Abstract
The pyrolysis behavior of Turkish biomass samples such as hazelnut shell, almond shell, and sunflower stalk residue was studied using a thermogravimetric analysis (TGA) laboratory-scale setup. Biomass samples were characterized using the standard method of the Van Soest detergent analysis, and both the virgin biomass and fractions were investigated. The reaction temperature was increased to 900 °C with a heating rate range between 2 and 60 °C min−1 in the TGA experiments. Seven solid-state reaction models were applied to evaluate the obtained experimental TGA results. The heating rate was not the only parameter affecting the values of activation energy and the ratio of the main components such as the cellulose and lignin of the virgin biomass samples (almond shell, sunflower stalk, and hazelnut shell) also affected the value of the activated energy values. It was determined that a model fitting mechanism gives limited information to determine the exact activation energy values for the samples. The reaction order model provided straightforward and decisive results for all the biomass and lignin samples. Models of two- and three-dimensional diffusion were better suitable for the cellulose devolatilization. It was also determined that the activation energy of the lignin samples was similar regardless of the types of biomass. According to the kinetic calculations, the cellulose samples showed the highest activation energy values and the lignin samples had the lowest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to a shortage of fossil fuels and the environmental effect of greenhouse gasses on climate changes, a newfound attention has been focused on alternative energy sources and biomass is an important energy source in this respect. The global energy needs in cities is increasing considerably and biomass resources are becoming increasingly important because of the economic potential and huge amount of the annual volumes of agricultural waste that can be used as a source of energy [1]. Biomass consists of lignocellulosic material produced through plant growth and can originate from natural regrowth forests, plantation forestry, annual field crops, algae production, or from residues of any of the above. Besides, it can also be derived from industrial processes, such as municipal waste, or land clearing operations. Convenient forms of energy from biomasses can be in the form of heat, power, and liquid, solid, and gaseous fuels [2].
The unedible parts of agricultural wastes can be converted to biofuels for example: heat, value-added products, and electricity. Energy is a very important issues for the socioeconomic development of any country, and depleting fossil sources have encouraged research and development on energy recovery from lignocellulosic wastes. According to the Global Status Report (GSR) on energy, the large majority, i.e., 78.4%, is supplied by non-renewable fossil fuels such as petroleum, coal, and natural gases and merely 19% from renewable resources such as solar, hydropower, wind, and biomass. Fossil fuels cause severe environmental problems in nature, and less emission of environmental pollutants is only possible by consuming more renewable energy carriers. The fossil fuels are not only consumed for energetic purpose, but also a source of different chemicals and the depleting fossil fuels reserve is an important issue for different chemical industries. Lignocellulosic biomass materials will become very important as a source of chemical feedstock because of their renewable nature [3].
Pyrolysis is one of the promising thermochemical conversion methods to convert biomass into a valuable commodity. Utilization of biomass resources using pyrolysis has attracted increasing interest, resulting in the production of biooil, different gaseous components, and carbon-rich solid residue. The pyrolysis process involves many complicated series reactions such as dehydration, depolymerization, and bond cleavage and reformation within certain temperature ranges and the behavior of the structural components of a biomass such as cellulose, hemicellulose, and lignin should be examined for optimum process conditions. There are many potential tools to investigate the thermal degradation mechanism of a biomass, and among the different analysis methods, the thermogravimetric analyzer coupled with the Fourier transform infrared (TG-FTIR) spectroscopy and pyrolysis gas chromatography/mass spectrometer (Pyro-GC–MS) are widely used techniques [4].
Biomass mainly consists of three components: cellulose, hemicellulose, and lignin, which have quite different thermal characteristics. The overall performance of the pyrolysis processes is closely related to three components and side components, extractives, and ash. It is possible to determine the pyrolysis product distribution according to the component proportion in a biomass sample [5].
Over the past several years, the thermal decomposition of a biomass using Pyro-GC–MS and TG-FTIR has been applied to determine pyrolysis products and their involvement with the structural parts of a biomass such as cellulose, hemicellulose, and lignin. The relevant literature about the three basic components of a biomass in pyrolysis includes many studies on the model components of the biomass structural units such as xylan, cellulose, and lignin (Sigma-Aldrich Chemie GmbH) [6], Alcell lignin [7], kraft lignin [8], cellulose (Toyoroshi Co.) [9], and xylan (Sigma Co.) [10].
The chemical characteristics of a biomass are varied depending on the ratios of the major constituents for example: holocellulose (cellulose and hemi cellulose), lignin, and the extractive mineral contents based on the location of the plantations. The greatest portion of the biomass structure is cellulose, the most abundant natural polymer in the world with lignin being another important phenolic component of the plant biomass. Because the pyrolysis behavior of the major constituents of a biomass is quite different from each other, thermogravimetric analysis (TGA) technique has been used in order to reveal the pyrolysis behavior of a biomass and the major constituents of a biomass material. Many investigations have been accomplished regarding the pyrolysis of a biomass and the major constituent cellulose, but there is a lack of information on the pyrolysis behavior of the lignin fractions of a biomass. The present studies on lignin are mainly on the pure standard of lignin components (organosolv lignin, kraft lignin, and soda lignin), and the pyrolysis behavior of lignin fractionated from the virgin biomass is relatively scarcer.
The TGA technique allows us to predict the properties of a biomass based on mathematical models, and the accurate characterization of samples in thermal decomposition is possible in this way. Various physical and chemical phenomena occur in the thermochemical conversion of a biomass such as evaporation, decomposition, and partial combustion, which require extensive investigation. Because of convenience, a rapid and cost-effective method is needed for large-scale biomass utilization; the thermogravimetric analysis technique has been accepted as an efficient technique that can be used to characterize the properties of a biomass. Thermogravimetric analysis (TGA) is a commonly used technique in the analysis of mass loss data for kinetic parameters [11]. This is important for the process design, feasibility of operation, and scaling for industrial manufacturing [12, 13]. The kinetic methods for the analysis of a biomass were considered by Gai et al. [11]. TGA was used as an effective tool to acquire the data for the proximate analysis of solid fuels [14]. There are many factors affecting the thermogravimetric analysis of biomass samples. Factors can be listed as: the heating rate, sample types, sample size, gas types, and the flow rate of gas, and these have been studied and reported in previous studies [11, 15,16,17,18,19,20]. In the present study, the pyrolytic behavior of lignin, cellulose, and virgin biomass has been investigated and the understanding of the pyrolysis characteristics of the major fractions of biomass is very important in the utilization of biomass waste for thermal processing units.
Biomass pyrolysis mechanisms can be best understood by measuring the biomass conversion, and the kinetics of pyrolysis reaction can be defined using three key parameters such as activation energy, pre-exponential factor, and the reaction model. Key parameters are determined from the results of thermogravimetric studies, and the activation energy value from the TGA data has been reported by many researchers. Agrawal and Chakraborty [21] treated the whole pyrolysis of biomass and used regression analysis to evaluate the key parameters. Jun-Ho et al. [22] determined the activation energy from the TGA data of food wastes, and the kinetic equations were obtained. The Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) methods were applied to calculate the activation energies of a biomass [23]. The activation energy and pre-exponential factors were determined from the TGA data of lignin, cellulose, and the whole agricultural biomass. Different lignin components (Hardwood lignin, kraft lignin, industrial lignin, alkali, hydrolytic, organosolv, and Klason lignin), agricultural wastes (corn cob, peanut shell, and pinecone), and cellulose were employed in these studies. The samples were pyrolyzed in wide range of heating rates (2–200 K/min), and reported activation energy values were varied in the range of 25–360 kJ/mol depending on the sample types and experimental conditions [12, 17, 24,25,26,27,28,29].
2 Experimental
2.1 Materials and Method
2.1.1 Material
Production and consumption of nuts is very important for human health, and nuts have been primarily recommended for problems related to coronary heart disease, blood sugar, cholesterol, diabetes, and also because of antioxidants, vitamin, and mineral sources characteristics. They are recommended to be consumed on a daily basis in suitable amounts. Turkey has the advantages of producing different types of nuts due to the existence of various climate conditions. The production of nuts is increasing because of the increasing domestic and international demand year by year. The most important and commonly produced types in Turkey are hazelnuts, walnuts, and almonds. The quality of hazelnuts is very high, and it is internationally accepted. Turkey is the most important country for the production and export of hazelnuts. According to the literature, 4 million inhabitants of Turkey depend directly upon the production, marketing or processing of this product.
Hazelnuts are cultivated in an area of around 350,000 ha in Turkey, and the main production area is spread all along the Black Sea Region and extends up to 30 km in land. The average annual hazelnut production during the last 5 years has been reported as around 600,000 tons in shell and is almost 70–75% of the hazelnut crops of the world [30].
Almond is produced in cities of the Aegean and Mediterranean region, and the main almond-producing provinces in Turkey are Muğla and Mersin. One quarter of the Turkish almond production is carried out in these cities. The Datça district of Muğla and Bozyazı and the Anamur district of Anamur have been reported as the important regions for almond productions. Besides these cities, Antalya, Isparta, Denizli, and Elazığ have a certain capacity for the production of almonds in Turkey. The average annual almond production has been reported as around 50,000 tons with an area of around 20,000 ha.
One of the most important raw materials of the vegetable oil sector is the sunflower, and it is also the most abundantly produced oil seeds in Turkey. The cultivation areas of sunflower have been around 500,000–600,000 ha for many years. The Thrace-Marmara Region is the most important area in Turkey for the production of sunflowers. Tekirdağ, Edirne, Konya, Kırklareli, and Adana are the cities where sunflowers are mostly produced. The average annual sunflower production has been reported as around 1,500,000 tons [31].
The agricultural crop residues in Turkey are very high due to climatic conditions and crop production with a wide range of diversity. The available energy potential of the agricultural crops is extremely high, and development of suitable technologies for energy and chemical feedstock is very important. Thermochemical conversion technologies are one of the promising technologies for utilization of biomass waste. Devolatilization is the first stage of all the thermochemical conversion processes, and the behavior of agricultural waste with different cellulose and lignin ratios should be examined under slow pyrolysis conditions to understand the structural effect of biomass in the thermochemical conversion. It is necessary to develop more efficient thermochemical processing technologies involving lignocellulosic waste conversion. Cellulose, hemicellulose, and lignin are the main constituents of plant biomass, and in this study, the sunflower stalk, almond shell, and hazelnut shell were selected as a sample of the waste biomasses because of the cellulose, hemicellulose, and lignin rich structures of the selected samples, respectively.
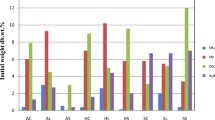
In this study, nine different biomass samples were analyzed: hazelnut shell (rich in lignin and poor in holocellulose), hazelnut lignin, hazelnut cellulose, sunflower stalk (rich in extractive and holocellulose and rich in ash), sunflower lignin, sunflower cellulose, almond shell (rich in holocellulose, poor in extractive), almond shell lignin, and almond shell cellulose using the TGA device. The origin of the virgin biomass samples hazelnut shell, almond shell, and sunflower stalk were collected from urban areas of Turkey. The hazelnut shell samples investigated in this study were produced in Giresun, located in the Black Sea Region of Turkey. The almond shell was obtained from the city of Muğla-Datça located in the Aegean region, of the western part of Turkey. Finally, the sunflower stalk sample was produced in the agricultural area around the city of İzmir located in the West or Aegean Region of Turkey. The biomass samples, after being collected, were ground and sieved to a particle size less than 100 µm. The composition of the biomass samples is given in Table 1. The fractionation of the biomass samples to lignin and cellulose was accomplished based on the standard test method of Van Soest detergent [32]. In this method, an NDF (neutral detergent fiber) analysis was used to determine the total cellulose, hemicellulose, and lignin as main components of the biomass samples, then an ADF (acid detergent fiber) analysis was employed to determine the total amount of cellulose and lignin, and finally an ADL (acid detergent lignin) analysis was accomplished to find the total amount of lignin in the biomass samples. The Van Soest process was repeated three times for each sample, and the average values of the results are presented in Table 1. There was no deviation among the results of the analysis.
2.1.2 Isolation of Cellulose
A sample of biomass (40 g) was extracted with toluene ethanol (2:1 volumetric ratio) for 6 h. The extractive materials were separated from the samples in this way. The residue of the extraction process was re-extracted with water at 70 and 80 °C, respectively. At the end of this operation, the water-insoluble fraction and filtrate (including water-soluble hemicelluloses fraction) were separated. Once the water was separated, the lignin content of the water insoluble part was removed by heating at 75 °C for 2 h in the presence of 3% NaClO with the pH value of the mixture adjusted to 3.5–4.0 using 6 M acetic acid. At the end of this process, the insoluble part including holocellulose (cellulose + hemicelluloses) and filtrate including soluble fraction (lignin) were separated. The holocellulose part was extracted with 10% KOH or 10% NaOH (600 mL) for 10 h, at 20 °C. Afterward, the insoluble part involving cellulose and filtrate including alkali-soluble hemicelluloses fraction were separated.
2.1.3 Isolation of Lignin
A 5 g biomass sample was added to and well-mixed with 10 mL of dimethylaniline in a round-bottom flask. Then, this primary mixture was mixed with an H2SO4 solution (78% by weight), and the secondary mixture was stirred for 4 h at room temperature. Thereafter, 312.5 mL of water was added and mixed for 4 h at room temperature and then 1.6 L of water was added, and the mixture was boiled for 6 h using a condenser connected to the round-bottom flask. Each of the water addition steps reduced the concentration of H2SO4 from 28.8 to 3.26%, respectively. At the end of this operation, the final mixture was filtered and the residue, including the lignin and, the filtrate involving cellulose degradation products were separated. Finally, the lignin fraction was dried in oven at 105 °C.
2.1.4 Method
The apparatus used in this study, regarding the characterization of biomass, is a combination of a Thermobalance SDT 2960, from TA Instruments, connected via a short stainless-steel heated line (at 150 °C) to a NEXUS FTIR spectrometer, manufactured by Thermo Nicolet. The TGA tests were carried out using a flowing inert gas, and gas was the carrier with a flow rate of 100 mL/min. The carrier gas flow was adjusted to prevent secondary reactions in the oven and to obtain suitable concentrations of the components in the pyrolysis gas.
The thermogravimetric analyzer (TGA) consists mainly of a balance with a sensitivity of ± 1 mg to which two beams are connected. One of the beams is loaded with the test sample, and the other is used as reference. On the tip of the beams, a thin thermocouple is placed in order to, at slow heating rates, to assume the possibility that this temperature is the actual temperature in the sample. Both beams are inserted into a small ceramic furnace which is heated electrically. The achieved heating rates are up to 100 °C/min. The process is remotely controlled via a PC. In this paper, tests are performed at 2, 20, 40, and 60 °C/min in order to characterize the pyrolysis behavior and data for the reaction kinetics.
The samples were ground and placed in an alumina cup in the TGA in amounts varying between 15 and 28 mg. Then, the TGA run started with selecting the parameters concerning the pyrolysis test and flushing the oven and the FTIR gas cell with nitrogen of 100 mL/min flow for 20 min at 30 °C in order to blow all atmospheric gases. Then, the temperature was increased by 20 °C/min to 120 °C and staying isothermally at this temperature for 20 min in order to evaporate all moisture. After this isothermal period, the temperature was increased by 20 °C/min up to 900 °C for all samples. Then, air was inserted into the TGA and the pyrolytic solid residue was combusted at 900 °C. During each experiment, the FTIR would stop collecting spectra after the TGA test finished. The result of the FTIR and the effect of heating rate on the identified gases from the setup (CO, H2O, CO2, and CH4) are not given in this paper because mass loss data from the TGA analysis were employed in the kinetic calculations. The heating rate dependence of the biomass structure and evaluation of gas products will be presented later in a separate paper.
2.1.5 Kinetic Studies
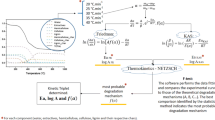
However, the decomposition process has a complicated reaction mechanism, and the decomposition process can be represented with the following reaction scheme in many research papers for the simplicity of calculation and interpretation of reaction parameters (activation energy and pre-exponential constant)
The conversion rate dx/dt regarding the TG experiment with a constant heating rate, β = dT/dt, can be expressed as follows:
where \( f(x) \) = function of conversion, such as (1 − x) for first-order reaction or other model equations as seen in Table 2, E = activation energy, A = Arrhenius factor, R = gas constant, x = conversion defined by:
where w = instantaneous sample weight at a certain time t, w0, wf = initial and final sample weight, respectively.
Integration of Eq. 2 gives the Coats–Redfern Equation:
where g(x) = integrated form of \( f(x). \)
In this study, the reaction mechanisms and expressions f(x) and g(x) regarding the description of the solid-state reactions were taken from the literature [14, 33] and are also presented in Table 2.
Coats and Redfern developed a graphical method concerning kinetic parameters determination of solid decomposition [13]. Their analysis is performed by plotting ln[g(x)/T2] versus 1/T. Thus, to determine the most probable model the Coats–Redfern method is used. All reaction mechanisms presented in Table 2 were fitted to the experimental data, and activation energies are found from the slope of the best fitted curve. The results of the calculations are given in Tables 3, 4 and 5.
3 Results and Discussion
3.1 Thermogravimetric Analysis of Samples
The TGA mass loss data of the biomass samples show a strong dependence on the both sample types and heating rate. The difference between the initial dry sample mass and the total mass of the pyrolytic residue (char) increases with an increased heating rate. The changes in mass as a function of temperature and time for each experiment are presented in Figs. 1, 2, 3, 4, 5, 6, 7, 8, and 9, and the combustion of char residue was carried out above 900 °C by switching the carrier gas nitrogen to air and a higher mass loss of char by forming CO2 gases was observed in the low heating rates. Among all the biomass samples, the highest derivative mass loss rate was observed in the lignin samples, and the almond lignin gave the highest mass loss for the same heating rates. The amount of char residue depends on not only the heating rates but also the types of biomass and lignin samples and also, whole biomass samples result in the largest char production. When comparing the percentages of extractive materials, lignin, and ash among the biomass samples, the larger percentages of ash and extractive was found in the sunflower stalk. However, the percentages of lignin are lower in the sunflower stalk. The higher percentages of ash in the sunflower stalk could be explained by the function of stalk in the plant biomass. The stalk conducts water, minerals, and food to other parts of the plant and the mineral content of the water can precipitate in the stalk structure over time. Among all the samples tested, the largest amount of conversion was obtained for the cellulose samples and lignin showed the lowest.
The cellulose extracted from the sunflower stalk resulted in a higher char residue because the alkali metal in ash tends to catalyze the depolymerization of cellulose polymer and in the absence of ash in cellulose, the decomposition rate was lowered yielding higher char residue. In the TGA analysis of the cellulose samples, it was observed that degradation occurred at relatively narrow peaks as stated in former studies in the literature [34]. Among the samples tested, the weight loss of sunflower cellulose starts first, below 200 °C and the other two samples of almond shell and hazelnut shell start at approximately 215 °C. It can be explained that the relatively high ash content and impurities are due to the isolation stages of the cellulose fraction from the sunflower stalk. In the literature as stated, cellulose degradation tends to form active species and subsequent reaction of it via decarboxylation and decarbonylation reactions result with the formation of gaseous species such as CO and CO2 [27, 35] and it may reflect as mass loss to TGA data. Mass loss around 400 °C is a small amount, and the highest mass loss was observed at 350 °C for the cellulose samples. This also agrees with the related findings in the literature [36]. Sunflower cellulose degradation represents two separate peaks: one at nearly 260 °C and the second at around 330 °C. This distinctive situation was explained by the presence of inorganic minerals that were present in the sunflower stalk. The chemical isolation of cellulose yielded a product including relatively small amounts of impurities in the forms of ash due to the high ash content of the virgin biomass from which the cellulose will be isolated. These mineral materials behave as an inorganic catalyst and tend to degrade the cellulose of the sunflower stalk at a relatively low temperature. The chemical composition of the ash was analyzed, and the potassium, calcium, and magnesium minerals were found higher for sunflower stalk than the other two virgin biomass samples (almond and hazelnut shell). The high K content of the sunflower stalk can be due to two reasons. The first reason is chemical contaminations during separation of cellulose and hemicellulose fractions from the virgin biomass where KOH was used in the operation; the second reason is that the axial structural parts of the stalk are responsible for conducting water to the plant and the mineral content of water tends to precipitates in the stalk structure. The mass loss of the lignin samples starts at around 170–200 °C and finishes the degradation at nearly 700 °C. The devolatilization and gas evolution stages of lignin occur in a wider temperature range [37] and show a single peak in contrast to the mass loss peak for the cellulose samples.
The complicated molecular structure of lignin requires high activation energy for decomposition and the reaction starting at relatively low temperatures continues to high temperatures by forming a char residue via hydrogen deficiency and condensation reactions resulting in a polyaromatic char formation in the lignin pyrolysis. This is the expected case as stated in the literature [38]. It was also observed of a high char formation in lignin pyrolysis in the study by Jensen et al. [39]. Among the samples tested, the mass loss peak of the almond and sunflower lignin samples decomposed at a similar temperature range, but the mass loss peak of hazelnut lignin was higher about 30–35 °C. It was explained by the lowest ash content of the hazelnut lignin among the isolated lignin from the biomass samples. Among the virgin biomass samples tested, mass loss started first for the sunflower stalk and hazelnut shell at around 180 °C, and the fastest reaction of sunflower stalk can be explained by the high mineral content of this sample and the catalytic activity of the mineral part in degradation. The reactivity of a biomass in thermal decomposition is not only related to the mineral content, and also the structural units show different reactivities. The hemicellulose structure is more reactive than the other structural components of a biomass and decompose relatively early in the stages of pyrolysis. Among the samples tested in this study, the almond shell has the highest hemicellulose content and it was observed that the TGA studies of the almond shell at the lowest heating rates give two visible shoulders at 260 and 320 °C, reaching a peak value at 372 °C. The peak shoulders were more observable at low heating rates because low reaction rates make hemicellulose degradation more distinctive and thus, two separate peaks were determined in contrast to the decomposition of the same samples at higher heating rates. According to samples studied, we determined a difference in the thermal behavior between isolated samples and the virgin biomass indicating a structural effect is important in pyrolysis.
Because the fractions tend to degrade at different temperature ranges, initial composition of biomass should be considered for utilization of agricultural waste in pyrolysis at industrial scale. High cellulose and hemicellulose but low in lignin containing biomass samples are much more susceptible for handling together in the operation. High lignin containing biomass gives more char fraction because of a complicated high molecular weight structure and high ash containing biomass ratio should be the control in the bulk of biomass because it degrades the pyrolysis vapor to a low molecular weight species, and may accelerate the reaction rate by catalytic activity and also may be the cause of clog in the system due to the sintering of ash with a low melting point.
3.2 Kinetic Evaluations
The investigation of the decomposition characteristics of a virgin biomass and a main component of the biomass can be understood using TGA method and model fitting method and with a second-order reaction model can give more straightforward and decisive results. Many different studies on different biomass samples and model components corresponding to cellulose and hemicelluloses are available in the literature. Activation energy values in the main decomposition range are varied in a wide range. In this study, isolated cellulose and lignin fractions from virgin biomass samples were used and it can be accepted as a unique work in this respect.
Biomass material is a popular source of renewable energy and can also be converted into biofuel with high energetic content. In all thermochemical processes, pyrolysis is always the first step and many complex series of reactions occur during pyrolysis. Pyrolysis reactions are affected by different factors such as the chemical composition of the biomass, heating rate, final temperature, and particle size. In this study, the activation energies for the conversion of the original biomass samples (almond shell, hazelnut shell, and sunflower stalk) and cellulose, lignin fractions isolated from the biomass samples were determined by thermogravimetric analysis. In the kinetic study of the biomass and isolated species from the biomass samples, 7 kinetic models for solid-state rate equations were employed at non-isothermal pyrolysis conditions. Models can be described as a reaction order-geometrical contraction and two- or three-dimensional diffusion. Reaction models applied in this study are given in Table 2.
The TGA results can be successfully used to understand the thermal degradation mechanism of solid fuels. There are many methods to process non-isothermal pyrolysis data for solid-state degradation. These methods can be described as a model fitting method and conversional method. The first gives the best statistical fit as the kinetic model from which activation energy can be calculated. The second needs several kinetic graphs to evaluate the analysis and to determine the kinetic parameters. Different TGA curves at different heating rates for the same conversion value allows for calculations of the kinetic parameters.
In this study, the model fitting method was used for the solid-state reaction kinetic and this method allows for the direct determination of the kinetic parameters using a single (TGA) measurement. In this method, different model functions can be applied as statistically equivalent by giving a high value of the regression coefficient (square of correlation coefficient). The model fitting methods give the data in comparable magnitude, and it can be applied as a straightforward method to at least discuss the effect of heating rates, composition of components, and particle size on the kinetic data, conveniently.
The activation energy and the square of the correlation coefficient revealed that the regression coefficient (R2) values are varied as a function of the conversion rate. We determined that the activation energy for the pyrolysis of all biomass samples was not similar for all of the conversion values between 10 and 95% and that it is also related to the reaction mechanism. It is varied in the whole pyrolysis process for each of the models studied depending on the conversion. In this respect, the second-order reaction model gives more stable values. Toward the end of the non-isothermal pyrolysis range in TGA when the conversion rate reached 95% conversion, the activation energy value decreases. It can be explained by different types of bond dissociation at higher temperature rather than more similar types of bond cleavage in at lower temperatures to the corresponding conversion range as 10–80%.
With the exception of the slow heating rate of the sunflower cellulose, activation energies are almost the same and independent to conversion when the second-order reaction model was applied within the conversion range of 10–80% and this model satisfactorily describes the pyrolysis process rather than the other methods which were used in the calculations. Activation energies for all of the samples were found as very close to each other when the second-order kinetic model was used. The average activation energy values within the conversion range, for which the model was giving a high value for the regression constant, were calculated and compared for all of the samples. The average activation energy values are given in Tables 3, 4 and 5.
The differences in the activation energy values of the virgin biomass samples and isolated cellulose and lignin fractions can verify the effect of the complicated nature of the biomass samples with different cellulose and lignin ratios on the pyrolysis process. The heating rate was not the only parameter affecting the values of the activation energy and the ratio of the main components such as cellulose and lignin of the virgin biomass samples (almond shell, sunflower stalk, and hazelnut shell) also affected the value of the activated energy values. The results of our investigation show that the reaction models do not give a linear for the conversion value of more than 80%, and the reaction mechanism cannot be recognized above this conversion value using the models from which the activation energies were calculated in this paper. Comparison of the calculated activation energy values using models 1–4 and models 5–8 shows that the magnitude of Ea calculated using two- and three-dimensional diffusion models is nearly twice the values of the reaction order and geometrical contraction models. It was determined that the model fitting mechanism gives a limited information to determine the exact activation energy values for the samples. Our result is in agreement with the literature, and it was explained by the change in the reaction mechanism with the extent of the reaction due to the solid–gas reaction and forming of a porous or dense layer on an unreacted solid surface [33].
Thermal decomposition of the biomass samples depends on the weight ratio of its components such as cellulose, hemicellulose, lignin, and extractive materials. We determined that activation energies of the almond shell were found slightly higher than that of the values for the hazelnut shell and sunflower stalk, whereas the values were quite similar for the hazelnut shell and sunflower stalk. The reason for this fact was poorly understood, and it was explained by the higher holocellulose (cellulose and hemicellulose) and less extractive materials contents of the almond shell with respect to the samples of the hazelnut shell and sunflower stalk. Thermal degradation of the biomass sample is affected by the extractive, hemicellulose, cellulose, and lignin ratios of the virgin biomass. According to the literature, higher extractive contents speed up the decomposition process, and the higher reactivity of the hemicellulose and lignin also shows the same effect on degradation. In our study, the higher activation energy values for all heating rates were calculated for low extractives and the lignin containing almond shell. It is also in agreement with the literature [12, 40].
We know that the heating rate affects the decomposition rates of the biomass and maximum decomposition rates shift toward higher temperatures. In this study, for the values of the conversion rate varying from 10 to 70% (or 80% for some samples) the heating rate has an influence on the temperature range for decomposition. Thus, high heating rates stimulate the thermal degradation and at higher heating rates, individual conversions are reached at higher temperatures. This can be explained as a result of heat and mass transfer limitations in TGA studies. Temperature gradients may occur at higher heating rates between the surface of the biomass particle and inside of the sample bulk and devolatilization requires higher temperatures for the same conversion range.
Decomposition of cellulose isolated from virgin biomass samples occurs at lower temperature range for all heating rates with respect to raw biomass and lignin at the same conversion range, but decomposition of isolated lignin samples goes through the widest temperature range. It was explained by various oxygenated functional groups in the structure with a different thermal stability and breakage of the bonds occurring at a different temperature range in the pyrolysis process. In our study, the thermal degradation of lignin samples isolated from different biomass samples showed lower activation energy values relative to the samples of cellulose and virgin biomass. Because of complex structure of lignin, thermal degradation behavior is affected by chemical constituents, heating rate, and degradation temperature.
Lignin samples isolated from three different biomass samples with different lignin ratios showed the same behavior in the pyrolysis process, and we obtained quite similar activation energies for each lignin samples at all heating rates. It verifies that there are no significant structural differences between the isolated lignin samples from each biomass type.
Among the three types of samples studied, the activation energy of the cellulose samples was found to be higher in value than that of lignin. The lignin degradation rate is slower than cellulose and occurs in a broader temperature range with respect to cellulose because of less thermally stability. The lower activation energy values of lignin were assigned to parallel, successive, and competitive reactions leading low activation energies. Many studies were carried out on pyrolysis of lignin and it was determined that thermal degradation of lignin is affected by heat and mass transfer effects and which also affect the activation energy of lignin [40]. Different types of functional groups in the structure of lignin with different thermal stabilities decompose at different temperatures giving relatively low molecular weight products and highly reactive free radicals aiding further reaction to form low molecular volatile products from the complicated lignin structure, and it may lower the activation energy of thermal decomposition. Although the degradation rate of lignin is slower because of its stability, residual fraction of lignin after pyrolysis was found to be higher with respect to the residual fraction of cellulose pyrolysis. It was determined in the TGA analysis by burning of the pyrolysis residue for each run when the pyrolysis was over by switching the gas flow from nitrogen to air. The weight derivative of the residual mass in combustion was always calculated higher than that of the cellulose residue in pyrolysis.
Finally, the synergistic effect of a structural unit of biomass was examined using the activation energy values of cellulose, lignin, and the weight fraction of each. Hypothetic activation energy values were calculated by multiplying of the activation energy values of cellulose and lignin with their weight ratios and the sum for each were compared with the activation energy values of the virgin biomass. Table 6 represents the hypothetic and real data for activation energies of the samples tested. The hypothetic activation energy was similar for sunflower stalk, but the high extractive ratio of sunflower stalk must be taken into account in comparison because cellulose and lignin fractions cover approximately 78 wt% of the entire mass. The related values for almond shell and hazelnut shell were approximately 95 wt% and 90 wt%, respectively. By assuming the activation energy of the holocellulose fraction (cellulose + hemicellulose) is roughly equal to the value of activation energy of cellulose for each of the biomass samples, we can get almost the same hypothetic activation energy values for almond and hazelnut shells with whole biomass indicating there is no specific synergistic effect between the organic structural parts of the samples. But the low activation energy for the sunflower stalks indicates the catalytic effect of the mineral content on the devolatilization process.
4 Conclusion
TGA was used to characterize whole Turkish Biomass samples (almond shell, hazelnut shell, and sunflower stalk samples and their isolated cellulose and lignin fractions) conversion under slow devolatilization conditions in a nitrogen atmosphere at different heating rates (2, 20, 40, and 60 K/min). The objectives of this work were to investigate devolatilization kinetics during pyrolysis and to identify characteristic differences in the pyrolysis behavior of three widely used biomass feedstocks from Turkey and their cellulose and lignin fractions. It was shown that the lignin content of any biomass feedstock is the key factor in the devolatilization process because of slower decomposition with a wide temperature range and high char residue with low yield of volatile component. A total of seven different devolatilization models were applied to estimate the kinetic parameters using the mass loss data from thermogravimetric analysis. The applicability of the findings of the TGA experiments can be explained as the characteristics that a biomass fraction plays in a fundamental role in the design and operation of a pyrolysis reactor. Biomass with a higher cellulose and hemicellulose content decomposed faster and produced a larger fraction of volatile products than the biomass with a higher lignin content. A biomass containing high amounts of lignin led to a larger fraction of char products than a biomass containing low amounts of lignin in pyrolysis. It can be concluded that an industrial pyrolysis can be optimized by temperatures in the reacting system with respect to the specific composition in cellulose, hemicellulose, and lignin of the biomass feedstock. We recommended that whole biomass containing a higher lignin content needs higher pyrolysis temperatures and longer retention time than a biomass with a higher holocellulose content. The mass loss of sunflower stalk samples was determined to be higher due to the significantly high ash content. However, the cellulose content in the sunflower stalk is the largest among the whole biomass samples tested, and the mineral content behaves as a catalyst to speed up the reaction rate and decomposition became faster among the biomass samples tested. The devolatilization behavior of cellulose and lignin fractions is quite different from each other with respect to temperature range for decomposition, rate of the reaction, and char yield, and it was also verified by TGA data based on activation energies. The fraction characteristics may affect the product composition in pyrolysis, but the data for the decomposition of a biomass fraction cannot be used to estimate the total mass loss behavior of the whole biomass. The holocellulose content of almond shell and sunflower stalk is almost similar, but the activation energy for these biomass samples is quite different. The activation energy for sunflower stalk was found to be 65.2 kJ/mol, whereas the value was 84.2 kJ/mol for almond shell. The difference was determined as the different lignin and ash contents of these biomass samples, especially ash, which tends to accelerate the decomposition by catalytic activity, and the cellulose samples did show similarities with a wood-derived cellulose, whereas whole biomass samples showed similarities only with other agricultural residues.
Based on the results obtained in the present study, it can be suggested that the pyrolysis behavior depends on the chemical structure of a biomass. The components of the biomass should be considered in the pyrolysis of comingled biomass waste for value-added product formation. There is a need to develop a preparation approach for the utilization of different biomass and facilitate industrial production of pyrolysis oil and other platform chemicals. Future perspective of the study will be the evaluation of the decomposition products of the virgin biomass and the cellulose and lignin samples isolated from the original biomass by TG-FTIR coupling. In this way, the volatile product evaluation rate and their relations with the structural units will be online monitored and examined in a detailed way.
References
Moreno, M.A.P.; Manzano, E.S.; Moreno, A.J.P.: Biomass as renewable energy. Worldwide research trends. Sustainability 11, 863 (2019)
Nunes, L.J.R.; Causer, T.P.; Ciolkosz, D.: Biomass for energy: a review on supply chain management models. Renew. Sustain. Energy Rev. 120, 109658 (2020)
Kumara, B.; Bhardwaja, N.; Agrawala, K.; Chaturvedib, V.; Vermaa, P.: Current perspective on pretreatment technologies using lignocellulosic biomass: an emerging biorefinery concept. Fuel Process. Technol. 199, 106244 (2020)
Chena, W.H.; Wanga, C.W.; Ongd, H.C.; Showe, P.L.; Hsiehf, T.H.: Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 258, 116168 (2019)
Qu, T.; Guo, W.; Shen, L.; Xiao, J.; Zhao, K.: Experimental study of biomass pyrolysis based on three major components: hemicellulose, cellulose, and lignin. Ind. Eng. Chem. Res. 50, 10424–10433 (2011)
Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C.: Characteristics of hemicellulose, cellulose, and lignin pyrolysis. Fuel 86(12–13), 1781–1788 (2007)
Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V.: Effect of the temperature on the composition of lignin pyrolysis products. Energy Fuels 24(8), 4470–4475 (2010)
Ohra-aho, T.; Linnekoski, J.: Catalytic pyrolysis of lignin by using analytical pyrolysis-GC–MS. J Anal Appl Pyrolysis 113, 186–192 (2015)
Hosoya, T.; Kawamoto, H.; Saka, S.: Cellulose–hemicellulose and cellulose–lignin interactions in wood pyrolysis at gasification temperature. J. Anal. Appl. Pyrolysis 80(1), 118–125 (2007)
Waters, C.L.; Janupala, R.R.; Mallinson, R.G.; Lobban, L.L.: Staged thermal fractionation for segregation of lignin and cellulose pyrolysis products: an experimental study of residence time and temperature effects. J. Anal. Appl. Pyrolysis 126, 380–389 (2017)
Gai, C.; Dong, Y.; Zhang, T.: The kinetic analysis of the pyrolysis of agriculture residue under non-isothermal conditions. Bioresour. Technol. 127, 298–305 (2013)
Ferdous, D.; Dalai, A.K.; Bej, S.K.; Thring, R.W.: Pyrolysis of lignins: experimental and kinetics studies. Energy Fuels 16, 1405–1412 (2002)
Giuntoli, J.; Arvelakis, S.; Spliethoff, H.; de Jong, W.; Verkooijen, A.H.M.: Quantitative and kinetic thermogravimetric fourier transform infrared (TG-FTIR) study of pyrolysis of agricultural residues: influence of different pretreatments. Energy Fuels 23, 5695–5706 (2009)
Aboulkas, A.; El Harfi, K.; El Bouadili, A.K.; Benchanaa, M.; Mokhlisse, A.; Outzourit, A.: Kinetics of co-pyrolysis of Tarfaya (Morocco) oil shale with high-density polyethylene. Oil Shale 24, 15–33 (2007)
Lin, Y.C.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W.: Kinetics and mechanism of cellulose pyrolysis. J. Phys. Chem. C 113, 20097–20107 (2009)
Lora, J.H.; Glasser, W.G.: Recent industrial applications of lignin: a sustainable alternative to nonrenewable materials. J. Polym. Environ. 10, 39–48 (2002)
Mani, T.; Murugan, P.; Mahinpey, N.: Determination of distributed activation energy model kinetic parameters using simulated annealing optimization method for nonisothermal pyrolysis of lignin. Ind. Eng. Chem. Res. 48, 1464–1467 (2009)
Brebu, M.; Cazacu, G.; Chirila, O.: Pyrolysis of lignin—a potential method for obtaining chemicals and/or fuels. Cellul. Chem. Technol. 45, 43–50 (2011)
Min, K.: Vapor-phase thermal analysis of pyrolysis products from cellulosic materials. Combust. Flame 30, 285–294 (1977)
Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.: Characterization and prediction of biomass pyrolysis products. Fuel Energy Abstr. 37(5), 611–630 (2011)
Aggarwal, P.; Dollimore, D.; Heon, K.: Comparative thermal analysis study of two biopolymers, starch and cellulose. J. Therm. Anal. 50, 7–17 (1997)
Jun-Ho, J.; Seung-Soo, K.; Jae-Wook, S.; Ye-Eun, L.; Yeong-Seok, Y.: Pyrolysis characteristics and kinetics of food wastes. Energies 10(8), 1191 (2017)
Wu, Z.; Wang, S.; Zhao, J.; Chen, L.; Meng, H.: Pyrolytic behavior and kinetic analysis of wheat straw and lignocellulosic biomass model compound. Adv. Mater. Res. 860–863, 550–554 (2013)
Chan, R.W.; Krieger, B.B.: Kinetics of dielectric-loss microwave degradation of polymers: lignin. J. Appl. Polym. Sci. 26, 1533–1553 (1981)
Nunn, T.R.; Howard, J.B.; Longwell, J.P.; Peters, W.A.: Product compositions and kinetics in the rapid pyrolysis of milled wood lignin. Ind. Eng. Chem. Process Des. Dev. 24, 844–852 (1985)
Caballero, J.A.; Font, R.A.; Marcilla, J.; Conesa, A.: New kinetic model for thermal decomposition of heterogeneous materials. Ind. Eng. Chem. Res. 34(3), 806–812 (1995)
Li, B.; Lv, W.; Zhang, Q.; Wang, T.; Ma, L.: Pyrolysis and catalytic pyrolysis of industrial lignins by TG-FTIR: kinetics and products. J. Anal. Appl. Pyrolysis 108, 295–300 (2014)
Caballero, J.A.; Font, R.; Marcilla, A.: Study of the primary pyrolysis of Kraft lignin at high heating rates: yields and kinetics. J. Anal. Appl. Pyrolysis 36, 159–178 (1996)
Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V.: A systematic study of the kinetics of lignin pyrolysis. Thermochim. Acta 498, 61–66 (2010)
Erdogan, C.;, Strossman, C.: Turkey tree nuts annual report. Global Agricultural International Network. Gain Report-TR8029, pp. 1–4 (2018)
Konyalı, S.: Sunflower production, consumption, foreign trade and agricultural policies in Turkey. Soc. Sci. Res. J. 6(4), 11–19 (2017)
VanSoest, P.J.; Robertson, J.B.; Lewis, B.A.: Symposium: carbonhydrate methodology, metabolism and nutritional implications in dairy cattle. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74(10), 3583–3597 (1991)
Kahrizsangi, R.E.; Abbasi, M.H.: Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans. Nonferrous Met. Soc. China 18, 217–221 (2008)
Várhegyi, G.; Antal, J.; Jakab, E.; Szabó, P.: Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 42, 73–87 (1997)
Yan, R.; Yang, H.P.; Chin, T.; Liang, D.T.; Chen, H.P.; Zheng, C.G.: Influence of temperature on the distribution of gaseous products from pyrolyzing palm oil wastes. Combust. Flame 142, 24–32 (2005)
Worasuwannarak, N.; Sonobe, T.; Tanthapanichakoon, W.: Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. J. Anal. Appl. Pyrolysis 78, 265–271 (2007)
Guerrero, M.B.; Beceiro, J.L.; Jiménez, P.E.S.; Cosp, J.P.: Comparison of thermal behavior of natural and hot-washed sisal fibers based on their main components: cellulose, xylan and lignin. TG-FTIR analysis of volatile products. Thermochim. Acta 581, 70–86 (2014)
Joffres, B.; Laurenti, D.; Charon, N.; Daudin, A.; Quignard, A.; Geantet, C.: Thermochemical conversion of lignin for fuels and chemicals: a review. Oil Gas Sci. Technol. 68, 753–763 (2013)
Jensen, A.; Dam-Johansen, K.; Wójtowicz, M.A.; Serio, M.A.: TG-FTIR study of the influence of potassium chloride on wheat straw pyrolysis. Energy Fuels 12, 929–938 (1998)
Brebu, M.; Vasile, C.: Thermal degradation of lignin—a review. Cellul. Chem. Technol. 44(9), 353–363 (2010)
Acknowledgements
The authors gratefully appreciate the financial support of The Scientific and Technological Research Council of Turkey (TÜBİTAK) (Project No: 106T748), and we would like to thank the Council of Higher Education (Turkey) for the scholarship. The authors appreciate the Karlsruhe Institute of Technology, for analyzing the elemental composition of the biomass samples. The authors also appreciate the TU Delft/Faculty of 3 mE, Department of P&E for the TGA-FTIR analysis of the biomass samples. Finally, the authors would like to thank Mr. G. Serin for his support in the isolation steps of the biomass samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ballice, L., Sert, M., Sağlam, M. et al. Determination of Pyrolysis Kinetics of Cellulose and Lignin Fractions Isolated from Selected Turkish Biomasses. Arab J Sci Eng 45, 7429–7444 (2020). https://doi.org/10.1007/s13369-020-04594-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04594-4