-wells with numbers ranging from 1–11 were also inoculated aseptically with the examined microbial strains. At the time of inoculation, the final concentrations of microbial cells were about 5 × 105 and 0.5–2.5 × 103 colony-forming unit (CFU)/ml for the examined strains of bacteria and C. albicans, respectively.
The benzoate compounds were tested against seven bacteria and one fungus strain that cause dermic and mucosal infections, besides other infections, in humans [26]. For all the tested bacteria, we had four controls: (1) positive control which contains media and bacteria; (2) negative control which only contains media; (3) compound control (compound + media) to be sure that there is no contamination and turbidity and that the changes are not due to the compound itself (so the compounds were serially diluted in this control), and (4) DMSO which were tested for every microbe separately to check the effect on each one, and the antimicrobial activity of DMSO was also considered.
2.5 Chemistry Method
This chemical method was applied in the synthesis of all compounds. Benzoic acid derivatives (Table 1) (3.062 g, 0.025 mol) and 2-thiophenylethanol (3.856 g, 0.025 mol) were refluxed for 2 h and then left for one night. The reaction was catalyzed by adding 2 ml sulfuric acid. The product was dissolved in 20 ml ethyl acetate and then extracted with saturated sodium bicarbonate. The phases were washed with saturated sodium chloride, dried with sodium sulfate, and the solvent was evaporated. All final products were purified using flash chromatography using the mobile phase of 40% n-hexane, 60% ethyl acetate, and a silica gel as the stationary phase.
2.6 Statistical Analysis
Statistical differences were analyzed either with the 2-tailed unpaired Student’s t test (For comparison between two groups) or one-way analysis of variance (one-way ANOVA with Newman–Keuls’ post tests among multiple groups) using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Data are shown as means ± SEM.
3 Results and Discussion
3.1 Chemistry
Four novel derivatives were synthesized via the esterification method, and their chemical structures have been characterized by using different chemical techniques. The chemical structures of the compounds along with their main chemical properties are listed in Table 1.
3.2 Antitumor Activity
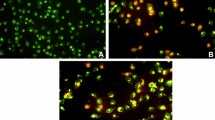
We have worked on the inhibitory effects of the compounds on the DNA cell cycle of MCF-7 cells. To investigate whether the compound could induce cell cycle perturbations in breast cancer cells, flow cytometry analysis of propidium iodide stained nuclei cells was performed, and the compounds were incubated with MCF-7 for 24 h at a concentration of 500 mg/ml as indicated in materials and methods. Figure 2 shows an increasing trend in the fraction of cells in the G1 phase following treatments with compounds. After treatment of 4 compounds (52–55%), average values were obtained in the G1 phase as compared to 41 ± 15.2% in the untreated Cells. Significant elevations in the proportion of cells in the S phase were obtained following 500 mg/ml concentration of compound 2a and 2d (9.3 ± 2.2, 10.3 ± 2.8, and 7.16 ± 1.7% of 2a, 2d and untreated cell, respectively); the other compounds showed a significant decrease in this phase. In the G2/M phase at the same concentration, all compounds showed a decrease in their averages in comparison with untreated cells and a significant decrease in the average of compound 2d (M4) in the S phase of 3.67 ± 1.2% in comparison with untreated cells (21.8 ± 6.5%). These data indicated a cell cycle arrest in this phase; the other compounds show a decrease in the average of this phase, and these results indicate that the four compounds as a potent inhibitor of cell cycle progression at the G2/M phase and might suggest anticancer properties (p value < 0.05).
Next was determined whether the four compounds that perturb DNA content induce apoptosis. Cells undergoing apoptosis have their phosphatidylserine (PS) phospholipid translocated from the inner face of the plasma membrane to the cell surface. As mentioned in materials and methods, the detection of PS was estimated by staining with a fluorescent conjugate of annexin-V, a protein that has a high affinity for PS, followed by flow cytometry analysis. Cells were stained with propidium iodide (PI), which can enter the cell only when the plasma membrane is damaged. Early apoptosis was evaluated by positive for PS, but negative for PI and was distinguished from late apoptotic and necrotic cells estimated by positive for both PS and PI.
Four compounds (2a–d) significantly decreased apoptosis to 47.3 ± 15.6, 29 ± 11.5, 10.6 ± 3.4, and 8.3 ± 2.6%, respectively. Figure 3 shows the averages of treatment of MCF-7 cells with four compounds, respectively (p value < 0.05 in all groups). Our data suggest that four compounds could have an anticancer potential through the G2-M cell cycle phase arrest of MCF-7 and shifting the cells to necrosis.
3.3 Antimicrobial Activity
The compounds studied in this work showed antibacterial activity as shown in Table 2. Compound 2c showed significant activity at concentrations of 3.125–6.25 mg/ml against all bacterial strains, except the Candida strain. Compound 2d showed good antibacterial activity at a concentration range of 3.125–6.25 mg/ml, except the MRSA and Candida. 2a was the only compound that showed some activity against Candida at a concentration of 1.56 mg/ml, and it had antibacterial activity against S. aureus, P. vulgaris, E. faecium, and MRSA at concentrations of 3.125, 6.25, 6.25, and 3.125 mg/ml, respectively. 2b compound showed the highest antibacterial activity for S. aureus, P. vulgaris, K. pneumonia, and MRSA with concentrations of 3.125, 3.125, 6.25, and 6.25 mg/ml, respectively. When we compared the MIC of the four compounds with good antibiotics, we found that the 2a compound has the same MIC as fluconazole (1.56 mg/ml) for the inhibition of the growth of Candida. Moreover, 2a, 2b, and 2d have the same MIC as ampicillin (3.125 mg/ml) for the inhibition of the growth of S. aureus bacteria.
Consequently, future studies are required to assess the active antimicrobials from the synthesis compounds as 2a, 2b, and 2d against a wide range of foodborne bacterial strains and other harmful pathogens for their possible application in the food, cosmetics, and pharmaceutical industries.
4 Conclusion
At the same concentration, 2a compound showed potent antifungal activity the same as the potential antifungal drug fluconazole. Moreover, compounds 2a, 2b, and 2d have the same antibacterial power against S. aureus as the conventional antibiotic ampicillin. In fact, the newly synthesized four compounds (2a–d) significantly decreased apoptosis p < 0.05 in all groups. Our data suggest that these compounds could have an anticancer potential through the G2-M cell cycle phase arrest of MCF-7 and shifting the cells to necrosis. Further studies investigating the antimicrobial and anticancer characters of these compounds in vivo are required to approve these results and to prepare suitable pharmaceutical dosage forms from the active molecules.
Data Availability
All utilized data to support the findings of the current study are included in the article.
References
Abu-Dahab, R.; Afifi, F.: Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7). Sci. Pharm. 75(3), 121–146 (2007)
Hawash, M.: Highlights on specific biological targets; cyclin-dependent kinases, epidermal growth factor receptors, ras protein, and cancer stem cells in anticancer drug development. Drug Res. 69(9), 471–478 (2019)
Mans, D.R.; Da Rocha, A.B.; Schwartsmann, G.: Anti-cancer drug discovery and development in Brazil: targeted plant collection as a rational strategy to acquire candidate anti-cancer compounds. Oncologist 5(3), 185–198 (2000)
Schwartsmann, G.: Marine organisms and other novel natural sources of new cancer drugs. Ann. Oncol. 11(3), 235–243 (2000)
Hawash, M.M.; Baytas, S.N.: Antiproliferative activities of some biologically important Scaffold. FABAD J. Pharm. Sci 43(1), 59–77 (2017)
Daniels, A.L.; Van Slambrouck, S.; Lee, R.K.; Arguello, T.S.; Browning, J.; Pullin, M.J.; Kornienko, A.; Steelant, W.F.: Effects of extracts from two Native American plants on proliferation of human breast and colon cancer cell lines in vitro. Oncol. Rep. 15(5), 1327–1331 (2006)
Hawash, M.M.; Kahraman, D.C.; Eren, F.; Cetin Atalay, R.; Baytas, S.N.: Synthesis and biological evaluation of novel pyrazolic chalcone derivatives as novel hepatocellular carcinoma therapeutics. Eur. J. Med. Chem. 129, 12–26 (2017). https://doi.org/10.1016/j.ejmech.2017.02.002
Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A.: Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomarkers Prev. 9(11), 1163–1170 (2000)
Siegers, C.-P.; Steffen, B.; Röbke, A.; Pentz, R.: The effects of garlic preparations against human tumor cell proliferation. Phytomedicine 6(1), 7–11 (1999)
Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.: Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1, 1046–1050, 1051 (1995)
Gottesman, M.M.: How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 53(4), 747–754 (1993)
Madhuri, S.; Pandey, G.: Some anticancer medicinal plants of foreign origin. Curr. Sci. 96(6), 779–783 (2009)
Aldomere, Y.A.: Synthesis, characterization, antibacterial activities of novel polydentate Schiff’s bases and their transition metal complexes. (2015)
Bhalodia, N.R.; Shukla, V.: Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: an ethnomedicinal plant. J. Adv. Pharm. Technol. Res. 2(2), 104–111 (2011)
Davies, K.; Pryor, W.: The evolution of free radical biology & medicine: a 20-year history. Free Radic. Biol. Med. 39(10), 1263–1264 (2005)
Hussein, A.I.A.: Modification of biologically active compounds from selected medicinal plants in Palestine (2009)
Havlickova, B.; Czaika, V.A.; Friedrich, M.: Epidemiological trends in skin mycoses worldwide. Mycoses 51, 2–15 (2008)
del Olmo, A.; Calzada, J.; Nuñez, M.: Nutrition: benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 57(14), 3084–3103 (2017)
Pizzorno, J.E.; Murray, M.T.; Joiner-Bey, H.: The Clinician’s Handbook of Natural Medicine E-Book. Elsevier, Amsterdam (2016)
Rahman, A.-U.: Studies in Natural Products Chemistry/Edited by Atta-ur-Rahman. Elsevier, Amsterdam (2012)
Patrick, G.L.: An Introduction to Medicinal Chemistry. Oxford University Press, Oxford (2013)
Debnath, J.; Muthuswamy, S.K.; Brugge, J.S.: Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30(3), 256–268 (2003)
Nunez, R.: DNA measurement and cell cycle analysis by flow cytometry. Curr. Issues Mol. Biol. 3, 67–70 (2001)
Forbes, B.A.; Sahm, D.F.; Weissfeld, A.S.: Study Guide for Bailey and Scott’s Diagnostic Microbiology-E-Book. Elsevier, Amsterdam (2016)
Wikler, M.A.: Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement. Clinical and Laboratory Standards Institute, New York (2007)
Evans, B.A.J.; Griffiths, K.; Morton, M.: Inhibition of 5α-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 147(2), 295–302 (1995)
Acknowledgements
The authors would like to acknowledge the Faculty of Graduate Studies, Pharmaceutical Sciences Program, at An-Najah National University for facilitating the accomplishment of the current study.
Funding
None.
Author information
Authors and Affiliations
Contributions
The current research was done by the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
N/A.
Rights and permissions
About this article
Cite this article
Jaradat, N., Khasati, A., Hawi, M. et al. In vitro Antitumor, Antibacterial, and Antifungal Activities of Phenylthio-Ethyl Benzoate Derivatives. Arab J Sci Eng 46, 5339–5344 (2021). https://doi.org/10.1007/s13369-020-05114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05114-0