Abstract
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease affecting the central nervous system as a result of reactivation of the John Cunningham (JC) polyomavirus and occurs almost exclusively in immunosuppressed individuals. The disease course of PML is variable but usually progressive and often fatal. Treatment is predominantly focused on immune restoration, although this is difficult to do outside of human immunodeficiency virus–associated PML. A recent case series demonstrated a potential role for programmed cell death protein 1 (PD-1) inhibitors, such as pembrolizumab, to contain and/or clear JC virus. Herein, we discuss the first reported Australian case of a 61-year-old female with PML secondary to chemoimmunotherapy demonstrating complete clearance of JC virus as well as clinical and radiological stabilisation following pembrolizumab treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case presentation
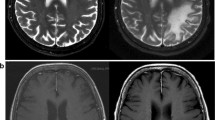
A 61-year-old female presented with a 3-week history of progressive homonymous hemianopia and deteriorating visual acuity. History was significant for treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) 14 years previously for diffuse large B cell lymphoma; rituximab monotherapy 8 years previously for low-grade follicular lymphoma; rituximab and bendamustine 3 years previously for relapsed symptomatic low-grade follicular lymphoma; and current treatment with maintenance rituximab and intravenous immunoglobulin for hypogammaglobulinaemia. She underwent magnetic resonance imaging (MRI) of the brain, which demonstrated bilateral occipital asymmetric expansile white matter enhancement consistent with a leukoencephalopathy. Due to concerns for posterior reversible encephalopathy syndrome (PRES) secondary to hypertension, antihypertensive therapy was commenced. Rituximab and immunoglobulin treatment was ceased. Total T cell count was 640/μL, with CD4 count of 304/μL and CD8 count of 340/uL. CD20 positive B cells were near absent. Lumbar puncture was performed, with cerebrospinal fluid demonstrating lymphocytosis (mixture of T cells with preserved CD4:CD8 ratio) and positive qualitative JC virus nucleic acid by real-time polymerase chain reaction (performed on JCV ELITe MGB assay, ELITechGroup). Plasma JC virus nucleic acid was also detected on the same assay. Visual acuity continued to deteriorate, with gross homonymous hemianopia. Repeat MRI performed 8 weeks later demonstrated extension into the right corpus callosum, bilateral temporal lobe, and left frontal lobe. Mefloquine was commenced with an initial dose of 750 mg followed by 250 mg weekly for 5 weeks with no clinical or radiological response. Three months after initial presentation, pembrolizumab was initiated at a dose of 2 mg/kg (150 mg) monthly and given for a total of three doses, having to be ceased due to a lichenoid drug eruption thought likely secondary to therapy and requiring corticosteroids and acitretin. There was subsequent stabilisation of symptom burden, with repeat MRI performed 3 months after commencement of pembrolizumab showed marginal improvement in occipital white matter changes and no progression of lesions elsewhere (Fig. 1). No clinical or radiological features of immune reconstitution syndrome were observed. Repeat CSF analysis was performed 5 months after commencement of therapy, with normal biochemical profile and no detectable qualitative JC virus. Plasma analysis was also negative for JC virus.
Discussion
PML occurs almost exclusively in immunosuppressed individuals,(Anand et al., 2019; Tan & Koralnik, 2010) with marked variance in clinical outcome seen in those able to achieve immune reconstitution.(Gasnault et al., 2011; Pavlovic et al., 2015) PML secondary to chemoimmunotherapy carries an extremely poor prognosis, with an overall median survival of 2 months and a mortality rate of 88%.(Anand et al., 2019; Adrianzen Herrera et al., 2019) A recent case series in the New England Journal of Medicine evaluated eight patients with PML treated with pembrolizumab, of which four had PML secondary to chemoimmunotherapy for a lymphoproliferative disorder.(Cortese et al., 2019) The cohort included two patients with chronic lymphocytic leukaemia, both having received rituximab and fludarabine as part of their protocol, one man treated for Hodgkin’s lymphoma over 40 years previously, and another with unspecified non-Hodgkin’s lymphoma treated with rituximab. A substantial decline in JC virus load, in addition to clinical response, was observed in two of these patients; one had no clinical, radiological, or virological response; and another had progressive disease. Our patient discussed here had previously been treated with the alkylating agent bendamustine, well as having concurrent treatment with the anti-CD20 monoclonal antibody rituximab. Both these agents can cause prolonged B lymphocyte depletion, with the latter particularly associated with PML.(Carson et al., 2009; Warsch et al., 2012) Following three doses of the PD-1 inhibitor pembrolizumab, our patient demonstrated complete clearance of JC virus, the first reported case using this novel therapy in Australia. Whilst clinical stabilisation was achieved, the patient did not achieve any improvement in visual acuity or visual fields, and the radiological improvement was minimal. The CSF lymphocytosis at diagnosis may have reflected a degree of immune reconstitution, although the patient had progressive disease despite this. We were unable to determine the burden of JC virus at time of diagnosis (as no quantitative level was performed and able to obtained retrospectively, although copy threshold was 26.5), and also were unable to determine PD-1 expression on CD4+ and CD8+ lymphocytes within the CSF, which is hypothesised to be increased in patients with PML. Whilst the evidence to date is limited, the complete clearance of JC virus in our patient supports the hypothesis that blocking the PD-1 pathway may reinvigorate T cell activity to mediate an antiviral effect,(Cortese et al., 2019) but further robust clinical trials are needed to demonstrate this.
References
Adrianzen Herrera D, Ayyappan S, Jasra S, Kornblum N, Derman O, Shastri A, Mantzaris I, Verma A, Braunschweig I, Janakiram M (2019) Characteristics and outcomes of progressive multifocal leukoencephalopathy in hematologic malignancies and stem cell transplant – a case series. Leuk Lymphoma 60(2):395
Anand P, Hotan GC, Vogel A, Venna N, Mateen FJ. (2019) Progressive multifocal leukoencephalopathy: a 25-year retrospective cohort study. Neurol Neuroimmunol Neuroinflamm. 6(6)
Carson K, Focosi D, Major E, Petrini M, Richey E, West D, Bennett C (2009) Monoclonal antibody-associated progressive multifocal leukoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a review from the Research on Adverse Drug Events and Reports (RADAR) project. Lancet Oncol 10(8):816–824
Cortese I, Muranski P, Enose-Akahata Y, Ha SK, Smith B, Monaco M et al (2019) Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med 3800:1597–1605
Gasnault J, Costagliola D, Hendel-Chavez H, Dulioust A, Pakianather S, Mazet AA, de Goer de Herve MG, Lancar R, Lascaux AS, Porte L, Delfraissy JF, Taoufik Y, for the ANRS 125 Trial Team (2011) Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PLoS One 6(6):e20967. https://doi.org/10.1371/journal.pone.0020967
Pavlovic D, Patera A, Nyberg F, Gerber M, Liu M (2015) Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord 8(6):255
Tan C, Koralnik I (2010) Beyond progressive multifocal leukoencephalopathy: expanded pathogenesis of JC virus infection in the central nervous system. Lancet Neurol 9(4):425–437
Warsch S, Hosein PJ, Morris MI, Teomete U, Benveniste R, Chapman JR, Lossos IS (2012) Progressive multifocal leukoencephalopathy following treatment with bendamustine and rituximab. Int J Hematol 96(2):274–278
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holmes, A., Wellings, T., Walsh, O. et al. Progressive multifocal leukoencephalopathy associated with a lymphoproliferative disorder treated with pembrolizumab. J. Neurovirol. 26, 961–963 (2020). https://doi.org/10.1007/s13365-020-00899-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-020-00899-0