Abstract
Cognitive and functional neural correlates of human immunodeficiency virus (HIV) are only partially understood at present. Variability in neural response, which has been noted in the literature, may relate to clinical factors associated with HIV, including time since HIV diagnosis, CD4 count and nadir, HIV viral load, and comorbid infectious processes, especially hepatitis C. The present investigation evaluated working memory-related functional neural activation in 26 HIV+ participants, 28 demographically matched HIV-seronegative individuals, and 8 HIV+ individuals with hepatitis C coinfection. Analyses examined impact of HIV infection duration, CD4 count and nadir, HIV viral load, and hepatitis C serostatus. Results showed that HIV-seronegative participants had fastest reaction times, and during the working memory task, HIV+ participants with hepatitis C coinfection showed strongest bias toward commission errors; however, signal detection (i.e., overall task performance) was equivalent across groups. Functional magnetic resonance imaging (fMRI) results showed HIV-related greater activation to an easier vigilance task and HIV-related lower activation to a more difficult working memory task, consistent with reduced cognitive reserve. Hepatitis C coinfection related to diffuse neural dysregulation. Correlational analyses suggested relationships of increasingly severe disease with poorer functioning in brain regions linked to error monitoring and attention regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the advent of combination antiretroviral therapy (cART), many HIV-seropositive (HIV+) people exhibit neurocognitive deficits (Heaton et al. 2010). Further, in vivo neuroimaging has shown evidence of structural and functional brain abnormalities in cART-treated, medically stable cohorts (Chang et al. 2001, 2004; Ernst et al. 2009; Gongvatana et al. 2009; Ances et al. 2012). Functional underpinnings of HIV’s deleterious effects on attention and working memory (Cohen et al. 2010b; Joska et al. 2010; Liner et al. 2010; Wendelken and Valcour 2012) remain of particular interest, as these basic cognitive skills play a role in higher level cognition such as memory and executive function. In addition, success of attention and working memory depends on healthy function of brain regions and white matter tracts directly or indirectly impacted by HIV infection (for recent reviews, see Burdo et al. 2010; Valcour et al. 2010).
In particular, structural or functional neural dysregulation in subcortical, frontal, and parietal regions may contribute to HIV+ individuals’ attention and working memory deficits (e.g., Clark and Cohen 2010). Research has shown HIV-related structural and metabolic abnormalities in subcortical regions including the basal ganglia (Ances et al. 2012; Becker et al. 2011; Cohen et al. 2010a; Muller-Oehring et al. 2010), and frontal and parietal cortex (Cohen et al. 2010a; Towgood et al. 2012; Gongvatana et al. 2013). Brain-wide gray and white matter changes have also been revealed (Thompson et al. 2005; Becker et al. 2012; Ragin et al. 2012).
Existing fMRI studies complement these volumetric and metabolic studies by suggesting inefficient neural processing in subcortical, frontal, and parietal networks in HIV+ individuals. For example, on tasks of visual attention, HIV+ participants show reduced activation in visual attention networks (i.e., prefrontal, dorsal parietal, and cerebellar regions) but increased compensatory activation elsewhere in the brain (Chang et al. 2004; Ernst et al. 2009). For progressively more complex visual attention tasks (i.e., tracking four rather than one moving targets), HIV+ individuals showed increased attention network response to the same task over a 1-year interval, despite equivalent performance, whereas seronegative participants show less activation over the same interval. Working memory tasks have shown increased parietal activation for simple conditions in HIV+ individuals (i.e., 1-back tasks) (Chang et al. 2001) and increased frontal activity, especially for more complex conditions (i.e., 2-back tasks) (Ernst et al. 2002). In addition, although HIV+ individuals show greater magnitude neural activation for more complex relative to simpler tasks in prefrontal and parietal cortices, this relative increase is significantly smaller than that seen in HIV-seronegative participants (Tomasi et al. 2006). This finding, which has been referred to as reduced dynamic range of activation, may suggest reduced cognitive reserve due to inefficient processing in HIV+ individuals, similar to what has been suggested in individuals with other CNS-impacting disorders such as multiple sclerosis (Chang et al. 2001; Penner et al. 2003; Sweet et al. 2006).
Importantly, fMRI investigations have also indicated that HIV+ participants have less stability in the blood oxygen level dependent (BOLD) response to cognitive challenge—or in other words, more variable neural activation during cognitive tasks (Ances et al. 2011). This reduced stability has been shown to relate to HIV disease severity markers, such as presence of HIV-associated neurocognitive disorders (i.e., HAND) (Chang et al. 2013), effectiveness of individual treatment (Ances et al. 2008), and presence of cART (Chang et al. 2008). Variability may also explain some discordance in fMRI investigation results within HIV+ individuals. These findings, along with known relationships of more severe HIV disease with more advanced cognitive impairment or structural brain changes (Cohen et al. 2010b; Sun et al. 2010; Ellis et al. 2011; Devlin et al. 2012; Crum-Cianflone et al. 2013) underscore the importance of examining the contribution of clinical factors to alteration in functional MRI responses.
HCV coinfection is another clinical factor that may impact neural function in HIV+ individuals. Up to 25 % of HIV+ individuals are coinfected with hepatitis C (HCV) (Kim et al. 2013), and studies within our research group and others suggest deleterious impact of comorbid HCV on cognition (Devlin et al. 2012; Rempel et al. 2013; Sun et al. 2013) and cytokine predictors of cognition (Cohen et al. 2011). Evidence has accumulated for impact of HCV coinfection on brain structure (Jernigan et al. 2011; Pfefferbaum et al. 2012) and brain metabolism (Forton et al. 2001; Taylor et al. 2004; Winston et al. 2010; Garvey et al. 2012). However, functional neural consequences of HIV/HCV coinfection during active tasks have not been explored.
Previous research has provided evidence for the impact of HIV on attention and working memory, with functional MRI tasks suggesting reduced activation due to low cognitive reserve in task-related regions and reliance on compensatory activation in additional neural regions. However, studies to date leave open questions regarding impact of HIV-related clinical factors, including time since HIV diagnosis, CD4 count and nadir, and viral load. Moreover, no active task fMRI studies have directly addressed the impact of HCV coinfection. Thus, the present study aimed to characterize the impact of HIV infection and HIV disease makers (i.e., hepatitis C coinfection, CD4 count and nadir and plasma viral load) on neural response to a working memory paradigm. We hypothesized that HIV+ individuals would show increased prefrontal cortical activation for a 2-back working memory task, as well as a reduced neural response to the working memory task, after accounting for response during the control task (i.e., reduced dynamic range), particularly in regions shown to have reduced dynamic range in previous investigations, such as prefrontal and parietal regions. We expected individuals with longer time since HIV diagnosis, detectable plasma viral load, and lower nadir and current CD4 levels to show more dysregulation of neural activation. We further hypothesized that HIV/HCV-coinfected individuals would show an exaggeration of the functional dysregulation in HIV+ individuals.
Method
Participants
Participants were 85 adults recruited from The Miriam Hospital Immunology Center as part of an NIH-sponsored study of HIV-associated brain dysfunction. Institutional review boards approved the study and informed consent was obtained from each participant before enrollment. All participants underwent a neurological examination and thorough medical history assessment. HIV infection was documented by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot. Active HCV infection was defined as positive anti-HCV by ELISA and positive qualitative HCV RNA by polymerase chain reaction.
Exclusion criteria included head injury with loss of consciousness of greater than 10 min; neurological complications including dementia, seizure, stroke, and opportunistic brain infection; severe psychiatric illness; and substance dependence during the 6 months prior to the baseline evaluation or positive urine toxicology for cocaine, opiates, stimulants, or sedatives at the time of the baseline evaluation.Footnote 1 Further exclusions were made due to lack of functional MRI data (one participant), scanner artifact or excessive head motion (three participants), failure to perform the N-back task to criterion level (i.e., <75 % correct 0-back trials or failure to follow instructions; five participants), and combined head motion and performance issues (nine participants). After all exclusions, the total sample size included in the present analysis was 62.
Basic demographic characteristics for our sample are as follows: Twenty-six participants were female. Participants included 28 HIV seronegative individuals, 26 HIV+ individuals, and 8 HIV/HCV seropositive individuals. Average participant age was 45 years. For HIV+ and HIV/HCV seropositive participants, the average amount of time since diagnosis of HIV infection was 7.6 years. For these same individuals, average current CD4 count was 550, average CD4 nadir was 201, and average HIV viral load was 30,375. The majority of HIV+ and HIV/HCV seropositive participants were on cART treatment at the time of the fMRI scan (i.e., 79 %). When directly comparing HIV seronegative, HIV+, and HIV/HCV seropositive groups, there were no significant differences in age, gender, or race (Table 1); however, HIV/HCV-coinfected participants showed a trend toward lower educational attainment than either HIV seronegative or HIV+ participants (χ 2 = 4.657; p = 0.097). Within HIV+ participants, those coinfected with HCV did not differ from the larger HIV+ group in terms of time since HIV diagnosis, current CD4 value, CD4 nadir, current viral load detectability, or current cART treatment (see Table 1 for additional participant demographic information).
Task
We employed a verbal N-back fMRI paradigm (Owen et al. 2005) during which a series of consonant letters was visually presented. Participants responded either Yes or No using a response button box following the presentation of each letter, indicating whether it is the same as or different from the letter presented N items earlier. Each letter was presented for 500 ms with a 2,500-ms interstimulus interval. Uppercase and lowercase letters were randomly presented. Zero-back and 2-back conditions were used in this experiment. Data were acquired in two separate task runs. Each run consisted of alternating blocks of three 36-s 0-back trials, three 45-s 2-back trials, and three 30-s resting block during which a stationary cross was presented; including instruction time, total task length was 12 min.
Statistical analysis
Performance on the 0-back and 2-back tasks was indexed by percentage correct target and total trials, A′ (a nonparametric measure of signal detection that takes into account both omission and commission errors) (Snodgrass and Corwin 1988), mean reaction time (RT), and reaction time variability (Simmonds et al. 2007) (Table 2). Due to differing sample sizes and non-normal distribution of several variables, nonparametric Mann-Whitney or Kruskall-Wallis tests were used to compare demographic, clinical, substance use, and performance data for HIV+ and HIV-seronegative individuals, as well as HIV+ and HIV/HCV-seropositive individuals.
fMRI data acquisition
Whole-brain, echo planar BOLD fMRI images were acquired in 42 interleaved axial 3 mm slices (TR = 2,500 ms, volumes = 147, TE = 28 ms, FOV = 192 × 192 mm, matrix = 64 × 64) on a Siemens 3 Tesla TRIM Trio magnet (Siemens Corporation 2013).
fMRI analysis
Analyses were completed primarily using FEAT (fMRI Expert Analysis Tool) version 5.98, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Cortical reconstruction and removal of non-brain tissue were performed with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/; Segonne et al. 2004). Registration to high resolution structural and standard space images was carried out using FLIRT (Jenkinson and Smith 2001; Jenkinson et al. 2002) and further refined using FNIRT nonlinear registration (Andersson et al. 2007; Andersson and Smith 2007).
Individual subject analysis
After reconstruction, functional data for the two task runs for each subject were entered into separate first-level mixed-effects analyses in FEAT. Data were motion corrected, smoothed (FWHM = 6 m), and high-pass temporal filtered (140 s). Film prewhitening was applied. Task instructions, 0-back, and 2-back trials, as well as their temporal derivatives, were included in the model. A double-gamma hemodynamic response function model was employed. Outlying data due to subject head motion were included as confound variables using fsl_motion_outliers. Contrasts of interest at the run level included activation to 0-back trials and 2-back trials relative to rest, as well as differences between these conditions (i.e., 0-back > 2-back; 2-back > 0-back). Data passing quality control thresholds were passed on to second-level fixed-effects GLMs averaging within subject, across runs. Second-level contrasts were identical to first-level contrasts.
Group analysis
Third-level group analysis employed FSL randomise (Bullmore et al. 1999; Nichols and Holmes 2002; Hayasaka and Nichols 2004), a permutation-based method for GLM analysis. For data display, all figures were created using MRIcron; text was overlaid on images using Adobe Photoshop.
The first aim of the group analysis was to examine group activation (i.e., HIV+, HIV-seronegative, and HIV/HCV coinfected) to each of the two task conditions (i.e., 0-back vs rest; 2-back vs rest) and for the contrast of the two task conditions (2-back > 0-back). This latter contrast was calculated as a measure of dynamic range in neural activation between the more difficult and an easier task. A second and related aim was to contrast neural activation differences between each of the three groups (i.e., HIV+, HIV-seronegative, and HIV/HCV coinfected) on all task conditions (i.e., 0-back vs rest; 2-back vs rest; 2-back > 0-back).
Based on these aims, three GLMs were run, modeling 0-back versus rest, 2-back versus rest, and 2-back > 0-back, respectively. Within each GLM, contrasts of interest were main effects for each clinical group (positive and negative activations for HIV+, HIV-seronegative, and HIV/HCV coinfected), and direct comparisons among the diagnostic groups. Probability of gray matter was entered into the matrix as a voxelwise covariate to control for potentially confounding anatomical variation (see Oakes et al. 2007). Familywise error rates of p < 0.01 or lower were employed (see tables for exact values).
Examination of additional clinical factors
Additional aims of the study were to examine the potential relationship of clinical disease factors with neural activation in HIV+ and HIV/HCV-coinfected participants. For this analysis, time since HIV diagnosis, CD4 and CD4 nadir were demeaned, and individual subject scores were entered into separate group-level GLMs in FSL FEAT. Again, separate GLMs were run for 0-back versus rest, 2-back versus rest, and 2-back > 0-back. Contrasts of interest included main effects for group (i.e., HIV+ and HCV coinfected) and main effects for clinical factors. In each GLM, outlier detection was used to eliminate effects of extreme data points and gray matter probability was included as a voxelwise confound covariate. Analyses were corrected for multiple comparisons using voxelwise thresholds of a minimum z >2.464, and cluster probability thresholds based on Gaussian random field theory of p < 0.05. Given the relatively smaller sample size for these analyses (N = 34), as well as the relatively small literature examining continuous HIV clinical factors with working memory response, thresholds were then relaxed to examine covariate trends for further study.
Impact of current HIV plasma viral load detectability was examined by grouping all individuals with HIV (including those with HCV coinfection) by plasma viral load detectability. As above, permutation-based GLM analyses were run for each condition of interest (i.e., 0-back versus rest, 2-back versus rest, and 2-back > 0-back); group main effects and contrasts of these two clinical groups were examined. Probability of gray matter was entered into the matrix as a voxelwise covariate. Familywise error rate of p < 0.01 or lower were employed. As with covariate analyses, thresholds were subsequently relaxed to examine covariate trends.
Results
N-Back fMRI response by clinical group
The first analytical goal was to examine activation in all three clinical groups (i.e., HIV+, HIV-seronegative, and HIV/HCV coinfected) for each of the two task conditions (i.e., 0-back and 2-back) and the contrast of these two conditions (2-back > 0-back), which was calculated as an assessment of dynamic range of neural activation between an easier task and a more challenging task. Regions of significant neural activation by task condition, for each clinical group, are provided in Table 3.
0-Back task
Activation for the 0-back task was observed for all groups in regions consistent with previous literature, including left motor cortex, left parietal regions, and bilateral supplementary motor area, frontal, occipital, and cerebellar regions (see Table 3).
2-back task
For the 2-back task, activation was observed in similar regions as observed during the 0-back task for all clinical groups. HIV-seronegative participants additionally showed activation in right superior temporal and right parietal regions, while HIV+ participants showed additional activation of right insula and basal ganglia, and HIV/HCV-coinfected participants showed additional activation in left inferior temporal regions and bilateral basal ganglia (see Table 3).
2-Back > 0-back
When the 2-back and 0-back tasks were directly compared as an assessment of dynamic range of neural activation, all three clinical groups showed greater activity in bilateral parietal, frontal, and supplementary motor areas. HIV-seronegative participants also showed greater activation in bilateral cerebellum and inferior temporal regions. HIV+ individuals also showed greater bilateral cerebellar activation. Both HIV+ and HIV/HCV-coinfected individuals also showed greater bilateral precuneus and basal ganglia activation (see Table 3).
N-Back fMRI activation differences between clinical groups
The second analytical goal was to assess N-Back fMRI response differences when comparing clinical groups (i.e., HIV+, HIV-seronegative, and HIV/HCV coinfected). Significant between-group differences in neural activation, by task condition, are presented in Table 4.
0-Back task
In a direct comparison, HIV+ and HIV-seronegative participants did not show differences in 0-back activation. HIV/HCV-coinfected participants showed greater 0-back activation in bilateral cerebellar and occipital regions than HIV-seronegative participants (see Table 4).
2-Back task
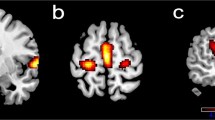
Direct comparisons of clinical groups again showed no significant activation differences between HIV+ and HIV-seronegative individuals. HIV/HCV-coinfected participants showed greater activation compared to HIV-seronegative participants in bilateral frontal regions, cerebellum, and basal ganglia, as well as left supplementary motor, and right postcentral and angular gyrus (see Table 4 and Fig. 1a). HIV/HCV-coinfected participants showed significantly greater 2-back task activation compared to HIV+ participants in bilateral cerebellum and right frontal, precentral, precuneus, and occipital regions (see Table 4 and Fig. 1a).
Illustration of between-group differences in neural activation to task. a Greater 2-back versus rest activation in HIV+ individuals with HCV coinfection, as compared to HIV-seronegative participants (yellow) and HIV+ participants (green). Colors range from t = 2 to t = 5 for both contrasts. Axial slices begin at z = 20. b Greater 2-back versus 0-back activation in HIV-seronegative versus HIV+ participants. Colors range from t = 2 (red) to t = 5 (yellow). Axial slices begin at z = 25 (Color figure online)
2-Back > 0-back
In a direct contrast of HIV+ and HIV-seronegative participants, HIV-seronegative individuals showed greater dynamic range (i.e., greater 2-back compared to 0-back activation) in right inferior temporal regions, bilateral thalamus, and bilateral occipital and parietal cortices (see Table 4 and Fig. 1b).
Additional clinical factors and neural activation
Time since HIV diagnosis
In HIV+ participants, longer time since HIV diagnosis was associated with greater 0-back task activation relative to rest in the bilateral basal ganglia (see Table 5). A trend-level relationship was revealed for longer estimated HIV duration with broadly greater activation during both tasks, with regions of note including precuneus, angular gyrus, posterior cingulate, occipital, temporal, and frontal regions (see Table 5). Within HIV/HCV-coinfected participants, positive trends were observed between time since diagnosis and greater 2-back task activation in the left hippocampus and temporal regions. Positive trends were also observed for HIV/HCV-coinfected participants’ time since diagnosis and activation in several regions for the 2-back > 0-back condition.
CD4 nadir
CD4 nadir in HIV+ individuals was associated with greater 2-back task activation relative to rest in the right inferior frontal cortex and bilateral cerebellum and occipital regions (Table 5). Several trend-level relationships were observed, including negative trends relating CD4 nadir with 0-back activation relative to rest in the posterior cingulate in HIV+ individuals and negative trends relating CD4 nadir with bilateral basal ganglia and cerebellum response in HIV/HCV-coinfected individuals (Table 5).
Current CD4 count
For HIV+ participants, higher current CD4 count was associated with significantly greater 0-back relative to rest responses in the orbitofrontal cortex, greater 2-back relative to rest responses in the right pre- and post-central gyrus (see Table 5), and lower responses in the bilateral occipital cortex for the 2-back > 0-back condition. HIV/HCV-coinfected participants showed a negative association of current CD4 with 0-back responses in the right occipital regions, and a positive association with 2-back responses in the right anterior cingulate (see Table 5). Trend-level negative associations were observed for CD4 count with 0-back task activation in the bilateral anterior cingulate and medial frontal gyrus for HIV+ participants. Additional trends included positive associations of CD4 count with 0-back task activation in the left middle and superior temporal gyri and bilateral anterior cingulate cortex (ACC′; 2-back task activation in right occipital, left inferior frontal, and bilateral basal ganglia; and 2-back > 0-back task activation in left frontal regions for HIV/HCV-coinfected participants).
Detectable viral load
Trends only were observed for viral load analyses. Specifically, HIV+ participants with detectable viral load showed a trend toward greater 0-back task activation relative to rest in the bilateral cerebellum, as well as broad and diffuse activation across the bilateral frontal cortex, precuneus, and posterior cingulate (see Table 6). Detectable viral load was also associated with trends toward greater 2-back activation relative to rest in the bilateral orbitofrontal and left basal ganglia and temporal cortex (see Table 6), and greater 2-back > 0-back increases in a diffuse network including bilateral temporal, orbitofrontal and subgenual cingulate cortices. Those with undetectable viral load showed trends toward greater 0-back task activation in the left superior parietal and post central regions; greater 2-back activation in the left superior parietal and lateral occipital cortex; and greater 2-back > 0-back increases in a diffuse network including but not limited to the right ACC and superior frontal regions.
N-Back task performance
See Table 2 for full details of performance results.
0-Back
Kruskall-Wallis tests showed no significant group differences in 0-back reaction time variability, bias (B′), or signal detection (A′). However, groups differed significantly in 0-back reaction time (χ 2 = 6.932, p = 0.031; Table 2), with HIV-seronegative participants having fastest reaction times.
2-Back
Kruskall-Wallis tests showed no significant group differences in 2-back reaction time variability or signal detection; however, groups differed significantly in terms of 2-back bias (χ 2 = 7.532, p = 0.023) and reaction time (χ 2 = 8.764, p = 0.013; Table 2). Again, HIV-seronegative participants had fastest reaction times. HIV/HCV-coinfected individuals showed a strong positive bias (i.e., tendency to make a false positive error), while HIV-seronegative participants had a mild positive bias (i.e., tendency to make a false positive error), and HIV+ participants had a mild negative bias.
Discussion
The results of the present study extend previous findings that have demonstrated functional brain disturbances on tasks requiring working memory in people infected with HIV (Chang et al. 2001, 2004; Ernst et al. 2002, 2009). The current 2-back task requires working memory along with sustained and focused attention. In contrast, the 0-back task requires sustained attention and vigilance but does not have significant working memory demands. Here, HIV-seropositive participants showed less difference in the brain activation between the 2-back and 0-back tasks, compared to the seronegative participants. Specifically, people with HIV had greater activation on the simpler sustained attention task (0-back) but less activation on the working memory task (2-back). This finding is consistent with previous work that suggests HIV affects the brain by causing a reduction in dynamic range in response to cognitive challenge (Tomasi et al. 2006). Reduced dynamic range is consistent with the hypothesis that even in the post-cART era, HIV continues to affect brain functioning (Ernst et al. 2009; Clark and Cohen 2010) and, in particular, results in reduced cognitive reserve that requires HIV-seropositive individuals to expend more cognitive resources on simpler tasks. In the present study, a reduction in functional range was observed despite comparable task performance, with the exception of slower reaction time for those with HIV.
The present investigation also showed that several clinical factors impact neural activation in the context of HIV infection. In particular, hepatitis C (HCV), which has been linked to poorer cognitive outcomes in previous work by our lab (Devlin et al. 2012) emerged as an important clinical variable. Individuals with HIV and HCV coinfection showed a broad and diffuse pattern of greater neural activation than HIV negative participants on both the 0-back and 2-back working memory tasks. When compared to HIV+ participants, coinfected individuals again showed diffuse, greater neural activation during the working memory task. The pattern of this latter contrast, which reflects the specific effect of hepatitis C, was not entirely straightforward, and some caution must be used in interpretation due to the relatively smaller size of our HIV/HCV-positive sample. However, results suggest broadly increased activation, including within regions overlapping with the working memory network, which may suggest that comorbid HCV does not have a simple additive impact over HIV serostatus in reducing of cognitive reserve, as we had predicted. In addition, HIV/HCV-coinfected participants showed greater working memory-related activation in task-irrelevant regions, including some—like the angular gyrus—which are typically associated with self-directed rather than task-directed attention (Raichle et al. 2001; Gusnard et al. 2001). Again, functional differences were observed despite comparable performance on the task, perhaps due to a behavioral response bias whereby the HIV/HCV-coinfected participants were more likely to make false positive errors. Such as response bias would be consistent with expectations for people with frontal lobe dysfunction affecting inhibitory control.
Other clinical factors tested, including detectability of HIV virus, time since HIV diagnosis, CD4 nadir, current CD4 count, and viral load detectability, also related to task activation. Results revealed that longer time since HIV diagnosis related to greater working memory activation in occipital cortex and the precuneus, a region involved in self-directed attention. In contrast, higher current CD4 count was associated with less activation in regions associated with self-directed thought and with greater activation in regions associated with error detection. Somewhat less clear positive associations emerged between CD4 nadir and cerebellar, occipital, and inferior frontal neural activation during the working memory task. In addition, trend-level results suggested a positive relationship of viral load detectability and time since HIV diagnosis with diffuse activation in a network of regions that included several important for self-directed attention: the precuneus, posterior cingulate, and frontal cortex. Greater CD4 nadir showed trend-level associations with lower activity in regions important for self-directed attention and greater activity in regions important for error detection and monitoring. Finally, analyses revealed a complex pattern of trend-level associations of current CD4 count with frontal cortical activation. These clinical factor results, while preliminary, warrant follow-up in larger samples.
The present finding that effect differences between the HIV seropositive and seronegative groups were primarily observed as a function of the dynamic range of cortical activation with increasing task demand suggests that the effects of HIV on the brain in the post-cART era may not be revealed by examination of brain response during individual tasks (e.g., attention/vigilance and working memory) in isolation or at a single level of difficulty. It also supports previous work showing that HIV+ individuals’ neural dysregulation is not limited to more difficult tasks (Chang et al. 2001, 2004; Ernst et al. 2002, 2009), but that impairment—or need for additional cognitive reserve—is evident in basic functions, such as attention. As intact attention is necessary for successful engagement of more complex cognitive functions such as working memory and executive functioning, continued work on functional neural correlates of attention in HIV, using sensitive measures, is needed.
In contrast to the HIV effect, the present study revealed a more complex picture regarding the effects of specific HIV-associated clinical factors. HIV/HCV-coinfected individuals and those with current detectable viral load exhibited a more diffuse pattern of activation, including in the brain regions not normally associated with working memory. This suggests that coinfected participants and those with most active disease experienced more diffuse neural dysregulation, a finding consistent with reduced neural efficiency in the face of comorbid or severe disease. HIV/HCV-coinfected individuals and those with detectable HIV viral load also showed greater activity in some regions important for self-directed attention. Time since HIV diagnosis, lower CD4 nadir, and lower current CD4 count also related to increased activity in these regions. Areas such as the posterior cingulate and angular gyrus are traditionally associated with default network—a network hypothesized to be important for self-directed attention, which is typically not active during cognitive tasks. Coupled with findings of increased activation in error-monitoring regions in those with less severe CD4 nadir, the present results suggest a simultaneous failure of self- and other-directed attention in more severe disease. If future work replicates this finding in larger HIV/HCV comorbid samples and those with participants who have detectable viral load, it may suggest that part of the impact of comorbidity and acute disease on cognitive functions (Devlin et al. 2012) is via not only failure of task-relevant attention networks and potentially compensatory activation in task-unrelated regions but also via dysregulation within self-directed attention networks that are traditionally functionally decoupled from active-task relevant networks (Fox et al. 2009).
Relative to previous work, the present study is unique in that it includes a large sample and examines several clinical variables in the context of HIV infection. The present study also adds to the literature in its control for gray matter probability at any given functional voxel; we can confidently say the present results are not solely a reflection of underlying disease-related structural neural changes. In addition, this study lends further support to previous work from our group and others that shows continued impact of HIV in the post-cART era, including impact on cognition (Clark and Cohen 2010; Devlin et al. 2012) and brain structure and function (Letendre et al. 2009; Cohen et al. 2010b; Clark and Cohen 2010; Gongvatana et al. 2013).
Limitations of the present study include a relatively small sample of HIV/HCV-coinfected individuals, the exploratory nature of some analyses with clinical factors, and loss of a number of participants due to poor performance and head motion during scanning. Future studies of larger samples of people with HCV and HIV/HCV coinfection are needed to confirm and expand our understanding of the impact of HCV on functional brain activation. More work is also required to ascertain relationship of HIV clinical factors with neural function, especially in light of the fact that several of these factors are derived from patient self-report (i.e., time of diagnosis) and may have inherent reporter error. Finally, in excluding participants who did not meet a standard performance threshold, we ensured that we are examining neural activation in participants who are attempting the task and showing some degree of ability to complete the task; however, there was variability in task success within the remaining sample. In future investigations, it may be more effective to employ parametric task manipulations, to equate performance across participants, and be able to define task difficulty on an individual subject basis. Nonetheless, findings from the current study provide evidence that brain function continues to be affected by HIV and comorbid factors and also suggest that fMRI could provide a useful biomarker for assessing these effects. Additional research is needed to test the value of these fMRI metrics for predicting functional outcome in people living with chronic HIV infection.
Notes
Participants who met substance dependence criteria at later time points (i.e., 12 or 24-month visits) were not excluded from participation. For time points most proximal to participant MRI scan, 44 % of individuals qualified for lifetime diagnosis of alcohol dependence (3 % proximal to MRI; no significant differences between clinical groups). Lifetime cocaine dependence was met in 42 % of participants (5 % proximal to MRI). Kruskall-Wallis tests showed significant group differences for endorsement of lifetime (χ 2 = 10.143, p = 0.006) and MRI proximal cocaine dependence (χ 2 = 8.345; p = 0.015; highest rates in HIV/HCV coinfected participants). Lifetime narcotic dependence was met in 15 % of participants (2 % proximal to MRI), with significant differences among clinical groups for lifetime (χ 2 = 22.463, p < 0.001; highest rates in HIV/HCV coinfected participants) but not MRI proximal dependence.
References
Ances BM, Roc AC, Korczykowski M et al (2008) Combination antiretroviral therapy modulates the blood oxygen level-dependent amplitude in human immunodeficiency virus-seropositive patients. J Neurovirol 14:418–424. doi:10.1080/13550280802298112
Ances B, Vaida F, Ellis R, Buxton R (2011) Test-retest stability of calibrated BOLD-fMRI in HIV- and HIV+ subjects. NeuroImage 54:2156–2162. doi:10.1016/j.neuroimage.2010.09.081
Ances BM, Ortega M, Vaida F, et al (2012) Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 1999 59:469–477. doi:10.1097/QAI.0b013e318249db17
Andersson J, Smith SM (2007) Non-linear optimisation. FMRIB technical report TR07JA1. Oxford
Andersson J, Jenkinson M, Smith SM (2007) Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. Oxford
Becker JT, Sanders J, Madsen SK et al (2011) Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 5:77–85. doi:10.1007/s11682-011-9113-8
Becker JT, Maruca V, Kingsley LA et al (2012) Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology 54:113–121. doi:10.1007/s00234-011-0854-2
Bullmore ET, Suckling J, Overmeyer S et al (1999) Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18:32–42
Burdo TH, Soulas C, Orzechowski K et al (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 6:e1000842. doi:10.1371/journal.ppat.1000842
Chang L, Speck O, Miller EN et al (2001) Neural correlates of attention and working memory deficits in HIV patients. Neurology 57:1001–1007
Chang L, Tomasi D, Yakupov R et al (2004) Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 56:259–272. doi:10.1002/ana.20190
Chang L, Yakupov R, Nakama H et al (2008) Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol Off J Soc NeuroImmune Pharmacol 3:95–104. doi:10.1007/s11481-007-9092-0
Chang L, Holt JL, Yakupov R et al (2013) Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging 34:1240–1253. doi:10.1016/j.neurobiolaging.2012.10.012
Clark US, Cohen RA (2010) Brain dysfunction in the era of combination antiretroviral therapy: implications for the treatment of the aging population of HIV-infected individuals. Curr Opin Investig Drugs 2000 11:884–900
Cohen RA, Harezlak J, Gongvatana A et al (2010a) Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol 16:435–444. doi:10.3109/13550284.2010.520817
Cohen RA, Harezlak J, Schifitto G et al (2010b) Effects of nadir CD4 count and duration of HIV infection on brain volumes in the HAART era. J Neurovirol 16:25–32. doi:10.3109/13550280903552420
Cohen RA, de la Monte S, Gongvatana A et al (2011) Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. J Neuroimmunol 233:204–210. doi:10.1016/j.jneuroim.2010.11.006
Crum-Cianflone NF, Moore DJ, Letendre S et al (2013) Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology 80:371–379. doi:10.1212/WNL.0b013e31827f0776
Devlin KN, Gongvatana A, Clark US et al (2012) Neurocognitive effects of HIV, hepatitis C, and substance use history. J Int Neuropsychol Soc JINS 18:68–78. doi:10.1017/S1355617711001408
Ellis RJ, Badiee J, Vaida F et al (2011) CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS Lond Engl 25:1747–1751. doi:10.1097/QAD.0b013e32834a40cd
Ernst T, Chang L, Jovicich J et al (2002) Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59:1343–1349
Ernst T, Yakupov R, Nakama H et al (2009) Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol 65:316–325. doi:10.1002/ana.21594
Forton DM, Allsop JM, Main J et al (2001) Evidence for a cerebral effect of the hepatitis C virus. Lancet 358:38–39. doi:10.1016/S0140-6736(00)05270-3
Fox MD, Zhang D, Snyder AZ, Raichle ME (2009) The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283. doi:10.1152/jn.90777.2008
Garvey LJ, Pavese N, Ramlackhansingh A et al (2012) Acute HCV/HIV coinfection is associated with cognitive dysfunction and cerebral metabolite disturbance, but not increased microglial cell activation. PLoS One 7:e38980. doi:10.1371/journal.pone.0038980
Gongvatana A, Schweinsburg BC, Taylor MJ et al (2009) White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 15:187–195. doi:10.1080/13550280902769756
Gongvatana A, Harezlak J, Buchthal S et al (2013) Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol. doi:10.1007/s13365-013-0162-1
Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001) Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A 98:4259–4264. doi:10.1073/pnas.071043098
Hayasaka S, Nichols TE (2004) Combining voxel intensity and cluster extent with permutation test framework. NeuroImage 23:54–63. doi:10.1016/j.neuroimage.2004.04.035
Heaton RK, Clifford DB, Franklin DR Jr et al (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. doi:10.1212/WNL.0b013e318200d727
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841
Jernigan TL, Archibald SL, Fennema-Notestine C et al (2011) Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol 17:248–257. doi:10.1007/s13365-011-0032-7
Joska JA, Gouse H, Paul RH et al (2010) Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol 16:101–114. doi:10.3109/13550281003682513
Kim AY, Onofrey S, Church DR (2013) An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis 207:S1–S6. doi:10.1093/infdis/jis927
Letendre SL, Ellis RJ, Everall I et al (2009) Neurologic complications of HIV disease and their treatment. Top HIV Med 17:46–56
Liner KJ 2nd, Ro MJ, Robertson KR (2010) HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS Rep 7:85–91. doi:10.1007/s11904-010-0042-8
Muller-Oehring EM, Schulte T, Rosenbloom MJ et al (2010) Callosal degradation in HIV-1 infection predicts hierarchical perception: a DTI study. Neuropsychologia 48:1133–1143. doi:10.1016/j.neuropsychologia.2009.12.015
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Oakes TR, Fox AS, Johnstone T et al (2007) Integrating VBM into the General Linear Model with voxelwise anatomical covariates. NeuroImage 34:500–508. doi:10.1016/j.neuroimage.2006.10.007
Owen AM, McMillan KM, Laird AR, Bullmore E (2005) N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. doi:10.1002/hbm.20131
Penner I-K, Rausch M, Kappos L et al (2003) Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol 250:461–472. doi:10.1007/s00415-003-1025-0
Pfefferbaum A, Rosenbloom MJ, Sassoon SA et al (2012) Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry 72:361–370. doi:10.1016/j.biopsych.2012.02.018
Ragin AB, Du H, Ochs R et al (2012) Structural brain alterations can be detected early in HIV infection. Neurology 79:2328–2334. doi:10.1212/WNL.0b013e318278b5b4
Raichle ME, MacLeod AM, Snyder AZ et al (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. doi:10.1073/pnas.98.2.676
Rempel H, Sun B, Calosing C et al (2013) Monocyte activation in HIV/HCV coinfection correlates with cognitive impairment. PLoS One 8:e55776. doi:10.1371/journal.pone.0055776
Segonne F, Dale AM, Busa E et al (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage 22:1060–1075
Siemens Corporation (2013) Germany
Simmonds DJ, Fotedar SG, Suskauer SJ et al (2007) Functional brain correlates of response time variability in children. Neuropsychologia 45:2147–2157. doi:10.1016/j.neuropsychologia.2007.01.013
Snodgrass JG, Corwin J (1988) Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117:34–50
Sun B, Abadjian L, Rempel H et al (2013) Differential cognitive impairment in HCV coinfected men with controlled HIV compared to HCV monoinfection. J Acquir Immune Defic Syndr 62:190–196. doi:10.1097/QAI.0b013e31827b61f1
Sun B, Abadjian L, Rempel H et al (2010) Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol 16:115–124. doi:10.3109/13550280903559789
Sweet LH, Rao SM, Primeau M et al (2006) Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp 27:28–36. doi:10.1002/hbm.20163
Taylor MJ, Letendre SL, Schweinsburg BC et al (2004) Hepatitis C virus infection is associated with reduced white matter N-acetylaspartate in abstinent methamphetamine users. J Int Neuropsychol Soc 10:110–113. doi:10.1017/S1355617704101161
Thompson PM, Dutton RA, Hayashi KM et al (2005) Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A 102:15647–15652. doi:10.1073/pnas.0502548102
Tomasi D, Chang L, de Castro Caparelli E et al (2006) The human immunodeficiency virus reduces network capacity: acoustic noise effect. Ann Neurol 59:419–423. doi:10.1002/ana.20766
Towgood KJ, Pitkanen M, Kulasegaram R et al (2012) Mapping the brain in younger and older asymptomatic HIV-1 men: frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex J Devoted Study Nerv Syst Behav 48:230–241. doi:10.1016/j.cortex.2011.03.006
Valcour VG, Shiramizu BT, Shikuma CM (2010) HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol 87:621–626. doi:10.1189/jlb.0809571
Wendelken LA, Valcour V (2012) Impact of HIV and aging on neuropsychological function. J Neurovirol 18:256–263. doi:10.1007/s13365-012-0094-1
Winston A, Garvey L, Scotney E et al (2010) Does acute hepatitis C infection affect the central nervous system in HIV-1 infected individuals? J Viral Hepat 17:419–426. doi:10.1111/j.1365-2893.2009.01198.x
Acknowledgments
Support for this project came from NIH R01MH074368, 403 P01AA019072, K99AA020235, and P30AI042853 (Lifespan/Tufts/404 Brown Center for AIDS Research). Many thanks to the current and past students, fellows, and staff of the Cohen and Tashima laboratories, especially Kate Devlin, Skye Ross, Matthew Pinna, and Anna Folkers.
Conflict of interest
The following authors declare that they have no conflict of interest: Jessica Caldwell, Assawin Gongvatana, Bradford Navia, Lawrence Sweet, Karen Tashima, Mingzhou Ding, and Ronald Cohen.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Caldwell, J.Z.K., Gongvatana, A., Navia, B.A. et al. Neural dysregulation during a working memory task in human immunodeficiency virus-seropositive and hepatitis C coinfected individuals. J. Neurovirol. 20, 398–411 (2014). https://doi.org/10.1007/s13365-014-0257-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-014-0257-3