Abstract
Progressive multifocal leukoencephalopathy (PML) is a rapidly progressive, often fatal viral infection of the brain without a known treatment. Recently, case reports have demonstrated survival from PML with therapies that improve cell-mediated immunity, including interleukin-7 (IL-7) or the chemokine receptor type 5 (CCR5) antagonist, maraviroc (MVC). We present the first known case of a patient with PML successfully treated with both IL-7 and MVC. A 63-year-old woman presented to our center with a 6-month history of progressive left hemiparesis. Extensive laboratory testing was negative except for a severe CD4 lymphocytopenia (140/μL). Serial brain MRIs done prior to presentation revealed an enlarging, non-enhancing T2-hyperintense lesion in the right fronto-parietal white matter. PML was confirmed through detection of the JC virus by PCR in the cerebrospinal fluid and by brain biopsy, and she was started on mirtazapine and mefloquine. She continued to deteriorate and was then given a course of recombinant IL-7. Though she remained clinically stable after IL-7 treatment and serum JCV PCR decreased from 1000 copies/mL to a nadir of 238 copies/mL, a repeat MRI 3 months later showed lesion enlargement. MVC was then initiated. Now, more than 2 years after initial presentation, she remains stable and serum JCV PCR is undetectable. This case demonstrates successful treatment of PML in a patient with idiopathic CD4 lymphocytopenia and highlights the potential benefits of IL-7 and MVC in the treatment of PML. Treatment with IL-7 and MVC led to clinical stability and improvement in JC virus titers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Case report and timeline
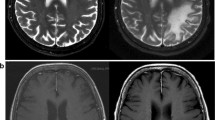
A 63-year-old previously healthy woman presented to our center with 6 months of progressive left hemiparesis, right-sided visual field cut, and mood instability. She had been given a diagnosis of stroke upon initial presentation and MRI at an outside facility. However, serial brain MRIs revealed an enlarging, non-enhancing, T2-hyperintense lesion in the right fronto-parietal white matter (Fig. 1a). The diagnosis of progressive multifocal leukoencephalopathy (PML) was confirmed through detection of JC virus (JCV) by PCR in the cerebrospinal fluid (1100 copies) and by brain biopsy showing infected oligodendrocytes and demyelination. Extensive laboratory testing on presentation revealed only a severe CD4+ lymphopenia (CD4+ count 140 cells/μL, CD8+ count 209 cells/μL, CD4/CD8 ratio 0.67), and the patient was diagnosed with idiopathic CD4+ lymphopenia (ICL). She was started on mirtazapine 30 mg daily and mefloquine (250 mg daily × 3 days, then 250 mg weekly) soon after diagnosis, but continued to deteriorate clinically with worsening hemiparesis and development of cognitive dysfunction. Recombinant interleukin (IL)-7 was obtained for compassionate use from RevImmune (Paris, France), and a course consisting of three consecutive weekly intramuscular doses of 10 μg/kg was administered. The patient remained stable after IL-7 treatment; her left-sided hemiparesis and visual field impairment did not improve, but her cognitive dysfunction returned to baseline. Serum JCV PCR decreased from 1000 copies/mL to a nadir of 238 copies/mL (Fig. 2). However, repeat MRI 3 months after treatment demonstrated enlargement of the right frontal lesion and new involvement of the left hemisphere (Fig. 1b). Maraviroc (MVC) (300 mg BID) was then initiated. CD4+ and CD8+ counts remained stable during treatment with IL-7 and MVC (Fig. 2c). Repeat MRI 3 months after MVC initiation demonstrated a decrease in size of the lesion (Fig. 1c). Due to severe claustrophobia, no further MRIs were performed. MVC was continued for 9 months and was subsequently stopped due to patient stability. Now, more than 2 years after initial presentation, she has remained clinically stable and serum JCV PCR has been undetectable.
MRI images of evolution of PML in our patient. a Brain fluid attenuated recovery (FLAIR) MRI sequence performed before initiation of any treatment, demonstrating a large right frontal white matter lesion without significant edema or mass effect. b Brain MRI performed 3 months after initiation of mefloquine, mirtazapine, and interleukin-7 demonstrated enlargement of the right frontal lesion and new involvement of the left hemisphere. c Brain MRI performed 3 months after initiation of maraviroc demonstrated a decrease in size of the white matter lesions
JC virus copy number and lymphocyte counts upon presentation and throughout course of treatment. a Timeline of treatment with mefloquine, mirtazapine, IL-7, and maraviroc. Dashed lines correspond with timing of MRIs performed throughout disease course. b Serum JC virus copy number during course of treatment, peaking at day 24 after presentation and steadily declining thereafter. c T cell lymphocyte counts stayed stable throughout treatment
Discussion
PML is a rapidly progressive, often fatal viral infection of the brain without existing treatment. ICL, diagnosed based on persistent CD4+ lymphocytopenia in the absence of other etiologies for an immunocompromised state, has been associated with several cases of PML in the literature, with variable clinical course and outcomes (Delgado-Alvarado et al. 2013). Stronger early T cell anti-JCV responses are associated with better prognosis after PML (Gheuens et al. 2011), and recent efforts in treating the disease have aimed at improving cell-mediated immunity with agents such as recombinant IL-7 or the chemokine receptor type 5 (CCR5) antagonist, MVC.
IL-7 is a cytokine with essential roles in early T cell development within the thymus, subsequent survival and proliferation in the periphery, and viral specific immunity (Mackall et al. 2011). As a principle driver of T cell activation and proliferation, IL-7 may provide benefit in immunocompromised patients with PML. Recent case reports have demonstrated successful use of IL-7 in the treatment of ICL-associated PML (Alstadhaug et al. 2014; Sospedra et al. 2014; Miskin et al. 2016), as well as in the setting of secondary lymphopenia (Sosperda et al. 2014; Gasnault et al. 2014), presumably via augmentation of the anti-JCV cellular response.
MVC, a chemokine receptor type 5 (CCR5) blocker, has also been suggested to be of benefit in PML, both in the setting of HIV (Ahuja et al. 2008) and ICL. A recent case series of three patients (Middel et al. 2015) demonstrated clinical remission and improvement of CD4+ cell counts in ICL-associated PML after starting MVC therapy. The mechanism of treatment remains unclear, though potential therapeutic effects may involve immune response modulation, direct inhibition of JCV replication, or both. It has been hypothesized that blocking CCR5 with MVC both promotes CD4+ T cell reconstitution and decreases excessive entry of immune cells into the infected brain, allowing for sufficient cellular immunity while at the same time preventing an excessive destructive response. While this is the rationale for the use of MVC treatment of PML-associated immune reconstitution inflammatory syndrome (IRIS) (Middel et al. 2015), a controlled study failed to show benefit in this setting (Sierra-Madero J et al. 2014). An alternative hypothesis is that CCR5 blockade disables viral propagation by preventing activation of downstream mediators such as nuclear factor of activated T cells 4 (NFAT4) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB), proteins implicated in JCV replication (Middel et al. 2015; Camargo et al. 2009, Spadaro et al. 2013).
Here, we report the first case of successful treatment of ICL-associated PML with combination IL-7 and MVC therapy. Initially started on mirtazapine and mefloquine, our patient continued to worsen neurologically and soon after received immunotherapy with recombinant IL-7. IL-7 was well tolerated with no adverse events noted. While serum JCV load decreased after IL-7 treatment and the patient remained clinically stable, a repeat MRI 3 months later demonstrated radiological progression of disease, suggesting insufficient viral load suppression, and MVC was then initiated. MVC treatment was well tolerated and 3 months after initiation, MRI showed lesion regression and serum JCV PCR became undetectable. Now, over 2 years after initial presentation, the patient remains clinically stable without any sign of recurrence of disease. While studies of AIDS-associated PML have demonstrated spontaneous remission in 7–9% of patients (Berger et al. 1998; Berger. 2000), prognosis in ICL-associated PML is unclear (Delgado-Alvarado et al. 2013) and there has been only one reported case of spontaneous long-term remission (Rueger et al. 2006). While we cannot know whether our patient may have achieved remission in the absence of therapy, we suspect this is highly unlikely based on the trajectory of her clinical and radiographic worsening.
This case highlights the potential benefits of using combination immunological therapy in the treatment of PML. In contrast to what has been described in prior reports (Alstadhaug et al. 2014; Sosperda et al., 2014; Miskin et al. 2016), CD4+ and CD8+ counts in our patient remained stable throughout treatment with IL-7 and MVC, while serum JCV titers decreased and correlated with response to treatment. The non-association of CD4+ and CD8+ counts with the serologic and radiographic course of illness suggests that MVC therapy has a direct effect on viral replication, an immunomodulatory effect independent of lymphocyte count, or both. Concordance of JCV titer with radiographic improvement suggests that serum JCV titer may represent a useful biomarker for treatment response. Future prospective studies of IL-7 and MVC combination therapy for the treatment of ICL-associated PML are warranted to further support the potential efficacy of this regimen.
References
Ahuja SK, Kulkarni H, Catano G, Agan BK, Camargo JF, He W, O'Connell RJ, Marconi VC, Delmar J, Eron J, Clark RA, Frost S, Martin J, Ahuja SS, Deeks SG, Little S, Richman D, Hecht FM, Dolan MJ (2008) CCL3L1-CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1 infected individuals. Nat Med 14:413–420
Alstadhaug KB, Croughs T, Henriksen S, Leboeuf C, Sereti I, Hirsch HH, Rinaldo CH (2014) Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurology 71(8):1030–1035
Berger JR (2000) Progressive multifocal leukoencephalopathy. Curr Treat Options Neurol 2(4):361–368
Berger JR, Pall L, Lanska D, Whiteman M (1998) Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neuro-Oncol 4(1):59–68
Camargo JF, Quinones MP, Mummidi S et al (2009) CCR5 expression levels influence NFAT translocation, IL-2 production, and subsequent signaling events during T lymphocyte activation. J Immunol 182:1550–6606
Delgado-Alvarado M, Sedano MJ, Gonzalez-Quintanilla V, de Lucas EM, Polo JM, Berciano J (2013) Progressive multifocal leukoencephalopathy and idiopathic CD4 lymphocytopenia. J Neurol Sci 327:75–79
Gasnault J, De Goer de Herve MG, Michot JM et al (2014) Efficacy of recombinant human interleukin 7 in a patient with severe lymphopenia-related progressive multifocal leukoencephalopathy. Open Forum Infect Dis 1(2):ofu074
Gheuens S, Bord E, Kesari S, Simpson DM, Gandhi RT, Clifford DB, Berger JR, Ngo L, Koralnik IJ (2011) Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol 85(14):7256–7263
Mackall CL, Fry TJ, Gress RE (2011) Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11(5):330–342
Middel A, Arends JE, van Lelyveld SFL, Otto S, Schuurman R, Frijns CJM, Tesselaar K, Hoepelman AIM (2015) Clinical and immunologic effects of MVC in progressive multifocal leukoencephalopathy. Neurology 85:104–106
Miskin DP, Chalkias S, Dang X, Bord E, Batson S, Koralnik I (2016) Interleukin-7 treatment of PML in a patient with idiopathic lymphocytopenia. Neurology: Neuroimmunology Neuroinflammation 3(2):e213
Rueger MA, Miletic H, Dorries K, Wyen C, Eggers C, Deckert M, Faetkenheuer G, Jacobs AH Long-term remission in progressive multifocal leukoencephalopathy caused by idiopathic CD4+ T lymphocytopenia: a case report. Clin Infect Dis 2006; 1;42(7):e53–6
Sierra-Madero J, Ellenberg S, Rassool MS et al (2014) A randomized, double-blind, placebo-controlled clinical trial of chemokine receptor 5 (CCR5) antagonist to decrease the occurrence of immune reconstitution inflammatory syndrome in HIV-infection: the CADIRIS Study. Lancet HIV 1(2):e60–e67
Sosperda M, Schippling S, Yousef S et al (2014) Treating progressive multifocal leukoencephalopathy with interleukin 7 and vaccination with JC virus capsid protein VP1. Clin Infect Dis 59(11):1588–1592
Spadaro F, Cecchetti S, Purificato C, Sabbatucci M, Podo F, Ramoni C, Gessani S, Fantuzzi L (2013) Nuclear phosphoinositide-specific phospholipase C ß1 controls cytoplasmic CCL2 mRNA levels in HIV-1 gp120-stimulated primary human macrophages. PLoS One 8(3):e59705
Acknowledgements
The authors thank Michel Morre and RevImmune for providing recombinant IL-7.
Funding
This study was supported by an Institutional Clinical Training Award from the National Multiple Sclerosis Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Asaff Harel has received consulting fees from Teva Pharmaceuticals.
Sam Horng reports no disclosures.
Tarah Gustafson reports no disclosures.
Anil Ramineni reports no disclosures.
Rebecca Straus Farber reports no disclosures.
Michelle Fabian has received consulting fees from Biogen pharmaceuticals.
Rights and permissions
About this article
Cite this article
Harel, A., Horng, S., Gustafson, T. et al. Successful treatment of progressive multifocal leukoencephalopathy with recombinant interleukin-7 and maraviroc in a patient with idiopathic CD4 lymphocytopenia. J. Neurovirol. 24, 652–655 (2018). https://doi.org/10.1007/s13365-018-0657-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-018-0657-x