Abstract
Human immunodeficiency virus (HIV) infection-associated neurocognitive disorders is accompanied with brain atrophy. In these patients, impairment of adult neurogenesis and neurite outgrowth in the hippocampus may contribute to cognitive dysfunction. Although running exercises can enhance neurogenesis and normalize neurite outgrowth, the underlying molecular mechanisms are not well understood. The HIV envelope protein, gp120, has been shown to impair neurogenesis. Using a gp120 transgenic mouse model, we demonstrate that exercise stimulated neural progenitor cell (NPC) proliferation in the hippocampal dentate gyrus and increased the survival rate and generation of newborn cells. However, sustained exercise activity was necessary as the effects were reversed by detraining. Exercise also normalized dendritic outgrowth of neurons. Furthermore, it increased the expression of hippocampal brain-derived neurotrophic factor (BDNF) and normalized hyperactivation of cyclin-dependent kinase 5 (Cdk5). Hyperactivated Cdk5 or gp120 treatment led to aberrant neurite outgrowth and BDNF treatment normalized the neurite outgrowth in NPC cultures. These results suggest that sustained exercise has trophic activity on the neuronal lineage which is mediated by Cdk5 modulation of the BDNF pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human immunodeficiency virus (HIV) infection causes neurocognitive impairment in nearly one third of individuals despite adequate antiretroviral therapy (Heaton et al. 2011). Considering the fact that over 30 million people are infected with the virus worldwide, the impact of this neurodegenerative process has important socioeconomic consequences (UNAIDS/WHO). However, despite a huge investment of resources in trying to understand the pathophysiology of HIV-associated neurocognitive disorders (HAND) and despite several clinical trials using neuroprotective drugs, there is no neuroprotective treatment available to date. Pathological studies have demonstrated that the virus infects glial cells while the neurons are not infected. Nevertheless, the neurons undergo synaptic pruning, neurite and axonal retraction, and eventually apoptosis (Ellis et al. 2007). Pathophysiological studies suggest that neuronal injury is mediated via viral proteins, cytokines, and other mediators released from HIV-infected and activated glial cells (Nath 2002). Based on these studies, clinical trials have been conducted using drugs that target excitotoxicity, oxidative stress, and neuroinflammatory pathways, all of which have failed (Lindl et al. 2010). Unfortunately, such neuroprotective strategies have failed in most neurodegenerative diseases where they have been tried (Ang et al. 2010). Current approaches are now focused on neuroregeneration. However, the delivery of stem cells and viral vectors will be limited to select patient populations hence other strategies are necessary for the population at large.
Exercise has been shown to improve neurocognitive function in a variety of disorders (Marzolini et al. 2012; Pontifex et al. 2012) as well as age-related cognitive decline (Stranahan et al. 2010). These effects are mediated via increased neurogenesis and improved synaptic plasticity (Vivar et al. 2012). Hence, we determined if voluntary exercise may have similar beneficial effects in an animal model of HAND. We used a transgenic mouse model in which the HIV envelope protein is expressed under the glial fibrillary acidic proten (GFAP) promoter. The transgenic mice were previously shown to display neuropathological changes, reduced hippocampal long-term potentiation, and age-dependent impairment in cognitive and neuromotor deficit seen in a proportion of HIV-1-infected individuals (D'Hooge et al. 1999; Krucker et al. 1998; Toggas et al. 1994). We determined if neurogenesis and neuronal morphology was impaired and if these effects could be reversed by exercise. We further determined if the effects of exercise persisted during a detraining period and explored the molecular basis of these effects.
Materials and methods
Ethics statement
All experiments involving mice were performed according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH). The study was approved by the Johns Hopkins Medicine Institutional Animal Care and Use Committee (permit number: MO02M269) and the NIH Animal Care and Use Committee (permit number: 1334-11).
Animals and drug treatment
Adult (8–9 weeks old) male heterozygous gp120 transgenic mice on a C57BL/6 background in which the expression of gp120 was driven by a GFAP promoter (Toggas et al. 1994) and littermate wild-type controls were housed in a standard facility. A minimum of five animals per group were used for each experimental condition. To assess cell proliferation, 200 mg/kg of bromodeoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA) or 50 mg/kg of 5-ethynyl-2′-deoxyuridine (EdU; Invitrogen, Carlsbad, CA, USA) was injected intraperitoneally (i.p.), and the animals were killed 24 h later. For studies of cell survival, BrdU (50 mg/kg) was injected i.p. four times at 2-h intervals, and then the animals were killed on either 1 or 21 days after the initial BrdU injection. dl-propranolol (Sigma-Aldrich) was dissolved in saline and injected i.p. at 5 mg/kg once daily for the last 3 days of a 10-day period of exercise, because receptor antagonist administration for longer than 4 days is reported to induce receptor supersensitivity (Nattel et al. 1979). Rolipram (Sigma-Aldrich) was dissolved in saline containing 2 % dimethyl sulfoxide and injected i.p. at 3 mg/kg once daily for 10 days.
Voluntary exercise
Mice were housed in a cage containing a running wheel. The voluntary wheel system consisted of an 11.5-cm-diameter wheel with a 5.0-cm-wide running surface (model 61390, Petsmart, Phoenix, AZ, USA) equipped with a digital magnetic wireless counter (model CC-MC100W, CatEye, Boulder, CO, USA) that was activated by wheel rotation. During the running period, running duration and distance were recorded daily for each cage. Two mice were housed per cage in all experiments. Littermates of the gp120 transgenic and wild-type mice were provided running wheels for 3, 10, or 20 days. Another group was provided running wheels for 10 days and then the wheels were removed and the mice were housed in standard cages for another 10 days.
Retroviral labeling of newly generated neurons
Engineered self-inactivating murine oncoretroviruses were used to express green fluorescent protein (GFP) specifically in proliferating cells and their progeny (Duan et al. 2007). Adult female gp120 transgenic and wild-type mice housed under standard conditions were anaesthetized (100 μg ketamine and 10 μg xylazine in 10 μL saline per gram) and oncoretroviruses were stereotaxically injected into the dentate gyrus at 4 sites (0.5 μL per site at 0.25 μL/min) with the following coordinates (posterior = 2 mm from Bregma, lateral = ±1.6 mm and ventral = 2.5 mm; posterior = 3 mm from Bregma, lateral = ±2.6 mm and ventral = 3.2 mm).
Immunohistochemistry, microscopy, and quantification
Animals were transcardially perfused with saline followed by 4 % (w/v) paraformaldehyde (PFA). After postfixing in PFA overnight, brains were immersed in a 30 % (v/v) sucrose solution. On the following day, brains were cryoprotected and cut in the coronal plane into 40-μm-thick sections on a sliding microtome. Every sixth brain section spanning the entire hippocampal dentate gyrus was washed in Tris-buffered saline (TBS; 10 mM Tris–HCl, pH 7.5, 150 mM NaCl) prior to incubation in blocking solution (TBS with 0.5 % (v/v) Triton-X and 2.5 % (v/v) donkey serum). Primary antibodies were applied overnight at 4 °C. Secondary antibodies were applied to sections for 2 h at room temperature followed by washing and counterstaining with 4′,6-diamidino-2-phenylindole (DAPI) to label all nuclei. Sections were mounted on Superfrost Plus glass slides (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −80 °C until they were imaged.
Primary antibodies were diluted in blocking solution as follows: anti-BrdU (rat, 1:1,000; Accurate, Westbury, NY, USA) or anti-doublecortin (DCX; goat, 1:250; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Secondary antibodies were conjugates of Alexa Fluor 488 or Alexa Fluor 594 (1:250, Invitrogen).
EdU cell proliferation assay was performed as described previously (Chehrehasa et al. 2009). Brain sections were treated with 0.5 % Triton X-100 for 20 min at room temperature. The sections were washed twice with 3 % BSA in phosphate-buffered saline (PBS) and then incubated with 500 μL of Click-iT® reaction cocktail (Invitrogen), containing 430 μL of 1× reaction buffer, 20 μL of CuSO4, 1.2 μL of Alexa Fluor azide, and 50 μL of reaction buffer additive for 30 min, protected from light. The sections were washed once with PBS and then incubated with Hoechst 33342 (5 μg/mL) for 30 min for nuclear staining, protected from light. After being washed twice with PBS, sections were mounted on Superfrost Plus glass slides and stored at −80 °C until they were imaged.
Sections were imaged using a Zeiss LSM 510 Meta multiphoton confocal system (Carl Zeiss Microimaging Inc., Thornwood, NY, USA). Z-stacks (2 μm thick, spanning the entire 40 μm thickness of each slice) were constructed for each image. BrdU- , EdU-, and DCX-positive cells within the subgranular zone and granule cell layer of every sixth slice spanning the hippocampal dentate gyrus were counted by a modified stereological method as previously described (Lie et al. 2005). Positively labeled cells were expressed as cells per volume of the granule cell layer, as assessed by staining of the nuclei with DAPI.
For dendritic analysis, three-dimensional reconstruction of the entire dendritic processes of each neuron was made from Z-series stacks of confocal images. The two-dimensional projection images were traced with NIH ImageJ. GFP + dentate granule cells with largely intact dendritic trees were analyzed for total dendritic length and branch number as described (Ge et al. 2006). Sholl analysis for dendritic complexity was carried out by counting the number of dendrites that cross a series of concentric circles from the soma as previously described (Ge et al. 2006). The mice were analyzed at either one or two weeks following retroviral labeling. Data shown are from a minimum of ten individual GFP + neurons from at least four animals in each group.
Immunoprecipitation and Cdk5 activity assay
Immunoprecipitation and Cdk5 kinase activity were performed as described previously (Veeranna et al. 1996). Briefly, protein G (+) A-agarose beads were washed 3 times with TBS and incubated with Cdk5 antibody (1–2 μg/500 μg of protein lysate, Santa Cruz Biotechnology) for 1 h at room temperature with gentle mixing. Beads were centrifuged and washed three times with TBS and suspended in TBS. The hippocampi of the gp120 transgenic and wild-type mice were lysed in ice-cold lysis buffer and incubated with antibody conjugated beads for 2.5 h at 4 °C on a rotating wheel. Beads were subsequently centrifuged and washed 3 times with TBS and suspended in kinase buffer (50 mM Tris/HCl pH 7.4, containing 1 mM EGTA and 5 mM MgC12). The immunoprecipitated beads were used as an enzyme source for the kinase activity. For the kinase assay, a total volume of 50 μL of kinase assay mixture was used, containing 50 μM Tris/HCl (pH 7.4) with EGTA, 1 mM dithiothreitol, 5 mM MgCl2, 0.5 μM microcystin LR, 10 μg of histone H1, and 10 μL of Cdk5 immunoprecipitates. The phosphorylation reaction was initiated by the addition of 0.1 mM[γ-32P]ATP and incubated at 30 °C for 30 min. The reaction was terminated by spotting 25 μL of the reaction mixture on P81 phosphocellulose pads that were washed five times in 75 mM phosphoric acid followed by rinsing with 95 % (v/v) ethanol. The radioactivity was measured in a liquid scintillation counter.
Quantitative Western blot analysis
For Western blot analysis, freshly dissected hippocampal tissues were weighted, homogenized and lysed directly in the sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris pH 6.8, 10 % (v/v) glycerol, 2 % (w/v) SDS, 0.01 % (w/v) bromphenol blue). Protein homogenates were boiled for 5 minutes and loaded onto 10-15 % SDS PAGE gel for electrophoresis (Bio-Rad, Philadelphia, PA, USA). Nitrocellulose membranes with transferred proteins were blocked with 4 % (w/v) BSA in TBS, incubated in TBST (TBS + 0.05 % Tween-20) with primary antibodies, washed and reacted with horse radish peroxidase-conjugated secondary antibodies (Roche Molecular Systems, Inc., Branchburg, NJ, USA). The following primary antibodies were used: anti-brain-derived neurotrophic factor (BDNF; rabbit, 1:500, Santa Cruz Biotechnology) and anti-p35 (rabbit, 1:500, Santa Cruz Biotechnology). Membranes were stripped and re-blotted with anti-actin antibody (mouse, 1:5,000, Santa Cruz Biotechnology) for a loading control. The relative intensities of the blots were measured by densitometry.
Cell cultures and immunocytochemistry
Cell culture media and supplements were purchased from Invitrogen if not specifically described. Human neural progenitor cells (NPCs) were cultured from human fetal brain specimens of 7–8 weeks gestation in accordance with NIH guidelines. The tissues were then triturated after removing meninges and blood vessels. After centrifugation at 1,000 rpm, cells were resuspended in DMEM/F12 media (containing 8 mM glucose, 1× N2 supplement, 1 % antibiotics, 0.1 % albumin (Sigma-Aldrich), human fibroblast growth factor-beta (20 ng/mL), and human epidermal growth factor (20 ng/mL)) and plated onto poly-d-lysine-coated (Sigma-Aldrich) T 25-cm2 tissue culture flasks. When cell cultures reached 60 % confluence, they were subcultured by treatment with 0.0125 % (w/v) trypsin (Sigma-Aldrich) and plated at a density of 2 × 104 cells/ml onto poly-d-lysine-coated 96-well plates or glass cover slips in 24-well plates. Media was replaced every other day. NPC cultures were ready for experiments 4–5 days after replating and >98 % of the cells expressed the neural stem cell marker, nestin while <1 % of the cells expressed the marker for astrocytes, GFAP, or the neuronal cell marker, beta-III tubulin as determined by immunocytochemistry. Primary antibodies were used as follows: anti-nestin (mouse, 1:1,000; Millipore, Billerica, MA, USA), anti-GFAP (rabbit, 1:2,000; Dako, Carpinteria, CA, USA), and beta-III tubulin (mouse, 1:2,000; Promega, Madison, WI, USA). Secondary antibodies were conjugates of Alexa Fluor 488, Alexa Fluor 594, or Alexa Fluor 633 (1:400, Invitrogen).
To measure neurite length, cells were fixed in 4 % PFA and rinsed with TBS with 0.5 % Triton-X. Cells were immunostained using anti-beta-III tubulin (mouse, 1:1,000; Promega) followed by incubation with the secondary antibody (anti-mouse Alexa Flour 594, 1: 400; Invitrogen). Cells were counterstained with DAPI, mounted in standard mounting media and imaged by a fluorescence microscope (model AMF-4302, Advanced Microscopy Group, Bothell, WA, USA). Dendritic length of neuronal images was analyzed with NIH ImageJ. All analyses were performed in a blind fashion.
Results
Effect of exercise and detraining on NPC proliferation in the hippocampal dentate gyrus
We first determined whether the effect of exercise on NPC proliferation in hippocampal dentate gyrus was time-dependent, and whether this effect could be maintained after cessation of exercise. Eight-week-old wild-type and gp120 transgenic mice were provided running wheels for 3, 10, or 20 days. Another group was provided running wheels for 10 days, the wheels were then removed, and the mice were housed for another 10 days (Fig. 1a). To assess NPC proliferation, a single dose of 200 mg/kg of BrdU was administered and the mice were killed 24 h later (Fig. 1a).
Exercise stimulated NPC proliferation in the hippocampal dentate gyrus which was reversed by detraining. a Experimental design: 8-week-old wild-type and gp120 transgenic mice were provided running wheels for 3, 10, or 20 days. Another group was provided running wheels for 10 days, and the wheels were then removed and the mice were housed for another 10 days. To assess NPC proliferation, a single dose of 200 mg/kg of BrdU was given and the mice were killed 24 h later. b Cumulative and average daily distance that mice ran for 3, 10, and 20 days showed no difference in wild-type versus gp120 transgenic mice. c Representative photomicrographs demonstrate immunostaining for BrdU-positive (green) newborn cells in dentate gyrus. SGZ subgranular zone, H hilus, GCL granular cell layer. Scale bar = 100 μm. d Quantitative analysis of BrdU-positive cells illustrates that numbers of newborn cells in dentate gyrus were increased by running while running followed by sedentary activity decreased the numbers. Values represent mean ± SEM (n = 4 or 5 animals in each group; *p < 0.05; **p < 0.01; ***p < 0.001, one-way analysis of variance (ANOVA) followed by Duncan's test). e Quantitative analysis of EdU-positive cells showed that the numbers of newborn cells in dentate gyrus were increased by running, whereas running followed by sedentary activity decreased the numbers. Values represent mean ± SEM (n = 5 animals in each group; *p < 0.05; **p < 0.01; NS not significant, one-way ANOVA followed by Duncan's test). f Correlation analysis shows that there was a positive correlation between the number of BrdU-positive cells and running distance
There was no difference in activity level of the wild-type compared with gp120 transgenic mice. The cumulative running distance at 3, 10, and 20 days groups was similar for the wild-type and gp120 transgenic mice. The average running distance during a 20-day period was 8.1 ± 0.9 and 7.9 ± 0.8 km/day in the wild-type and gp120 transgenic mice, respectively (Fig. 1b). Quantitative analysis of BrdU-positive cells resulted in a bell-shaped curve with an initial increase and a subsequent decrease in the wild-type mice. For the wild-type mice, running increased the number of BrdU-positive cells by 60 % within 3 days and 100 % by day 10, yet continuous exercise for 20 days resulted in a return of the number of BrdU-positive cells back to baseline (Fig. 1c, d). The gp120 transgenic mice also showed an increase in BrdU-positive cells at days 3 and 10 and a decrease at day 20 by running. However, there were also important differences between the two groups. At baseline, the number of BrdU-positive cells was lower in the gp120 transgenic mice and the effects of exercise were more pronounced (Fig. 1c, d). At days 3 and 10, the total number of BrdU-positive cells reached levels comparable to the wild-type mice, and did not return back to baseline by day 20 (Fig. 1c, d). Interestingly, detraining, i.e., running followed by sedentary activity, completely reversed the effect of exercise on NPC proliferation in both the wild-type and gp120 transgenic mice. In the wild-type mice, detraining led to a much greater drop in the number of BrdU-positive cells compared with the control sedentary group. By contrast, in the gp120 group, BrdU-positive cell levels returned to that of the control sedentary group after detraining (Fig. 1c, d). We confirmed these observations on the effect of exercise on NPC proliferation by EdU cell proliferation assays (Fig. 1e). There was a positive correlation between the number of BrdU-positive cells and running distance (Fig. 1f).
Effect of exercise on the survival rate of newly generated cells
To determine whether exercise regulates the survival rate of newborn cells in the adult hippocampus of the wild-type and gp120 transgenic mice, BrdU (50 mg/kg) was injected i.p. at 2-h intervals for 6 h. The mice were then killed either 1 or 21 days after the initial BrdU injection, and the number of BrdU-positive cells was compared between the two groups (Fig. 2a).
Exercise increased the survival rate of newly generated cells. a Experimental design. To study the survival of NPCs, 50 mg/kg injections of BrdU were given at 2-h intervals for 6 h and then the mice were killed either 1 or 21 days after the initial BrdU injection. b Representative confocal images demonstrate immunostaining of BrdU-positive (green) newborn cells in dentate gyrus of sedentary control and 20-day running group in gp120 transgenic mice. Antibody to NeuN was used to stain the nuclei of the neurons (red). c Quantitative analysis of BrdU-positive cells demonstrated that in both wild-type and gp120 transgenic mice 80 % of newborn NPCs survived after 20 days of running, compared with 30 % in the sedentary group. Partial protection was seen following 10 days of exercise. Values represent mean ± SEM (n = 5 animals in each group; **p < 0.01; ***p < 0.001; NS, not significant, one-way ANOVA followed by Duncan's test)
The total number of BrdU-positive cells decreased over time in both the wild-type and gp120 transgenic mice, reflecting a loss of newly generated cells (Fig. 2b, c). There was no difference in the survival rate of newborn cells between the wild-type (32 %) and gp120 transgenic mice (26 %); 85 and 79 % of newborn NPCs in the wild-type or gp120 transgenic mice, respectively, survived at 21 days of running, showing significant increases compared with the sedentary groups. Conversely, the group that was sedentary for a period of 10 days and then subjected to 10 days of running, as well as the group that initially ran for 10 days and then remained sedentary for 10 days, provided partial protection to the newly generated cells. There was no significant difference in the survival rate between the wild-type and gp120 transgenic mice (Fig. 2c).
Effect of exercise on neurogenesis
It has been shown that DCX expression levels in adult brain tissue reflect neurogenesis (Couillard-Despres et al. 2005). To determine whether exercise enhances adult hippocampal neurogenesis, we analyzed DCX-positive cells in the dentate gyrus of the hippocampus of sedentary animals, after 10 or 20 days of running, and following detraining. Twenty days of running resulted in a marked increase in the total number of DCX-positive cells in both the wild-type and gp120 transgenic mice. Ten days of running also showed increased DCX-positive cells in both wild-type and gp120 transgenic animals to comparable levels. Interestingly, 10 days of running when followed by sedentary activity for 10 days resulted in a decreased number of DCX-positive cells, consistent with a decrease in NPC proliferation (Fig. 3a, b).
Exercise enhanced neurogenesis. a Representative photomicrographs demonstrate the expression of DCX-positive immature neurons (red) in sedentary control, 20-, 10-, and 10-day running followed by 10-day sedentary groups. Nuclei were stained with DAPI (blue). SGZ subgranular zone, GCL granular cell layer, ML molecular layer. Scale bar = 50 μm. b DCX-positive immature neurons in the dentate gyrus were increased by running while running following sedentary activity decreased the number of these cells. Values represent mean ± SEM (n = 5 animals in each group; *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA followed by Duncan's test)
Effect of exercise on dendritic development of newborn neurons
We next examined the role of gp120 in the dendritic development of newborn neurons. Retroviruses expressing GFP were stereotaxically injected into the dentate gyrus of adult wild-type and gp120 transgenic mice to label proliferating NPCs and their progeny (Ge et al. 2006). The mice were provided access to running wheels for 14 days following retroviral injection, and then analyzed. Quantitative analysis demonstrated markedly elaborated dendritic trees in the gp120 transgenic mice as compared with wild-type mice. Dendritic length was increased in the gp120 transgenic mice (mean ± SEM = 569.0 ± 48.0 μm) compared with the wild-type mice (mean ± SEM = 332.7 ± 59.6 μm). Dendritic lengths were similar in exercised the gp120 transgenic mice (mean ± SEM = 356.0 ± 49.9 μm) as compared with the exercised wild-type littermates (mean ± SEM = 393.9 ± 44.3 μm). In addition, dendritic length was similar in exercised gp120 transgenic mice, when compared with sedentary wild-type mice (Fig. 4a, b). Thus, voluntary exercise normalized dendritic development in the gp120 transgenic mice.
Exercise normalized dendritic outgrowth of neurons. a Shown are samples of projected trajectories of Z-series confocal images of GFP-positive new neurons in the dentate gyrus. GFP-positive neurons with retrovirus-mediated genetic manipulation were examined at 14 days post-injection (dpi). Scale bar = 20 μm. b Analysis of total dendritic length of GFP-positive neurons demonstrated that gp120 transgenic mice had increased dendritic lengths at 14 dpi. Running results in normalization of total dendritic length in gp120 transgenic mice at 14 dpi. Values represent mean ± SEM (n = 5 animals in each group; *p < 0.05, one-way ANOVA followed by Duncan's test)
Effect of exercise on hippocampal BDNF levels
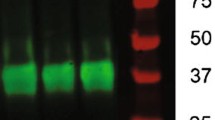
To determine whether gp120 alters the level of BDNF in the adult mouse brain, we performed a Western blot analysis. BDNF expression was downregulated in the entire brain, but more so in the hippocampus of the gp120 transgenic mice (25 and 45 %, respectively; p < 0.05, Fig. 5a, b). Additionally, proBDNF expression was increased by approximately 10 % in the hippocampus of the gp120 transgenic mice (Fig. S1a, b). We next determined whether exercise could restore the alteration of hippocmapal BDNF levels in the gp120 transgenic mice. Hippocampal BDNF levels in the gp120 transgenic mice were increased by 56 % after running for 10 days, but were still lower than those in the wild-type mice (p < 0.05) (Fig. 5c, d). By contrast, although BDNF levels in the wild-type mice also tended to increase with running, this change was not statistically different (Fig. 5d). Ten days after cessation of exercise, hippocampal BDNF levels were no longer significantly elevated in the gp120 transgenic mice (Fig. 5e). There was a positive correlation between the expression level of hippocampal BDNF and running distance (Fig. 5f).
Exercise increased hippocampal BDNF expression. Shown are sample immunoreactive bands from Western blots. Lysates from the entire brain or the hippocampus of wild-type and gp120 transgenic mice were subjected to Western blot analysis for brain-derived neurotrophic factor (BDNF; 15 kDa) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; as an internal control, 35 kDa). The ratio of BDNF/GAPDH was quantified. Values represent mean ± SEM (n = 3 animals in each group; *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA followed by Duncan's test). a, b BDNF expression was downregulated in the entire brain and the hippocampus of gp120 transgenic mice. c, d Hippocampal BDNF levels were increased in gp120 transgenic mice after running for 10 days. e Hippocampal BDNF levels were increased by running for 10 or 20 days but decreased by detraining in both wild-type and gp120 transgenic mice. f There was a positive correlation between the expression level of hippocampal BDNF and running distance
Effect of exercise on Cdk5 hyperactivity in the gp120 transgenic mice
To determine whether Cdk5 is activated in the gp120 transgenic mice, we performed a kinase activity assay following immunoprecipitation of Cdk5, using histone H1 as a substrate. Cdk5 kinase activity in the gp120 transgenic mice was 38 % higher than in the wild-type controls (Fig. 6a). As Cdk5 hyperactivity is regulated by p25, we determined levels of p25 and its precursor p35. The amount of p35 in the hippocampus as determined by Western blot analysis was 22 % lower in the gp120 transgenic mice compared with the wild-type controls. The corresponding levels of p25 in the gp120 transgenic mice increased by 25 % (Fig. 6b, c).
Hyperactivation of Cdk5 in gp120 transgenic mice was reversed by exercise. a Mouse brain lysates were immunoprecipitated with anti-Cdk5 antibody and subjected to a kinase activity assay using histone H1 as a substrate. Cdk5 is activated in gp120 transgenic mice. Values represent mean ± SEM (n = 5 animals in each group; *p < 0.05, Student's t test). b, c Shown are sample bands of p35 (b) and p25 (c) from Western blot analysis. Quantitative analysis demonstrated a downregulation of p35 and upregulation of p25 in the hippocampus of gp120 transgenic mice. Values represent mean ± SEM (n = 3 animals in each group; *p < 0.05; **p < 0.01, Student's t test). d Cdk5 activity in the hippocampus of gp120 transgenic mice was normalized after running for 20 days. Values represent mean ± SEM (n = 3 animals in each group; **p < 0.01; NS not significant, one-way ANOVA followed by Duncan's test)
We next determined whether the hyperactivation of Cdk5 observed in the gp120 transgenic mice could be reversed by voluntary exercise. The mice were provided access to running wheels for 20 days. Cdk5 kinase activities were similar in the exercised gp120 transgenic mice (mean ± SEM = 11,832.5 ± 1,670.2 counts/min) as compared with the exercised wild-type littermates (mean ± SEM = 11,211.0 ± 1,302.3 counts/min; mean wild-type runner) (Fig. 6d). Thus, exercise normalized the Cdk5 kinase activity in the gp120 transgenic mice.
Effect of BDNF treatment on aberrant neurite outgrowth in gp120-treated fNPCs
To determine the effect of gp120 and hyperactivated Cdk5 on neurite outgrowth, human fetal-derived neural precursor cells (fNPCs) were transfected with a GFP expression plasmid or co-transfected with p25 and Cdk5. The transfected cells were then exposed to recombinant gp120 protein (30 pM) for 24, 48 or 96 h. gp120 exposure resulted in an increase in neurite outgrowth in a time-dependent manner with significant effects at > 24 h. The average neurite lengths were (mean ± SEM) 88.8 ± 3.2 μm in untreated controls, 105.4 ± 4.5 μm after 24-h treatment with gp120, 117.9 ± 4.8 μm after 48-h treatment with gp120 and 127.9 ± 6.8 μm after 96-hour treatment with gp120, respectively. In fNPCs co-transfected with p25 and Cdk5, the average neurite length (mean ± SEM = 116.3 ± 3.4 μm) was increased by a similar extent compared with that in the gp120-treated cells. We next determined whether gp120-induced aberrant neurite outgrowth could be reversed by BDNF which is a factor upregulated by exercise (Neeper et al. 1996). Recombinant BDNF (100 ng/mL) with the gp120-treated fNPCs were treated for 24, 48, or 96 h. BDNF treatment completely abolished the gp120-induced neurite outgrowth. The average neurite lengths were (mean ± SEM) 104.5 ± 2.8 μm after 24-h treatment with BDNF, 79.0 ± 2.1 μm after 48-h treatment with BDNF, and 75.5 ± 2.1 μm after 96-h treatment with BDNF, respectively (Fig. 7a, b).
BDNF treatment normalized neurite outgrowth in gp120-treated fNPCs. a Representative photomicrographs demonstrate immunostaining of beta-III tubulin-positive (red) neurons in human fNPCs under different conditions. fNPCs were transfected with a GFP expression plasmid or co-transfected with plasmids encoding p25 and Cdk5. The transfected cells were then exposed to recombinant gp120 protein (30 pM) with or without recombinant BDNF (100 ng/mL) for 24, 48, or 96 h. Cells were immunostained using anti-beta-III tubulin (red). Scale bar = 200 μm. b Quantitative analysis of neurite length demonstrated that gp120 exposure resulted in an increase in neurite outgrowth in a time-dependent manner by a similar amount compared with that in fNPCs co-transfected with p25 and Cdk5. BDNF treatment completely abolished gp120-induced neurite outgrowth. Values represent mean ± SEM (*p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA followed by Duncan's test)
Rolipram failed to reinforce NPC proliferation and survival effect of exercise on newly generated cells
We determined whether the administration of rolipram, which exerts effects specifically upon the activation of the cyclic adenosine 3′,5′-monophosphate (cAMP) cascade and has been reported to increase blood flow and promote recovery from spinal cord trauma (Costa et al. 2013), led to a restoration of the defects in NPC proliferation and survival in the gp120 transgenic mice subjected to 10 days of running followed by 10 days of a sedentary period; 3 mg/kg of rolipram was given intraperitonially once a day for either 10 or 20 days. To study the effect on NPC proliferation, the mice were administered a single dose of BrdU and killed 24 h later (Fig. S2a). We found that rolipram treatment after 10 days of running failed to counteract the effect of detraining on NPC proliferation (Fig. S2b).
Another group of animals was given 50 mg/kg of BrdU at 2-h intervals for 6 h to study the effect of rolipram on the survival of NPCs. The mice in the control group were killed 1 day after the initial BrdU injection. The other three groups were either sedentary for 20 days, running for 10 days and sedentary for 10 days, or running for 10 days and received rolipram (3 mg/kg/day intraperitoneally) for 10 days while they were sedentary. All mice were killed on day 21 (Fig. S2c). Approximately 30 % of newborn cells survived 20 days after proliferation both in the wild-type and gp120 transgenic mice. The survival rate was increased by 85 % in the wild-type and 79 % in the gp120 transgenic mice with 20 days of running. A 10-day period of running protected 53 and 74 % of newborn cells in the wild-type and gp120 transgenic mice, respectively. These proportions were not affected by rolipram treatment after 10 days of running (Fig. S2d). Taken together, we demonstrate that the rolipram treatment did not reinforce the proliferative and survival effect of the 10-day running on newborn cells.
The effect of exercise on NPC proliferation was independent of norepinephrine signaling
We next determined whether running increases hippocampal NPC proliferation through enhanced norepinephrine (NE) neurotransmission via blockade of the NE receptors during the animals' active period. The mice were allowed to run for 10 days and received intraperitoneal injections of the β-adrenergic antagonist, propranolol, at 5 mg/kg for the last 3 days of a 10-day period of exercise (Fig. S3a). Considering receptor antagonist administration for longer than 4 days is reported to induce receptor supersensitivity, mice were given propranolol for 3 days and analyzed 2 h after BrdU injection. We found that running for 10 days increases proliferating NPCs both in the wild-type and gp120 transgenic mice, but propranolol failed to block any exercise-induced increase in NPC proliferation (Fig. S3b).
Discussion
In this study, we found that exercise reversed impaired NPC proliferation, survival, and neurogenesis in an HIV-gp120 transgenic model, and the effect was mediated by BDNF production and Cdk5 regulation.
NPC proliferation is decreased in the hippocampus of the gp120 transgenic mice
NPCs diminished in number in the gp120 transgenic mice, which is consistent with previous reports (Lee et al. 2011; Okamoto et al. 2007). The mechanism by which gp120 regulates NPC proliferation is not well understood, but several possibilities exist. Previous studies showed that gp120 perturbed intracellular signaling pathways, such as Src family kinase Lyn, PI3K, Akt, the focal adhesion-related proline-rich tyrosine kinase Pyk2, and proteins of the MAPK family which are involved in gp120-induced macrophage activation and neurotoxicity (Cheung et al. 2008; Kaul et al. 2007; Perfettini et al. 2005). gp120 may regulate intracellular proteins through direct interactions with surface-expressed molecules on NPCs. Of these, MAPKs are involved in biological activities of proliferation and differentiation (Ono and Han 2000). In fact, gp120 activated the p38 MAPK cascade in NPCs, thereby causing arrest in cell cycle progression of NPCs at the G1 phase, resulting in decreased proliferation (Okamoto et al. 2007). Stromal cell-derived factor-1 (SDF-1) acts as a chemoattractant and a mitogenic stimulus for NPCs. gp120 may block the effects of SDF-1 by interacting with CXCR4 receptors on NPCs (Tran and Miller 2003). Additionally, gp120 reduces levels of BDNF (Nosheny et al. 2004), which is a tropic factor for adult neurogenesis and neuroplasticity. We confirmed that a significant decrease in hippocampal BDNF was also seen in the gp120 transgenic mice. Autopsy studies show that HIV can infect NPCs in the brains of HIV-infected children (Schwartz et al. 2007). Thus, expression of gp120 within progenitor cells could potentially impair neurogenesis.
Exercise induces NPC proliferation which is reversed by detraining
We found that the ability to run was not impeded in the gp120 transgenic mice. The average running distance of the gp120 transgenic mice was similar to that of the wild-type mice. Thus, the effect of exercise on neurogenesis could be reliably compared in the two sets of animals. Running for 3 or 10 days increased NPC proliferation in the adult dentate gyrus in both the wild-type and gp120 transgenic mice. This acute effect wore off, if exercise was continued. In the wild-type mice, there was no further increase in NPC proliferation under sustained physical activity, as previously shown (Kronenberg et al. 2006). Thus, continuous exercise kept NPCs in balance. In the gp120 transgenic mice, the effect of exercise was more pronounced with marked increase in NPC proliferation and a slower decline with prolonged exercise. Interestingly, the benefits of exercise on NPC proliferation were lost by abrupt cessation of exercise in both types of animals. However, this was more pronounced in the wild-type animals where the numbers of proliferating NPC were lower than animals not subjected to exercise. These observations suggest a loss of adaptive responses in the gp120 transgenic animals. Thus, an abrupt cessation of exercise may be more detrimental than maintaining a sedentary state.
Exercise enhances neurogenesis and normalizes dendritic outgrowth of neurons
Most newborn cells in the adult hippocampus die quickly. Consequently, the outcome of neurogenesis is not only based on expansion of the precursor cell pool but also by increased survival of newborn neurons (Lledo et al. 2006). The number of newborn neurons decreased over time in both the wild-type and gp120 transgenic mice. gp120 did not impair survival of newly generated cells. Importantly, there was a steady increase in survival with continued exercise. Similarly, previous studies show a 20–35 % increase in survival of newly generated cells after a 3-week exercise period (Snyder et al. 2009; Wu et al. 2008).
In contrast to cell proliferation, the number of newborn neurons did not return to control values at 20 days after the initiation of physical activity. Thus, the effect of running on NPC proliferation is short lived and wears off over a number of weeks. However, once the NPCs differentiate to neurons, that effect is long lived in both the wild-type and gp120 transgenic animals.
Both the population of proliferating cells and the number of newborn granule neurons were decreased in gp120 transgenic mice. Surprisingly, the dendritic trees of newborn neurons in the gp120 transgenic mice were longer and more complex than those in the wild-type mice, suggesting a compensatory increase in dendrite formation in remaining neurons. Similar increases in dendritic arborization have been seen in acute models of neuronal injury such as seizures and hypoxia (Overstreet-Wadiche et al. 2006; Walter et al. 2007). The morphology of the dendrites and dendritic spine appeared normal. This is in contrast to the dendritic abnormality seen in HIV-1-infected individuals (Ellis et al. 2007). In normal conditions, physical and electrical activity accelerate neuronal maturation through enhancing neuronal circuit activity by acting directly on developing neurons (Piatti et al. 2011) or by inducing secretion of BDNF from mature neighboring cells (Ma et al. 2009). Consistent with these studies, we demonstrated that running increased dendritic development in the wild-type mice. Strikingly, exercise normalized dendritic arborization in newborn neurons in the gp120 transgenic mice. This suggests that exercise-enhanced neurogenesis may inhibit the compensatory increase in dendritic formation.
Exercise restores BDNF levels which normalize dendritic arborization
The mechanisms underlying NPC proliferation and survival have focused on growth factors including BDNF in the adult hippocampus of running animals (Kobilo et al. 2011; Marlatt et al. 2012; Vivar et al. 2012). In a normal rodent's brain, physical exercise is well known to increase BDNF expression (Neeper et al. 1996). In this study, although BDNF levels in the wild-type mice tended to increase with running for 10 days, this change was not statistically significant. BDNF levels are decreased in brains of an HIV transgenic rat model (Rao et al. 2011). Furthermore, gp120 promotes accumulation of pro-BDNF concomitantly with a decrease in mature BDNF. An altered ratio of proBDNF to mature BDNF was also seen in postmortem brain tissue of HAND subjects (Bachis et al. 2012). We confirmed these observations in the gp120 transgenic mice. We observed a dramatic increase in BDNF levels in the hippocampus after running and the significantly decreased BDNF levels in the gp120 transgenic mice could be restored by running. Consistent with other studies we found that cessation of exercise results in decay of BDNF levels (Berchtold et al. 2005; Hopkins et al. 2011). Interestingly, the dendritic arborization in the dentate gyrus of gp120 transgenic mice was abnormal. While the cells were fewer, they showed longer processes and increased branching. These effects could be duplicated in vitro by treatment of NPCs with gp120. We used a subtoxic dose (30 pM) of gp120 which is not able to trigger cell death. The higher dose of gp120 (>200 pM) inhibited neurite outgrowth and induced apoptotic cell death (Alirezaei et al. 2007; Hoke et al. 2009; Zhang et al. 2012). The in vivo findings could be reversed by exercise and the in vitro effects were blocked by BDNF.
BDNF also effects synaptic structure and function (Lu et al. 2008). Application of BDNF to hippocampal slices in culture increases the densities of dendritic spines and synapses (Tyler and Pozzo-Miller 2001). However, molecular mechanisms by which dendritic arborization is altered by gp120 and rescued upon exercise or by treatment of BDNF remain to be elucidated.
Effect of exercise on NPC proliferation is independent of NE neurotransmission or cAMP signaling
Animal studies have implicated increased 5-HT/NE neurotransmission in exercise-induced BDNF expression (Ivy et al. 2003). Furthermore, a major signal transduction pathway mediating 5-HT and NE action utilizes cAMP. Herein, we administered the β-adrenergic antagonist, propranolol, with exercise to determine whether enhancement of NE neurotransmission is required for the increased NPC proliferation during exercise. We also determined whether the administration of rolipram, which activates the cAMP cascade, led to restoration of the defects in NPC proliferation and survival. Inhibition of NE neurotransmission did not block exercise-induced upregulation of NPC proliferation and activation of cAMP signaling did not facilitate the proliferative and survival effect of running on newborn cells.
Exercise normalizes Cdk5 hyperactivation
Extensive studies have demonstrated an important role for Cdk5 kinase in neuronal function. We observed that gp120 causes Cdk5 hyperactivation leading to breakdown of p35 into p25. Accordingly, Cdk5 activity and p25 protein expression levels were increased with a concomitant decrease in p35 levels in the gp120 transgenic mice. These effects were rescued by exercise.
Dysfunction of Cdk5 has been implicated in a number of neurological disorders including Alzheimer's disease (Zheng et al. 2010), Parkinson's disease (Smith et al. 2003), Huntington's disease (Paoletti et al. 2008), amyotrophic lateral sclerosis (Nguyen et al. 2001), and ischemia (Wang et al. 2003). The activity of Cdk5 and p25 was found to be increased in cortical cultures exposed to supernatants from HIV-infected macrophages (Wang et al. 2007). Overexpression of Cdk5 was also present in the brains of patients with HIV encephalitis and the gp120 transgenic mice (Patrick et al. 2011). In adults, Cdk5 plays a role in learning and memory (Hawasli et al. 2007). Dysregulation of Cdk5 is implicated in neurodegenerative disorders, which are accompanied by deficiencies in neurogenesis where it regulates dendritic growth and spine development, through modulating the cytoskeletal dynamics (Cheung and Ip 2007). BDNF prevents the degradation of p35 in neurons by activation of PKC delta, thus stabilizing the Cdk5/p35 complexes (Zhao et al. 2009). Thus, the cognitive impairments that are hallmarks of these diseases may involve the deleterious effects that Cdk5 dysregulation may affect hippocampal neurogenesis.
There are some limitations to our study. The gp120 transgenic model is based on a single HIV protein and hence may not fully replicate effects of the virus. In fact this model does not display inflammation in the brain hence the effects of exercise on macrophage and glial cell activation n could not be studied. Our study was focused on the fate of neuronal lineage. Effects of exercise on other cell types need to be determined. All the same, the model allows the study of the effects of gp120 and exercise on NPC proliferation and differentiation independent of the inflammatory pathways. Furthermore, it may be reasonable to assume that enhanced neurogenesis will lead to improved neurocognitive function (Bechara and Kelly 2013). However, future behavioral studies may be needed to determine if that is the case in this model system as well.
In summary, gp120 has profound effects on the adult hippocampus which includes decreased proliferation and differentiation of NPC and aberrant neurite formation. This is mediated by its effect on the Cdk5/p35 pathway via BDNF. These effects can be reversed by exercise as it increases BDNF levels, but is independent of effects of exercise on NE neurotransmission or cAMP signaling. However, sustained exercise is necessary as abrupt cessation of exercise not only reverses the effects of exercise but also has a negative effect on each of these phenomena. Recently, some flavonoids were shown to increase BDNF production and had neuroprotective effects against HIV proteins (Nath et al. 2012) and paroxetine also increases neurogenesis (Lee et al. 2011). However, exercise has several advantages over pharmacological approaches. It is cost effective and can be performed over the life of the individual. Furthermore, exercise exerts its effects on neurogenesis in a highly controlled manner where as pharmacological approaches may lead to over stimulation of receptors with untoward effects. If the effects of detraining could be tempered by pharmacological intervention, then a combination of exercise and drug therapy could be considered as a therapeutic approach.
References
Alirezaei M, Watry DD, Flynn CF, Kiosses WB, Masliah E, Williams BR, Kaul M, Lipton SA, Fox HS (2007) Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci 27:11047–11055
Ang ET, Tai YK, Lo SQ, Seet R, Soong TW (2010) Neurodegenerative diseases: exercising toward neurogenesis and neuroregeneration. Front Aging Neurosci. doi:10.3389/fnagi.2010.00025
Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I (2012) Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci 32:9477–9484
Bechara RG, Kelly AM (2013) Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav Brain Res 245:96–100
Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW (2005) Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 133:853–861
Chehrehasa F, Meedeniya AC, Dwyer P, Abrahamsen G, Mackay-Sim A (2009) EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods 177:122–130
Cheung ZH, Ip NY (2007) The roles of cyclin-dependent kinase 5 in dendrite and synapse development. Biotechnol J 2:949–957
Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG (2008) Signaling mechanism of HIV-1 gp120 and virion-induced IL-1beta release in primary human macrophages. J Immunol 180:6675–6684
Costa LM, Pereira JE, Filipe VM, Magalhaes LG, Couto PA, Gonzalo-Orden JM, Raimondo S, Geuna S, Mauricio AC, Nikulina E, Filbin MT, Varejao AS (2013) Rolipram promotes functional recovery after contusive thoracic spinal cord injury in rats. Behav Brain Res 243C:66–73
Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21:1–14
D'Hooge R, Franck F, Mucke L, De Deyn PP (1999) Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci 11:4398–4402
Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H (2007) Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130:1146–1158
Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8:33–44
Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439:589–593
Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA (2007) Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci 10:880–886
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16
Hoke A, Morris M, Haughey NJ (2009) GPI-1046 protects dorsal root ganglia from gp120-induced axonal injury by modulating store-operated calcium entry. J Peripher Nerv Syst 14:27–35
Hopkins ME, Nitecki R, Bucci DJ (2011) Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience 194:84–94
Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA (2003) Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav 75:81–88
Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA (2007) HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ 14:296–305
Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H (2011) Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem 18:605–609
Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G (2006) Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging 27:1505–1513
Krucker T, Toggas SM, Mucke L, Siggins GR (1998) Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience 83:691–700
Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, Song H, Nath A, Venkatesan A (2011) Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol Dis 41:678–687
Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437:1370–1375
Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL (2010) HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol 5:294–309
Lledo PM, Alonso M, Grubb MS (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7:179–193
Lu Y, Christian K, Lu B (2008) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89:312–323
Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H (2009) Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323:1074–1077
Marlatt MW, Potter MC, Lucassen PJ, van Praag H (2012) Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol 72:943–952
Marzolini S, Oh P, McIlroy W, Brooks D (2013) The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabil Neural Repair 27(5):392–402
Nath A (2002) Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 186(Suppl 2):S193–S198
Nath S, Bachani M, Harshavardhana D, Steiner JP (2012) Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J Neurovirol 18:445–455
Nattel S, Rangno RE, Van Loon G (1979) Mechanism of propranolol withdrawal phenomena. Circulation 59:1158–1164
Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW (1996) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726:49–56
Nguyen MD, Lariviere RC, Julien JP (2001) Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron 30:135–147
Nosheny RL, Bachis A, Acquas E, Mocchetti I (2004) Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci 20:2857–2864
Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA (2007) HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell 1:230–236
Ono K, Han J (2000) The p38 signal transduction pathway: activation and function. Cell Signal 12:1–13
Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL (2006) Seizures accelerate functional integration of adult-generated granule cells. J Neurosci 26:4095–4103
Paoletti P, Vila I, Rife M, Lizcano JM, Alberch J, Gines S (2008) Dopaminergic and glutamatergic signaling crosstalk in Huntington's disease neurodegeneration: the role of p25/cyclin-dependent kinase 5. J Neurosci 28:10090–10101
Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E (2011) Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. Am J Pathol 178:1646–1661
Perfettini JL, Castedo M, Nardacci R, Ciccosanti F, Boya P, Roumier T, Larochette N, Piacentini M, Kroemer G (2005) Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J Exp Med 201:279–289
Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, Schinder AF (2011) The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31:7715–7728
Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, Hillman CH (2013) Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. J Pediatr 162(3):543–551
Rao JS, Kim HW, Kellom M, Greenstein D, Chen M, Kraft AD, Harry GJ, Rapoport SI, Basselin M (2011) Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflammation 8:101
Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DM, Hazra R, Major EO (2007) Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol 13:274–283
Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, O'Hare MJ, Callaghan S, Slack RS, Przedborski S, Anisman H, Park DS (2003) Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 100:13650–13655
Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA (2009) The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus 19:898–906
Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP (2010) Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging 31:1937–1949
Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L (1994) Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367:188–193
Tran PB, Miller RJ (2003) Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci 4:444–455
Tyler WJ, Pozzo-Miller LD (2001) BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21:4249–4258
Veeranna, Shetty KT, Amin N, Grant P, Albers RW, Pant HC (1996) Inhibition of neuronal cyclin-dependent kinase-5 by staurosporine and purine analogs is independent of activation by Munc-18. Neurochem Res 21:629–636
Vivar C, Potter MC, van Praag H (2013) All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci15:189–210
Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC (2007) Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci 27:7541–7552
Wang J, Liu S, Fu Y, Wang JH, Lu Y (2003) Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci 6:1039–1047
Wang Y, White MG, Akay C, Chodroff RA, Robinson J, Lindl KA, Dichter MA, Qian Y, Mao Z, Kolson DL, Jordan-Sciutto KL (2007) Activation of cyclin-dependent kinase 5 by calpains contributes to human immunodeficiency virus-induced neurotoxicity. J Neurochem 103:439–455
Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM (2008) Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol 105:1585–1594
Zhang Y, Shi Y, Qiao L, Sun Y, Ding W, Zhang H, Li N, Chen D (2012) Sigma-1 receptor agonists provide neuroprotection against gp120 via a change in bcl-2 expression in mouse neuronal cultures. Brain Res 1431:13–22
Zhao CT, Li K, Li JT, Zheng W, Liang XJ, Geng AQ, Li N, Yuan XB (2009) PKCdelta regulates cortical radial migration by stabilizing the Cdk5 activator p35. Proc Natl Acad Sci U S A 106:21353–21358
Zheng YL, Amin ND, Hu YF, Rudrabhatla P, Shukla V, Kanungo J, Kesavapany S, Grant P, Albers W, Pant HC (2010) A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J Biol Chem 285:34202–34212
Acknowledgments
We thank Dr. Valerie Toodle for technical assistance. This work was supported by grants from the NIH (R01-DA024593; R01-NS039253) and NINDS intramural funds.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
proBDNF expression was increased in the gp120 transgenic mice. a Shown are sample immunoreactive bands from Western blot analysis. Lysates from the hippocampus of the wild-type and gp120 transgenic mice were subjected to Western blot analysis for proBrain-derived neurotrophic factor (proBDNF; 34 kDa) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; as an internal control, 35 kDa). b The ratio of proBDNF/GAPDH was quantified. proBDNF expression was upregulated in the hippocampus of the gp120 transgenic mice. Values represent mean ± SEM (n = 3 animals in each group; *p < 0.05, one-way ANOVA followed by Duncan's test). (PDF 23 kb)
Fig. S2
Rolipram did not reinforce NPC proliferation and survival effect of exercise on newly generated cell. a Experimental design. To study the effect of the PDE4 inhibitor, rolipram on NPC proliferation, mice received rolipram (3 mg/kg) daily for ten consecutive days at 11 days after treatment (running or sedentary activity) for 10 days. Mice were administered a single dose of BrdU and killed 24 h later. b Rolipram treatment after 10 days of running failed to counteract the effect of detraining on NPC proliferation. Values represent mean ± SEM (n = 5 animals in each group; one-way ANOVA followed by Duncan's test). c Experimental design. For survival studies, mice were given 50 mg/kg of BrdU at 2-h intervals for 6 h. Rolipram (3 mg/kg) was administered to mice daily for ten consecutive days at 11 days after BrdU labeling. The mice were then killed either 1 or 21 days after the initial BrdU injection. d The survival rate was not increased by rolipram treatment after 10 days of running. Values represent mean ± SEM (n = 5 animals in each group; one-way ANOVA followed by Duncan's test). (PDF 91 kb)
Fig. S3
The effect of exercise on NPC proliferation was independent of NE signaling. a Experimental design. Mice were allowed free access to a running wheel for 10 days. Intraperitoneal injections of the β-adrenergic antagonist, propranolol at 5 mg/kg were given for the last 3 days of a 10-day period of exercise. All the animals were analyzed 24 h after BrdU injection. b Exercise increased proliferating NPCs both in the wild-type and gp120 transgenic mice, but propranolol failed to block exercise-induced increase in NPC proliferation. Values represent mean ± SEM (n = 5 animals in each group; one-way ANOVA followed by Duncan's test). (PDF 45 kb)
Rights and permissions
About this article
Cite this article
Lee, MH., Amin, N.D., Venkatesan, A. et al. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J. Neurovirol. 19, 418–431 (2013). https://doi.org/10.1007/s13365-013-0194-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-013-0194-6