Abstract
MicroRNAs (miRNAs) are 20–22 nucleotide length noncoding RNA molecules that represent key regulators of many normal cellular functions. miRNAs undergo two processing steps which transform a long primary transcript into the mature miRNA. Available literatures demonstrate the association between alterations in the expression of miRNAs and the progression of numerous human disorders. Even though significant advances have been made, many fundamental questions about their expression and function still remain unanswered. Identifying factors that block the negative action of drugs of abuse on the miRNAs could help in identifying new therapeutic strategies. In this review, we briefly discuss the importance of miRNAs on HIV, strategies used by virus to avoid the cells' antiviral miRNA defenses, and how HIV might control and regulate host cell genes by encoding viral miRNAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
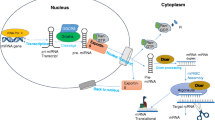
As the name suggests, microRNAs (miRNA) are tiny regulatory RNA molecules ~20–22 nucleotides (nt) in length. miRNAs are encoded by the genome but are not translated into protein. MicroRNAs are produced from a single arm of imperfect, 80-nt-long RNA hairpins located within polymerase II-derived transcripts referred to as a primary transcript (pri-miRNA) which is then trimmed in the nucleus by the microprocessor complex containing RNase Drosha and DGCR8. The resulting product is ~70-nucleotide-long stem-loop precursor miRNA (pre-miRNA). Pre-miRNA is exported out of the nucleus to the cytoplasm via the exportin-5. In the cytoplasm, pre-miRNA is processed by type III RNase Dicer to miRNA/miRNA* duplex of 19 to 25 nucleotides. miRNA/miRNA* is incorporated into the RNA-induced silencing complex (RISC) where miRNA* is degraded, while miRNA serves as a “guide strand” for messenger RNA (mRNA) targeting. RISC is a multiprotein complex consisting of argonautes, RNA processing/degrading enzymes, Dicer, HIV-1 TRBP, a cofactor, and several other proteins that is required for miRNA-mediated silencing (Bartel 2004; Kim et al. 2009; Lee et al. 2004).
miRNAs control the gene expression by contributing significantly to posttranscriptional regulation by binding to the 3′ untranslated region (UTR) of mRNAs, thereby repressing the translation or inducing mRNA degradation. The seed sequence, 5′ nucleotides 2–7 of the mature miRNA, must be complementary to the viral target for silencing or inhibiting virus replication (Bartel 2004).
miRNAs in disease
There are evidences from recent reports that miRNAs are critical key regulators for normal cellular functions like proliferation, differentiation, development, apoptosis, signal transduction, and establishment of cell lineage (Eisenberg et al. 2007; Kloosterman and Plasterk 2006; Lu et al. 2005; Wang et al. 2009). Expression of several miRNAs has been shown to be elevated or repressed in activated T cells in vitro (Cobb et al. 2006). Appealing evidences have accumulated to suggest the role of aberrant miRNA expression in various human diseases, such as cancer (Calin and Croce 2006; Croce and Calin 2005; Huang et al. 2007), autoimmune disease (Dai et al. 2007; Stanczyk et al. 2008; Zhao et al. 2010), neurodegenerative disease (Kim et al. 2007; Wang et al. 2008), inflammatory diseases (Sonkoly et al. 2007), muscular (Eisenberg et al. 2007) and cardiovascular disorders (Care et al. 2007; Ikeda et al. 2007), and developmental abnormalities and psychiatric disorders, such as schizophrenia (54). miRNAs can influence host–virus interaction in a variety of ways such as direct modulation of viral replication, by affecting viral susceptibility, and also by indirect modulation of cellular genes that influence viral propagation (Kumar and Jeang 2008; Scaria et al. 2007; Yeung et al. 2007).
Cellular miRNA in AIDS
The first evidence for the antiviral activity of cellular miRNAs came from a study, where researchers showed knockdown of miR-32 expression or deletion of the miRNA target sequence from the viral genome significantly enhanced primate foamy virus type 1 (PFV-1) replication suggesting that miR-32 inhibited infection by PFV-1 (Lecellier et al. 2005). Significant advances have been made in the field of miRNA since then. Recent findings of cellular change in miRNA expression during human immune deficiency virus (HIV) infection (Triboulet et al. 2007; Yeung et al. 2005a), contributing to HIV-1 latency in primary CD4+ T lymphocytes (Huang et al. 2007) and distinct patterns of miRNA in HIV-1 provirus plasmid-transfected HeLa cells (Sung and Rice 2009), provide supporting evidences to the fact that different expression profiles of cellular miRNAs associate with different stages in HIV-1 disease progression.
Several cellular miRNAs which potentially target a set of accessory genes of HIV-1 have recently been identified. Using computational approach, five human T-cell miRNAs targeting the highly conserved regions across all clades of HIV-1 were predicted (Hariharan et al. 2005). Significant change in at least 62 miRNAs from HIV-infected PBMC (Houzet et al. 2008) and change in miRNA profile in human cells after HIV-1 protein expression have also been reported (Yeung et al. 2005b). Table 1 summarizes the list of cellular miRNAs identified in relation to HIV/AIDS, the functions of which are discussed in this review.
HIV suppresses miRNA silencing pathway
Both Dicer and Drosha have been shown to contribute to the suppression of HIV-1 replication, suggesting a role of miRNA silencing machinery in the control of HIV-1 in vitro. Even though it is not clear whether this effected through direct or indirect mechanism, it is proposed that miRNAs are involved in negatively controlling HIV-1 replication (Triboulet et al. 2007).
Upregulation of 11 miRNA, including miR-122, miR-370, miR-373, and miR-297, moieties detected only in HIV-1 infected cells was noted during infection in Jurkat cells (Triboulet et al. 2007). HIV-1 also suppresses expression of the polycistronic miRNA cluster, miR-17/92, which encodes for miR-17-(5p/3p), miR-18, miR-19a, miR-20a, miR-19b-1, and miR-92-1. There was enhancement of HIV-1 production in Jurkat cells after the knockdown of specific siRNA against pri-miR-17/92, which suggests that downregulation of miR-17/92 affects virus replication. Since miR-17/92 does not directly target the viral genome, it seems that the effect of miR-17/92 on HIV-1 replication might be by targeting cellular protein(s). miRNA cluster miR-17/92 inhibits HIV-1 replication through repression of the histone acetyltransferase P300/CBP-associated factor (PCAF), an important cofactor for Tat in HIV-1 gene expression (Triboulet et al. 2007). miR-17-5p and miR-20a target the 3′ UTR region of histone acetylase PCAF to inhibit mRNA translation (Kiernan et al. 1999; Ott et al. 2004). This miRNA inhibition pathway is required for efficient viral replication (Lagos-Quintana et al. 2001; Triboulet et al. 2007).
Cellular miRNA contributes to HIV latency
During HIV-1 latency, the provirus gets stably integrated into the host genome without producing any viral transcripts or proteins. This latency helps HIV-1 to evade immune responses and the action of antiretroviral drugs (Berkhout 2008). HIV-1 uses miRNA-mediated downregulation of viral gene expression to its own advantage by allowing it to be in a state of viral latency.
Several cellular miRNAs target a set of accessory genes of HIV (Ahluwalia et al. 2008; Nathans et al. 2009; Sung and Rice 2009; Wang et al. 2009). The potential miRNA target sites were mapped in the 3′ UTR of HIV-1 RNAs in resting T cells (Huang et al. 2007). Among the cellular miRNAs with predicted binding sites in these regions, five (miR-28, miR-125b, miR-150, miR-223, and miR-382) are abundant in resting T cells compared to activated T cells (Huang et al. 2007) and monocytes (Wang et al. 2009). Increased HIV-1 production from pNL4-3-transfected cells upon neutralization of inhibitory effects of these five miRNAs suggests that these differentially expressed cellular miRNAs inhibit HIV-1 expression in primary resting CD4+ T cells through their interactions with the 3′ end of HIV-1 RNA, thereby contributing to viral latency observed in quiescent cells. However, these miRNA inhibitors do not affect cellular proliferation status (Huang et al. 2007).
Altered expression of cellular miRNA on immune cell differentiation and viral infection
In addition to their direct effect on HIV-1 replication, miRNAs also have significant roles in host innate immune defense regulation. Monocyte differentiation into dendritic cells is regulated and coordinated by different miRNAs (Noorbakhsh et al. 2010; Wang et al. 2009). Even though cells of the monocyte/macrophage lineage are susceptible to HIV-1 infection, monocyte-derived dendritic cells (MDDCs) are poor producers of HIV-1 compared with monocyte-derived macrophages. Wang et al. (2009) recently reported that although both monocytes and macrophages expressed anti-HIV-1 miRNAs like miR-28, 150, 223, and 382, their levels were different in monocytes and macrophages, freshly isolated monocytes expressing significantly higher levels of anti-HIV miRNAs than donor-matched macrophages. The modulation of the anti-HIV-1 miRNA levels in monocytes/macrophages could alter the cell's susceptibility to HIV-1 infection (Wang et al. 2009). The suppression of these anti-HIV-1 miRNAs in monocytes facilitates HIV-1 infectivity, whereas increase of the anti-HIV-1 miRNA expression in macrophages inhibited HIV-1 replication. It is suggested that monocyte differentiation and HIV-1 susceptibility are linked by a common set of miRNAs. The negative association of intracellular miRNA expression and cell differentiation from monocytes to either macrophages or MDDCs may confer resistance to HIV infection in monocytes. The miRNA's profiling analysis showed miR-223 to target HIV-1 in the 3′ end of the viral genome repressing its expression (Sung and Rice 2009).
miR-198, another miRNA which is downregulated during monocyte to macrophage differentiation, is capable of repressing HIV-1 replication through downregulation of cyclin T1 protein expression without affecting cyclin T1 mRNA levels supporting its anti-HIV-1 function (Sung and Rice 2009). The expression of cyclin T1, required for transactivation by HIV-1 Tat, increases during macrophage differentiation and enhances HIV-1 replication within macrophages (Liou et al. 2002). It is suggested that a cellular negative-feedback loop is activated that results in elevated levels of miR-198 following cyclin T1 upregulation and a subsequent dampening of the induction of cyclin T1 (Sung and Rice 2009).
Out of the five miRNAs identified against HIV targets, miR-29a, miR-29b, and miR-149 are expressed in T cells. miR-29a and miR-29b, which have highly related sequence similarity, not only inhibit Nef expression but also HIV virus replication in HEK293T cells and Jurkat T cells (Ahluwalia et al. 2008). miR-29a and miR-29b target the nef gene, whereas miR-149, miR-324-5p, and miR-378 target vpr, vif, vpu (Hariharan et al. 2005), nef, vpr, vif, and vpu being the four accessory genes of HIV-1. Their predicted target site is highly conserved in the sequences from all clades of HIV-1 (A, B, C, D, F, and H), except clade O.
Target prediction analysis by Nathans et al. suggested that the HIV-1 3′-UTR can be targeted by 11 miRNAs (miR-29a, 29b, 29c, 149, 147, 138, 513, 516-5p, 581, 644, and 646, Nathans et al. 2009). Even though the inhibition of miR-29a, b, or c increased HIV-1 production, highest effect was associated with miR-29a.
miRNA in neuro-AIDS
Activation of macrophages/microglia is thought to play a key role in development and progression of neuro-AIDS. Increased expression of miR-146a has been documented in T cells following proinflammatory stimuli (Taganov et al. 2006), or during viral infection (Motsch et al. 2007). Similarly, HIV-1-infected primary human fetal microglia also expresses increased level of miR-146a (Rom et al. 2010). Monocyte chemotactic protein-2 (MCP-2), a proinflammatory cytokine secreted by the acute HIV-infected microglia, is a ligand for C–C chemokine receptor type 5 (CCR5). There is a negative correlation between increased expression of miR-146a and MCP-2 inhibition, which suggests a possible role for miR-146a in modulating expression of MCP-2 during the course of HIV infection in cultured microglial cells (Rom et al. 2010). The activity of miR-146a does not interfere with viral replication in this cellular system.
miR-146a, together with miR-155, is thought to be involved in innate immunity by regulating the acute immune response after pathogen recognition by TLR. miR-155 participates in the maturation of human dendritic cells (DC). Since miR-155 decreases dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) expression, by downregulating transcription factor PU.1, miR-155 could be developed as a therapeutic target to prevent entrance of HIV through DC-SIGN binding (Martinez-Nunez et al. 2009).
HIV-associated neurocognitive disorders (HAND) develop in a subset of individuals infected with HIV-1. Since HIV does not infect neurons in the brain, but infects macrophages and microglia, it is suggested that HAND results from an indirect neurotoxicity. Significant upregulation of three miRNAs: miR-21, miR-142-3p, and miR-142-5p has been identified in brains of HIV/SIV-infected humans and monkeys (Yelamanchili et al. 2010). miR-21 expression has been found in a number of cell types, whereas miR-142 expression is largely confined to the hematopoietic system. Myocyte enhancer factor 2C (MEF2C), which is a CNS transcription factor and a target of miR-21 in neurons, expression in cells is repressed by miR-21. Repression of MEF2C could lead to deficits in neurocognitive functions seen in HIV and is a potential pathogenic factor in neurodegenerative disorders such as HAD and HAND (Yelamanchili et al. 2010).
Eletto and colleagues reported that a group of six miRNAs (374, 128a, 128b, 100, 25, and 99a) were upregulated and seven miRNAs (let-7e, 298, let-7f, let-7c, let-7b, 320, and 214) were downregulated by Tat in rat primary cortical neurons. Among these, miR-128a has been found to be enriched in the brain, preferentially in the mature neurons. Synaptosomal-associated protein 25 (SNAP25) is one of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors which are key regulators of membrane fusion. HIV-Tat promotes miR-128a activity, leading to a reduction in SNAP25 expression in neurons (Berkhout 2008; Eletto et al. 2008). Tat-mediated the downregulation of SNAP25 through miR-128a, suggesting that increased upregulation of specific microRNAs could precede neuronal damage.
Both miR-125a and miR-22 were found to be upregulated in HIV or HIV/major depressive disorder (MDD, Tatro et al. 2010). Induction of these dysregulated miRNAs leads to decreased protein translation of interferon-induced transmembrane protein 3 (IFITM3), an intracellular membrane protein, and soluble tumor necrosis factor receptor (sTNFR1A), a secreted protein in primary human neuronal cultures. IFITM protein is shown to inhibit HIV replication (Lu et al. 2011), and sTNFR1A is involved in neuroinflammation. miR219, which was shown to modulate NMDA receptor-mediated neurobehavioral dysfunction in schizophrenia (Kocerha et al. 2009), is also shown to be upregulated in HIV/MDD (Tatro et al. 2010).
HIV encephalitis (HIVE) brains show altered expression of multiple miRNAs (miR-129, miR-129-3, and miR-130). HIV-induced miRNA dysregulation in brain targets diverse biological processes, including neuroinflammation, metabolic processes, and cell death. Caspase-6, -7, -8, and -9 were associated with multiple miRNAs that were suppressed in HIVE brains (Noorbakhsh et al. 2010).
MicroRNA expression and drugs of abuse
The effect of different drugs of abuse and anti-HIV miRNA expression by different target cells is poorly understood. Among the five known anti-HIV miRNAs which are abundant in monocytes, expression of four (miRNA-28, miRNA-125b, miRNA-150, and miRNA-382) was found to be lower in monocytes treated with morphine compared with untreated cells, with little effect on the expression of miRNA-223. The reason for why anti-HIV miRNA-223 was not affected by opioid use is not clear (Wang et al. 2011). Combined effect of naltrexone (pan-opioid receptor antagonist) and CTAP (specific μ-opioid receptor antagonist) completely abrogated the suppressing effect of morphine on anti-HIV miRNA expression, whereas either of them alone had little effect on anti-HIV miRNA expression. Heroin-dependent subjects had significantly lower levels of anti-HIV miRNAs (miRNA-28, 125b, 150, and 382) in PBMCs than the healthy subjects. These findings paralleled the observation that morphine treatment of monocytes enhanced HIV replication.
Hollander et al. showed that rats had less addiction to cocaine with increase in microRNA-212 (miR-212) levels. This cocaine addiction behavior is modulated by miR-212 through its effect on a group of genes (Hollander et al. 2010). They showed that increase in miR-212 reduces cocaine intake through a stimulatory effect on striatal cAMP response element binding (CREB) signaling through Raf-1-mediated sensitization of adenylyl cyclase activity and increased transducer of regulated CREB (TORC) expression. They suggest that spree use of cocaine by human addicts activates CREB, which acts together with its cofactors to regulate the transcription of miR-212. This initiates a positive-feedback loop, further stimulating CREB–TORC activity and thereby limiting cocaine intake. Their group also showed the evidences for the regulation of cocaine intake by MeCP2 through interactions with miR-212 and striatal BDNF levels. These findings might help in reasoning the factor behind the vulnerability of some people to cocaine addiction compared to others (Im et al. 2010).
In our studies, we have shown that cocaine and methamphetamine significantly downregulated miR-155 and miR-20a in MDDC, thereby enhancing the HIV-1 infectivity. Transfection of MDCC with miR155 mimic specifically reversed the cocaine or methamphetamine-induced effects which in turn reduced HIV-1 infectivity (Unpublished data; presented at CPDD 2011).
Viral miRNA in HIV
To date, a total of 48 viral miRNAs have been described in herpes viruses (Pfeffer et al. 2004), polyomaviruses (SV40), and retroviruses (HIV-1, Omoto and Fujii 2005). Recent reports have demonstrated the existence of HIV-1-derived miRNAs, derived from coding and noncoding regions of the viral genome, which regulate both viral and host gene expression so that the repression can be relieved when no longer needed by the virus (Hariharan et al. 2005; Yelamanchili et al. 2010).
Tar miRNA
Functionally TAR-derived miRNA would target the complementary sequence of the TAR region itself, resulting in posttranscriptional or possibly even transcriptional inhibition of HIV-1 (Weinberg and Morris 2006). Downregulation of viral gene expression by TAR-derived miRNA has been demonstrated. HIV-1 miRNA derived from the TAR RNA sequence called TAR miRNA, expressed at all stages of viral life cycle and affects the host cell cycle (Klase et al. 2007). TAR miRNA have been shown to be anti-apoptotic independent of the source of the miRNA. TAR miRNA expression makes the infected cells resistant to apoptosis. Cellular genes, ERCCI and IER3, involved in apoptosis are downregulated by TAR miRNA, a mechanism by which a virus is able to extend the life span of the infected cell for increasing viral replication (Klase et al. 2009). It is suggested that the miRNAs produced by the TAR element contribute to the maintenance of the latent state.
TAR-derived miRNA can downregulate gene expression by recruiting the HDAC-1 to the HIV-1 long terminal repeat (LTR) promoter to silence transcription by chromatin remodeling. It was recently shown that CR8#13—third-generation cdk inhibitor—an effective inhibitor of HIV-1 LTR at the viral promoter, does so by increasing 3′ and 5′ TAR microRNA production in HIV-1-infected cells (Carpio et al. 2010).
Nef miRNA (miR-N367)
nef-derived miRNAs, a 70-nucleotide structure located in the nef/LTR overlap of the HIV genome, have been shown in HIV-1-infected cells (Yamamoto et al. 2002). nef miRNAs (miR-N367) which show perfect complementarity with nef have been shown to inhibit Nef expression and to downregulate the transcription and replication of HIV-1 (Omoto and Fujii 2005; Omoto et al. 2004). nef miRNAs have been detected in HIV-1-infected long-time nonprogressors that display low viremia.
HIV-miRH1
miR-H1, an 81-nucleotide stem-loop structure located immediately downstream of the two NF-kB sites in the LTR, has been reported to downregulate the AATF gene product (Kaul et al. 2009), accompanied lowered Bcl-2, c-myc, Par-4, and Dicer levels. miR-H1 seems to be antagonistic to the anti-apoptotic effect afforded by TAR miRNA. It was also noted further that HIV miR-H1 downregulate expression of the cellular miRNA miR149, which is considered to target the Vpr gene encoded by HIV-1.
Conclusion
Many fundamental questions regarding the expression and function of miRNAs still remain unanswered, even though considerable advances have been made in this field. A better understanding on miRNAs associated with diseases, their mRNA targets, and associated changes in protein products will lead to a better perception of the miRNA's regulatory effects and its association to different diseases. miRNAs may be involved in fine-tuning the transition from latency to activation, the clearance of latent HIV-1 reservoirs, and the reduction of virion production. More studies on the role of miRNAs associated with HIV-1 latency mechanisms could help in developing new strategies that will intervene in the mechanism of viral persistence. Until and unless there is reversal of HIV-1 gene silencing in every latently infected cell, complete cure for HIV-1 will remain impossible. This is because of the fact that virus from a single cell could restart the infection. Polymorphisms in miRNA sequences targeting HIV-1 may also contribute to disease progression. Available literature does not report polymorphisms in the mature miRNAs. Detailed studies in future about the targets of miRNAs and their regulatory function in cell physiology may help us to develop more sophisticated technologies which in turn may be beneficial to treat infectious diseases in humans. Also, identifying impact of drugs of abuse on miRNA expression, knowledge about the role of miRNAs as regulators of complex actions of cocaine, and strategies to block their effect on miRNA expression will help in developing therapeutics for drug addiction by manipulating the actions of miRNAs.
Abbreviations
- AATF:
-
Apoptosis-antagonizing transcription factor
- BDNF:
-
Brain-derived neurotrophic factor
- CCR5:
-
C–C chemokine receptor type 5
- CNS:
-
Central nervous system
- CREB:
-
cAMP response element binding
- DC-SIGN:
-
Dendritic cell-specific adhesion molecule-3-grabbing non-integrin
- HAD:
-
HIV-associated dementia
- HAND:
-
HIV-associated neurocognitive disorders
- HDAC-1:
-
Histone deacetylase-1
- HIV:
-
Human immune deficiency virus
- HIVE:
-
HIV encephalitis
- IFITM3:
-
Interferon-induced transmembrane protein 3
- LTR:
-
Long terminal repeats
- MCP-2:
-
Monocyte chemotactic protein-2
- MDD:
-
Major depressive disorder
- MDDC:
-
Monocyte-derived dendritic cells
- MEF2C:
-
Myocyte enhancer factor 2C
- MeCP2:
-
Methyl CpG-binding protein-2
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger RNA
- nt:
-
Nucleotide
- PBMC:
-
Peripheral blood mononuclear cells
- PCAF:
-
P300/CBP-associated factor
- PFV-1:
-
Primate foamy virus type-1
- RISC:
-
RNA-induced silencing complex
- SNAP25:
-
Synaptosomal-associated protein 25
- sTNFR1A:
-
Soluble tumor necrosis factor receptor
- TLR:
-
Toll-like receptor
- TORC:
-
Transducer of regulated CREB
- TRBP:
-
TAR binding protein
- UTR:
-
Untranslated region
References
Ahluwalia JK, Khan SZ, Soni K, Rawat P, Gupta A, Hariharan M, Scaria V, Lalwani M, Pillai B, Mitra D, Brahmachari SK (2008) Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology 5:117
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Berkhout B (2008) A balancing act: viruses and miRNAs. J Formos Med Assoc 107:1–3
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6:857–866
Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G (2007) MicroRNA-133 controls cardiac hypertrophy. Nat Med 13:613–618
Carpio L, Klase Z, Coley W, Guendel I, Choi S, Van Duyne R, Narayanan A, Kehn-Hall K, Meijer L, Kashanchi F (2010) microRNA machinery is an integral component of drug-induced transcription inhibition in HIV-1 infection. J RNAi Gene Silencing 6:386–400
Cobb BS, Hertweck A, Smith J, O'Connor E, Graf D, Cook T, Smale ST, Sakaguchi S, Livesey FJ, Fisher AG, Merkenschlager M (2006) A role for Dicer in immune regulation. J Exp Med 203:2519–2527
Croce CM, Calin GA (2005) miRNAs, cancer, and stem cell division. Cell 122:6–7
Dai Y, Huang YS, Tang M, Lv TY, Hu CX, Tan YH, Xu ZM, Yin YB (2007) Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus 16:939–946
Eisenberg I, Eran A, Nishino I, Moggio M, Lamperti C, Amato AA, Lidov HG, Kang PB, North KN, Mitrani-Rosenbaum S, Flanigan KM, Neely LA, Whitney D, Beggs AH, Kohane IS, Kunkel LM (2007) Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci U S A 104:17016–17021
Eletto D, Russo G, Passiatore G, Del Valle L, Giordano A, Khalili K, Gualco E, Peruzzi F (2008) Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. J Cell Physiol 216:764–770
Hariharan M, Scaria V, Pillai B, Brahmachari SK (2005) Targets for human encoded microRNAs in HIV genes. Biochem Biophys Res Commun 337:1214–1218
Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ (2010) Striatal microRNA controls cocaine intake through CREB signalling. Nature 466:197–202
Houzet L, Yeung ML, de Lame V, Desai D, Smith SM, Jeang KT (2008) MicroRNA profile changes in human immunodeficiency virus type 1 (HIV-1) seropositive individuals. Retrovirology 5:118
Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, Huang W, Squires K, Verlinghieri G, Zhang H (2007) Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med 13:1241–1247
Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT (2007) Altered microRNA expression in human heart disease. Physiol Genomics 31:367–373
Im HI, Hollander JA, Bali P, Kenny PJ (2010) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13:1120–1127
Kaul D, Ahlawat A, Gupta SD (2009) HIV-1 genome-encoded hiv1-mir-H1 impairs cellular responses to infection. Mol Cell Biochem 323:143–148
Kiernan RE, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang KT, Benkirane M, Van Lint C (1999) HIV-1 tat transcriptional activity is regulated by acetylation. EMBO J 18:6106–6118
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A (2007) A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10:126–139
Klase Z, Kale P, Winograd R, Gupta MV, Heydarian M, Berro R, McCaffrey T, Kashanchi F (2007) HIV-1 TAR element is processed by Dicer to yield a viral micro-RNA involved in chromatin remodeling of the viral LTR. BMC Mol Biol 8:63
Klase Z, Winograd R, Davis J, Carpio L, Hildreth R, Heydarian M, Fu S, McCaffrey T, Meiri E, Ayash-Rashkovsky M, Gilad S, Bentwich Z, Kashanchi F (2009) HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression. Retrovirology 6:18
Kloosterman WP, Plasterk RH (2006) The diverse functions of microRNAs in animal development and disease. Dev Cell 11:441–450
Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, Orum H, Kauppinen S, Kenny PJ, Wahlestedt C (2009) MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A 106:3507–3512
Kumar A, Jeang KT (2008) Insights into cellular microRNAs and human immunodeficiency virus type 1 (HIV-1). J Cell Physiol 216:327–331
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T (2001) Identification of novel genes coding for small expressed RNAs. Science 294:853–858
Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Saib A, Voinnet O (2005) A cellular microRNA mediates antiviral defense in human cells. Science 308:557–560
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23:4051–4060
Liou LY, Herrmann CH, Rice AP (2002) Transient induction of cyclin T1 during human macrophage differentiation regulates human immunodeficiency virus type 1 Tat transactivation function. J Virol 76:10579–10587
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435:834–838
Lu J, Pan Q, Rong L, He W, Liu SL, Liang C (2011) The IFITM proteins inhibit HIV-1 infection. J Virol 85:2126–2137
Martinez-Nunez RT, Louafi F, Friedmann PS, Sanchez-Elsner T (2009) MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN). J Biol Chem 284:16334–16342
Motsch N, Pfuhl T, Mrazek J, Barth S, Grasser FA (2007) Epstein–Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biol 4:131–137
Nathans R, Chu CY, Serquina AK, Lu CC, Cao H, Rana TM (2009) Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell 34:696–709
Noorbakhsh F, Ramachandran R, Barsby N, Ellestad KK, LeBlanc A, Dickie P, Baker G, Hollenberg MD, Cohen EA, Power C (2010) MicroRNA profiling reveals new aspects of HIV neurodegeneration: caspase-6 regulates astrocyte survival. FASEB J 24:1799–1812
Omoto S, Fujii YR (2005) Regulation of human immunodeficiency virus 1 transcription by nef microRNA. J Gen Virol 86:751–755
Omoto S, Ito M, Tsutsumi Y, Ichikawa Y, Okuyama H, Brisibe EA, Saksena NK, Fujii YR (2004) HIV-1 nef suppression by virally encoded microRNA. Retrovirology 1:44
Ott M, Dorr A, Hetzer-Egger C, Kaehlcke K, Schnolzer M, Henklein P, Cole P, Zhou MM, Verdin E (2004) Tat acetylation: a regulatory switch between early and late phases in HIV transcription elongation. Novartis Found Symp 259:182–193, discussion 193–196, 223–225
Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T (2004) Identification of virus-encoded microRNAs. Science 304:734–736
Rom S, Rom I, Passiatore G, Pacifici M, Radhakrishnan S, Del Valle L, Pina-Oviedo S, Khalili K, Eletto D, Peruzzi F (2010) CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB J 24:2292–2300
Scaria V, Hariharan M, Pillai B, Maiti S, Brahmachari SK (2007) Host-virus genome interactions: macro roles for microRNAs. Cell Microbiol 9:2784–2794
Sonkoly E, Wei T, Janson PC, Saaf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, Stahle M, Pivarcsi A (2007) MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One 2:e610
Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 58:1001–1009
Sung TL, Rice AP (2009) miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog 5:e1000263
Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103:12481–12486
Tatro ET, Scott ER, Nguyen TB, Salaria S, Banerjee S, Moore DJ, Masliah E, Achim CL, Everall IP (2010) Evidence for alteration of gene regulatory networks through microRNAs of the HIV-infected brain: novel analysis of retrospective cases. PLoS One 5:e10337
Triboulet R, Mari B, Lin YL, Chable-Bessia C, Bennasser Y, Lebrigand K, Cardinaud B, Maurin T, Barbry P, Baillat V, Reynes J, Corbeau P, Jeang KT, Benkirane M (2007) Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 315:1579–1582
Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, Martin ER, Vance JM (2008) Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet 82:283–289
Wang X, Ye L, Hou W, Zhou Y, Wang YJ, Metzger DS, Ho WZ (2009) Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood 113:671–674
Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ (2011) Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol 178:41–47
Weinberg MS, Morris KV (2006) Are viral-encoded microRNAs mediating latent HIV-1 infection? DNA Cell Biol 25:223–231
Yamamoto T, Omoto S, Mizuguchi M, Mizukami H, Okuyama H, Okada N, Saksena NK, Brisibe EA, Otake K, Fuji YR (2002) Double-stranded nef RNA interferes with human immunodeficiency virus type 1 replication. Microbiol Immunol 46:809–817
Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS (2010) MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis 1:e77
Yeung ML, Bennasser Y, Le SY, Jeang KT (2005a) siRNA, miRNA and HIV: promises and challenges. Cell Res 15:935–946
Yeung ML, Bennasser Y, Myers TG, Jiang G, Benkirane M, Jeang KT (2005b) Changes in microRNA expression profiles in HIV-1-transfected human cells. Retrovirology 2:81
Yeung ML, Benkirane M, Jeang KT (2007) Small non-coding RNAs, mammalian cells, and viruses: regulatory interactions? Retrovirology 4:74
Zhao X, Tang Y, Qu B, Cui H, Wang S, Wang L, Luo X, Huang X, Li J, Chen S, Shen N (2010) MicroRNA-125a contributes to elevated inflammatory chemokine RANTES levels via targeting KLF13 in systemic lupus erythematosus. Arthritis Rheum 62:3425–3435
Acknowledgments
This work was supported in part by grants from the National Institute of Drug Abuse (1R01MH085259, RO1-DA025576, RO1-DA021537, and RO1-DA027049) to Dr. Madhavan Nair.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilakka-Kanthikeel, S., Saiyed, Z.M., Napuri, J. et al. MicroRNA: implications in HIV, a brief overview. J. Neurovirol. 17, 416–423 (2011). https://doi.org/10.1007/s13365-011-0046-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-011-0046-1