Abstract
To assess the role of the phosphatase and tensin homologue on chromosome 10 (PTEN) in mediating envelope glycoprotein 120 (gp120)-induced neurotoxicity in the striatum, PTEN was silenced using short interfering RNA (siRNA) vectors. PTEN activity directs multiple downstream pathways implicated in gp120-induced neuronal injury and death. PTEN is a negative regulator of Akt (protein kinase B) phosphorylation, but has also been shown to directly activate extrasynaptic NMDA receptors and dephosphorylate focal adhesion kinase. Rodent striatal neurons were nucleofected with green fluorescent protein (GFP)-expressing siRNA constructs to silence PTEN (PTENsi-GFP) or with negative-control (NCsi-GFP) vectors, and exposed to HIV-1 gp120IIIB using rigorously controlled, cell culture conditions including computerized time-lapse microscopy to track the fate of individual neurons following gp120 exposure. Immunofluorescence labeling showed that subpopulations of striatal neurons possess CXCR4 and CCR5 co-receptor immunoreactivity and that gp120IIIB was intrinsically neurotoxic to isolated striatal neurons. Importantly, PTENsi-GFP, but not control NCsi-GFP, constructs markedly decreased PTEN mRNA and protein levels and significantly attenuated gp120-induced death. These findings implicate PTEN as a critical factor in mediating the direct neurotoxic effects of HIV-1 gp120, and suggest that effectors downstream of PTEN such as Akt or other targets are potentially affected. The selective abatement of PTEN activity in neurons may represent a potential therapeutic strategy for the CNS complications of HIV-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human immunodeficiency virus type-1 (HIV-1) glycoprotein 120 (gp120) is neurotoxic through a variety of incompletely understood mechanisms. Soluble gp120 can interact with chemokine (C-X-C motif or α-chemokine) receptor 4 (CXCR4) and chemokine (C-C motif or β-chemokine) receptor 5 (CCR5) in human and murine neural cells (Madani et al. 1998; Hesselgesser et al. 1998; Meucci et al. 1998; Huang et al. 1999; Kaul et al. 2007), as well as other potential targets (Berger et al. 1999), via CD4-dependent or independent mechanisms to induce CNS toxicity and inflammation (Jordan et al. 1991; Hesselgesser et al. 1998; Kaul and Lipton 1999; Khan et al. 2004; Kaul et al. 2007). Gp120 activates/overactivates macrophages/microglia imparting a generalized state of excitotoxicity through indirect (Dreyer et al. 1990; Lipton et al. 1991) and, perhaps, direct (Hesselgesser et al. 1998; Khan et al. 2004; Kaul et al. 2007) mechanisms of action. Findings that N-methyl-D-aspartate (NMDA) receptor antagonists can mitigate the neurotoxic effects of gp120 (Lipton 1992, 1996; Lannuzel et al. 1995) support the concept that excessive glutamate signaling contributes to neuronal injury. In addition, gp120 exposure disrupts ion homeostasis (Pulliam et al. 1993; Benos et al. 1994; Holden et al. 1999) and reduces the expression of excitatory amino acid transporters (Wang et al. 2003) in astrocytes, which is likely to inhibit extracellular glutamate uptake and further compromise neuronal function.
We previously demonstrated that silencing the phosphatase and tensin homologue on chromosome 10 (PTEN) could allay HIV-1 Tat-mediated neuronal death (Zhao et al. 2007). Not only is PTEN a well-known negative regulator of Akt/protein kinase B (PKB) phosphorylation, PTEN is also reported to directly activate extrasynaptic NMDA receptors (Ning et al. 2004), interact with p53 (Kurose et al. 2002), and dephosphorylate focal adhesion kinase (Tamura et al. 1999), each of which has been reported to mediate the neurotoxic effects of gp120. The phosphatidylinositol-3-kinase–Akt pathway is a critical downstream mediator of CXCR4 or CCR5 activation and has been proposed to positively and/or negatively regulate key aspects of gp120 function in a variety of cell types (Kapasi et al. 2001; Francois and Klotman 2003; Khan et al. 2004; Trushin et al. 2007). Factors that elevate Akt phosphorylation, such as platelet-derived growth factor (Peng et al. 2008a, b), lithium (Everall et al. 2002), fibroblast growth factor-2 (Langford et al. 2005), and fractalkine (Meucci et al. 2000) are neuroprotective against gp120 neurotoxicity. Based on the above evidence, we theorized that targeting PTEN might similarly protect against gp120-induced neuronal death. The findings herein demonstrate that gp120-induced toxicity in striatal neurons is markedly attenuated by silencing PTEN. Collectively, findings that PTENsi blocks neurotoxicity due to both gp120 and Tat exposure suggest that PTEN is an important target for therapeutic intervention against neurotoxicity associated with HIV.
Results
Chemokine receptor immunoreactivity on neurons
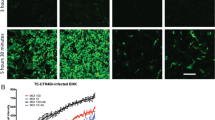
CXCR4 and CCR5 immunofluorescence was co-localized in untreated striatal neurons to assess the presence and cellular distribution of these principal gp120 co-receptors. Receptors were co-localized in untreated neurons (Fig. 1a, b) with antibodies against microtubule-associated protein 2 (MAP2) and counterstained with Hoechst 33342. In neurons, CXCR4 receptor immunoreactivity tended to be confined to the cell body (Fig. 1a), while CCR5 antigenicity extended into the dendrites. Unlike CXCR4, CCR5 appeared to be associated with the axolemma of striatal neurons (Fig. 1b).
Cellular localization of CXCR4 and CCR5 immunofluorescence in striatal neurons (a,b). CXCR4 (a) or CCR5 (b) immunoreactivity (red) was co-localized with MAP2 (green) and counterstained with Hoechst 33342 (blue). CXCR4 immunoreactivity was preferentially confined to the cell body, while CCR5 antigenicity extended into the dendrites and appeared to associate with some axons. Phase-contrast images showing time-lapse tracking of neuronal injury and death in the same neuron before and during exposure to gp120 (c). A within-subjects design is used to compare the survival of the same neuron before (0 h) and at 20-min intervals throughout treatment (24-h intervals shown). Neuron death is denoted here by a destruction of the cell body (arrow) and neurites, and has been confirmed by the inability to exclude viability markers such as ethidium homodimer, ethidium monoazide, or trypan blue (Singh et al. 2004); scale bars in a–c = 20 μm
Neurotoxicity
Time-lapse, digital images of cells were recorded at regular intervals for at least 48 h using a microscope and automated, computer-controlled stage encoder with environmental control (37°C, 95% humidity, 5% CO2) to repeatedly track the same neurons (Zeiss AxioObserver Z.1) as previously described (Bakalkin et al. 2010). Rigorous criteria were used to monitor injury and/or death in individual cells (see Fig. 1), including the pruning of dendrites and axons, dissolution of the Nissl substance, transient cytoplasmic swelling and vacuolization, nuclear damage and pyknosis, and eventual destruction of the cell body (Singh et al. 2004, 2005). In some cases, neuron death was confirmed by viability markers such as ethidium homodimer, ethidium monoazide, or trypan blue (not shown); however, these markers all possess some inherent cytotoxicity with prolonged exposure, which precludes their use to continuously monitor cells. Analyzing the response of individual neurons at set intervals throughout the experiment eliminates inter-subject variability and permits subtle treatment effects to be revealed (Singh et al. 2004, 2005). The use of computer-aided, near-continuous tracking of large numbers of neurons greatly facilitates this task, and increases the sensitivity of the assay (Fig. 1c).
Not surprisingly, gp120 alone was toxic to striatal neurons (Fig. 2). This has been previously published by our lab (Singh et al. 2004, 2005) and is in agreement with the toxic effects of gp120 seen in other neuron types (Meucci and Miller 1996; Kaul et al. 2007; Robinson et al. 2007). Also, as previously reported, our analysis revealed a modest background loss of neurons in control cultures treated with vehicle alone during 60 h (Singh et al. 2004; Zhao et al. 2007). Finally, exposure to denatured or deglycosylated gp120 (500 pM) was not neurotoxic (not shown), suggesting that the toxic effects of gp120 were selective as previously published (Singh et al. 2004, 2005).
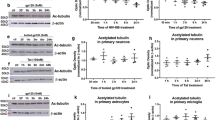
Gp120 caused significant reductions in p-Akt (Ser473) at 5, 10 and 45 min (* P < 0.05 vs. vehicle-treated controls; Mann–Whitney U test) and a near-significant decline in p-Akt at 30 min (§ P < 0.065 vs. vehicle-treated controls; Mann–Whitney U test; solid bars = Bio-Plex assay; striped bar = AlphaScreen assay) (a). Transfection (nucleofection) with GFP reporter-tagged PTEN (PTENsi-GFP), but not GFP-labeled control (NCsi-GFP), silencing constructs protected striatal neurons against the toxic effects of gp120 (b–d). Appearance of PTENsi-GFP fluorescence in striatal neurons at 7 days post-transfection at the onset of the experiments; scale bar = 10 μm (b). PTEN mRNA (c) and protein levels (not shown) were reduced only in PTENsi striatal neurons, as previously reported (Zhao et al. 2007). Gp120-induced neuronal death was evident in non-transfected (* P < 0.001) and NCsi-GFP-transfected (* P < 0.05) controls; however, transfection with PTENsi-GFP caused significant protection after 24 h or 48 h of gp120 exposure (# P < 0.05 vs. other gp120 treatment groups; repeated measures ANOVA; post hoc Duncan’s test (d); after the initial neuron losses caused by the nucleofection procedure itself, neither transfection with control NCsi-GFP nor PTENsi-GFP constructs affected neuron survival (d)
Gp120 causes transient reductions in p-Akt
Akt phosphorylation at Ser473 (p-Akt) was measured in isolated neuron cultures at 2, 5, 10, 20, and 30–45 min following gp120 exposure using (1) an amplified, luminescent proximity homogeneous assay (AlphaScreen®; PerkinElmer, Waltham, MA) and (2) a suspension array-based assay (Bio-Plex™; Bio-Rad, Hercules, CA). Both assays yielded similar results: p-Akt levels were significantly decreased from pretreatment values at 10 min (P < 0.05) irrespective of the assay used and marginally decreased at 30 min (P < 0.065) or 45 min (P < 0.05) after gp120 exposure compared to controls (Fig. 2a). By contrast, at 20 min following gp120 exposure, the response of p-Akt trended above control levels irrespective of assay procedure (AlphaScreen® or Bio-Plex™); however, this response was highly variable and not statistically significant (Fig. 2a).
PTENsi protects neurons against gp120 neurotoxicity
To assess the role of PTEN in gp120-induced neuronal death, control (NCsi green fluorescent protein (NCsi-GFP)) and PTEN-green fluorescent protein (PTENsi-GFP) silencing construct-transfected striatal neurons were exposed to vehicle or gp120IIIB (500 pM), and the same neurons were repeatedly assessed for viability from 0–48 h (Fig. 2d). The PTEN silencing construct effectively reduces expression of the PTEN gene (Fig. 2c) as previously described (Zhao et al. 2006, 2007). At 24 and 48 h, two-way analysis of variance (ANOVA) revealed a significant main effect of gp120 on neuron viability (P < 0.001; gp120 vs. non-gp120 treatment) and a marked interaction effect of PTEN silencing × gp120 exposure (P < 0.05; PTENsi × gp120 exposure; Fig. 2d). Gp120 caused an approximate twofold increase in the rate of neuronal death in control (non-transfected) neurons (P < 0.0005) and in neurons transfected with the control vector (NCsi-GFP; P < 0.05). However, transfection with the PTENsi-GFP fully protected striatal spiny neurons against gp120-mediated death (P < 0.05 vs. other gp120-treated groups).
As noted in the “Materials and methods” section, the transfection (nucleofection) efficiency for these constructs in striatal neurons is 41–55% and retained for at least 9 days post-transfection (Zhao et al. 2007). Although some neurons die during the nucleofection procedure, neurons are permitted to differentiate for about 7 days before experiments are started, and marginal or damaged neurons are not sampled as part of the within-subjects design; therefore, it is unlikely that the transfection procedure has any residual confounding effect in these studies. Moreover, the procedure itself did not sensitize the neurons to gp120 toxicity. In our hands, background neuronal losses of about 7–12% are typical for untreated, neuron-enriched striatal cultures (Fig. 2d).
Discussion
The results provide evidence that manipulation of PTEN levels can modulate the effects of gp120 on neurons. Since PTEN exerts control over the level of Akt phosphorylation, it seemed likely that enhanced neuron survival might result from reversing reductions in p-AKT caused by gp120 exposure. We therefore evaluated p-Akt levels in gp120-treated neurons. Transient and relatively minor reductions in p-Akt were observed at acute time points with exposure to high concentrations of gp120. Thus, while PTEN silencing might work by stabilizing small changes in p-Akt levels that are biologically significant, there may be other mechanisms at play. For example, recent evidence indicates that PTEN interacts with targets such as extrasynaptic NMDA receptors (Ning et al. 2004) and p53 (Stambolic et al. 2001; Freeman et al. 2003), which may also mediate the neurodegenerative effects of gp120 (Lipton, 1994; Lannuzel et al. 1997; Garden et al. 2004) and prompts speculation that PTEN can act via multiple neurotoxic effectors. Alternatively, Akt function may not be solely determined by phosphorylation at Ser473. For example, despite a high degree of regulation of phosphorylation/dephosphorylation at the Ser473 (Sarbassov et al. 2005; Gao et al. 2005) and Thr308 positions (Sato et al. 2002; Barry and Gibbins 2002), dramatic fluctuations in the phosphorylation levels at both sites are reported. Paradoxical increases in Akt phosphorylation have been reported in neurons destined to die, which may reflect a defensive response (Endo et al. 2006; Miyawaki et al. 2009). Though Akt phosphorylation is generally inversely proportional to PTEN activity, this is not always the case (Kurose et al. 2001) and may differ among cell types (Yoo et al. 2006).
Although the direct neurotoxic effects of gp120 were explored in the present study, it is well established that microglia mediate key aspects of gp120 neurotoxicity (Kaul et al. 2001; Gonzalez-Scarano and Martin-Garcia 2005; Kaul et al. 2007). As noted, gp120 can indirectly modify regulated neuron death through activation of CXCR4 (Meucci et al. 1998; Zheng et al. 1999; Kaul and Lipton 1999; Kaul et al. 2007) or CCR5 (Kaul et al. 2007) through actions in microglia. When considering neurons only, the deleterious consequences of CXCR4 activation do not appear to be limited to gp120, since stromal cell-derived factor-1 (SDF-1) also increases neuron death through CXCR4 (Kaul et al. 2007). Alternatively, SDF-1 (Khan et al. 2004) or RANTES can be protective at CCR5 (Kaul et al. 2007), although SDF-1–CCR5 interactions do not exclusively promote survival depending on context and the particular cell target involved. Gp120-evoked neuronal injury is reportedly mediated through increased p38 mitogen-activated protein kinase (MAPK) phosphorylation (Kaul and Lipton 1999; Yi et al. 2004; Hu et al. 2005; Singh et al. 2005; Wan et al. 2006; Kaul et al. 2007), by suppressing pro-survival pathways involving Akt (Kolson 2002; Kaul et al. 2007) and/or elevated ERK phosphorylation (Lannuzel et al. 1997). Irrespective of the particular MAPKs implicated, gp120 neurotoxic signals converge on a traditional regulated cell death effector pathway involving caspase-3 (Kaul et al. 2001; Garden et al. 2002; Bachis et al. 2003; Singh et al. 2004).
Direct, CD4-independent actions of gp120 in neurons have been controversial, since binding to CD4 is requisite for the conformational changes necessary for gp120 to interact with HIV co-receptors (Bhattacharya et al. 2003). Despite this, there are reports of CXCR4 (Hesselgesser et al. 1997, 1998; Huang and Bond 2000; Zhang et al. 2003; Khan et al. 2004; Bachis et al. 2009; Medders et al. 2010), and, to a lesser extent, CCR5 (Huang et al. 1999; Zhang et al. 2003; Bachis et al. 2009) activation being directly neurotoxic. While acute exposure to R5-tropic gp120 can directly activate p38 MAPK in neurons, microglia are reportedly required for R5-tropic gp120 to subsequently result in neuronal injury and death (Medders et al. 2010). Based on the present study, which exclusively examined X4-tropic gp120IIIB, it is uncertain whether PTENsi would protect against the more typical, indirect cytotoxic effects of gp120-exposed microglia or whether PTENsi would be more widely protective against the direct neurotoxic effects of alternative X4, R5, or dual-tropic gp120 strains (Ohagen et al. 1999; Gabuzda and Wang 2000; Zhang et al. 2003; Kaul et al. 2007). Importantly, PTENsi limits Tat-induced neuronal lethality (Zhao et al. 2007), suggesting that the neuronal injury and death accompanying neuroAIDS may be widely amenable to mitigation by PTENsi.
HIV-1 exposure activates multiple direct and indirect signaling pathways that are injurious to neurons, and that includes multiple, toxic, viral protein interactions. Targeting a single factor, protein, or pathway is therefore unlikely to block neuron dysfunction. A therapeutic strategy that is designed to intercept multiple detrimental processes is more likely to be successful. PTEN, as a major regulator of Akt phosphorylation and other key effectors, is properly positioned to be such a target. In situations where dominant-negative PTEN mutations occur naturally, as well as in global PTEN knockout mice, the risk of multiple types of cancer or developmental defects is increased due to sustained elevation in p-Akt levels and aberrant interactions with p53 (Wang et al. 1997; Stambolic et al. 2001; Degtyarev et al. 2008). This clearly limits the utility of PTEN silencing as a therapeutic strategy in cells with the potential to proliferate. However, mature neurons might be a more viable silencing target, since they are post-mitotic and have limited potential for transformation. Our evidence that PTEN silencing can block neuron death due to both HIV-1 gp120 and Tat makes PTEN an attractive candidate for further therapeutic assessment.
Materials and methods
Neuron-enriched cultures
Striatal neurons were prepared from E15-E16 ICR (CD-1) mouse embryos. Striata were dissected, minced, and incubated with 10 ml trypsin (2.5 mg/ml) and DNase (0.015 mg/ml) in Neurobasal Medium with 25 μM glutamate (30 min, 37°C). Tissue was triturated, resuspended in 10-ml medium, and cells filtered twice through 70-μm pore nylon mesh. Cultures of purified neurons were plated on poly-l-lysine-coated coverslips and maintained in Neurobasal media supplemented with B27 (Invitrogen, Carlsbad, CA), 0.5 mM l-glutamine, 0.025 mM glutamate, and allowed to mature for about 1 week prior to the start of experiments.
PTENsi of enriched striatal neuron cultures
Construction of the DNA-based PTEN silencing (PTENsi) and nonspecific, negative-control silencing (NCsi) vectors and silencing expression cassettes (SECs) has been previously described (Zhao et al. 2006, 2007). In brief, siRNA SECs for PTEN and NCsi used the mouse U6 gene promoter to drive a gene-specific or nonspecific hairpin DNA using the Silencer Express Kit (Ambion, Austin, TX) protocol. PTENsi or NCsi vectors were formed from SECs cloned into the pSEC hygromycin (hygro) vector. The GFP expression cassette, containing the human cytomegalovirus promoter driving the GFP coding region, was amplified by PCR from the pMaxGFP plasmid as previously described and used to construct GFP-tagged PTENsi (PTENsi-GFP) and NCsi (NCsi-GFP) vectors (Zhao et al. 2006, 2007). Embryonic day 15 striatal neurons were transfected prior to plating as previously described using the Amaxa Mouse Nucleofector kit (Amaxa Technology, catalog number VPG-1001) (Zhao et al. 2007). DNA (10 to 20 μg) was delivered to (3 × 106) neurons in 75 μl of transfection buffer, to which was added 35 μl of Ca2+-free Dulbecco’s modified Eagle’s medium. Neurons were plated immediately after transfection and grown in Neurobasal medium with supplements as described above. About a third of the neurons die during or soon after the transfection procedure. However, because neurons are permitted to differentiate for about 7 days after plating before experiments are started, and marginal or damaged neurons are not sampled at the onset of the within-subjects design, any possible long-term effects of the transfection procedure itself are likely to be inconsequential in these studies. Notably, nucleofection results in the prolonged expression of the control and silencing vectors; 41–55% of nucleofected striatal spiny neurons remain transfected with control and PTENsi constructs for at least 9 days (Zhao et al. 2007).
gp120 treatment
Cultures were treated with recombinant X4-tropic gp120 HIV-1IIIB (ImmunoDiagnostics) for 2 min, 5 min, 10 min, 20 min, 30–45 min, or 24 h or 48 h as indicated.
CXCR4 and CCR5 immunofluorescence
Striatal neurons were fixed for 15 min in 4% paraformaldehyde, permeabilized for 15 min in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 and 0.1% bovine serum albumin (BSA), and subsequently blocked for 1 h at room temperature in PBS supplemented with 0.1% BSA and 1% horse serum. Dual labeling was performed for 2 h at room temperature in anti-sera to CXCR4 (1:100; Abcam, Cambridge, MA) or CCR5 (1:100; BD Pharmingen, San Diego, CA) combined with anti-MAP2 (1:1,000; Chemicon, Temecula, CA) antibodies. Addition of goat anti-rabbit IgG (Alexa Fluor® 488) and donkey anti-mouse IgG (Alexa Fluor® 594) (Invitrogen; Eugene, OR) against the primary antibodies was performed for 1 h at room temperature, followed by counterstaining for 20 min with 0.5 μg/ml Hoechst 33342 (Invitrogen), which labels DNA and permits identification of cell nuclei. Cells were mounted in ProLong Gold Antifade reagent (Invitrogen) and visualized and digitally photographed using a Zeiss microscope (AxioObserver Z1) with AxioVision software and a MRm digital camera (Zeiss Inc., Thornwood, NY). Deconvolution of fluorescent images was performed using AutoQuant X version X2.2.0 (Media Cybernetics, Bethesda, MD).
Assessment of neuron viability
Repeated measures ANOVA was used to assess the effect of gp120 on the same neuron throughout the experiment (percentage of pretreatment value; Statistica. StatSoft, Tulsa, OK). In each experiment, treatments were distributed across cells pooled from the same pups. Approximately 50 healthy neurons with well-defined dendritic and axonal arbors were identified within ≥ 8 overlapping fields (×40 magnification; Mark&Find, Time Lapse, and MosaiX modules, Zeiss AxioVision 4.6) in individual culture wells in each experiment prior to treatment (0 h). The effect of each treatment on neuron survival was analyzed statistically at 4-h intervals using repeated measures ANOVA from n = 5–7 experiments (250–350 neurons in total per treatment) and reported as mean neuron survival ± the standard error of the mean (SEM). In the PTENsi-GFP and NCsi-GFP experiments, neuron survival was tracked as above; however, fluorescent images were additionally taken of the same neurons to identify transfected versus non-transfected neurons at 48 h. The findings were reported as the mean percentage of surviving neurons (relative to pretreatment values) ± SEM from n = 4–6 separate experiments.
Akt phosphorylation
Akt phosphorylation at Ser473 (p-Akt) was measured in isolated neuron cultures using two independent assays: (1) an amplified, luminescent proximity homogeneous assay (AlphaScreen®; PerkinElmer, Waltham, MA) and (2) a commercial suspension array assay against p-Akt and total Akt (Bio-Plex; Bio-Rad, Hercules, CA). In the case of the AlphaScreen assay, Akt/PKB phosphorylation at Ser473 was assayed in white, non-binding 96-well plates using a SureFire Phospho-AKT 1/2 Ser473 Kit (TGRAS500; AlphaScreen, PerkinElmer) and measured using a PHERAstar FS platereader (BMG LABTECH, Cary, NC) equipped with 680-nm laser excitation. In the case of the Bio-Plex assay, p-Akt and Akt were each normalized to protein concentration, and p-Akt and Akt fluorescent signals were analyzed using a dual-laser, flow cytometry-based microplate reader system (Bio-Plex) and Bio-Plex Manager™ software (Version 5, Bio-Rad). Data are presented as the ratio of p-Akt/Akt. The effects of 2, 5, 10, 20, and 30 min (AlphaScreen®) or 45 min (Bio-Plex) of exposure to control medium or 100 pM, 500 pM, or 1 nM gp120IIIB were assessed in neuron-enriched cultures at 37°C. Gp120-induced changes in p-Akt were compared to vehicle-treated controls at each time point (mean ± SEM from six experiments).
Statistical analyses
Differences in neuron survival were assessed by repeated measures ANOVA followed by Duncan’s post hoc testing (StatSoft, Statistica, Tulsa, OK). The Mann–Whitney U nonparametric test (Statistica, Version 9, Tulsa, OK) was used to compare the mean change in the p-Akt signal in control versus gp120-treated neurons at each time following exposure.
References
Bachis A, Major EO, Mocchetti I (2003) Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci 23:5715–5722
Bachis A, Biggio F, Major EO, Mocchetti I (2009) M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol 4:150–160
Bakalkin G, Watanabe H, Jezierska J, Depoorter C, Verschuuren-Bemelmans C, Bazov I, Artemenko KA, Yakovleva T, Dooijes D, Van de Warrenburg BPC, Zubarev RA, Kremer B, Knapp PE, Hauser KF, Wijmenga C, Nyberg F, Sinke RJ, Verbeek DS (2010) Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet 87:593–603
Barry FA, Gibbins JM (2002) Protein kinase B is regulated in platelets by the collagen receptor glycoprotein VI. J Biol Chem 277:12874–12878
Benos DJ, Hahn BH, Bubien JK, Ghosh SK, Mashburn NA, Chaikin MA, Shaw GM, Benveniste EN (1994) Envelope glycoprotein gp120 of human immunodeficiency virus type 1 alters ion transport in astrocytes: implications for AIDS dementia complex. Proc Natl Acad Sci USA 91:494–498
Berger EA, Murphy PM, Farber JM (1999) Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol 17(657–700):657–700
Bhattacharya J, Peters PJ, Clapham PR (2003) CD4-independent infection of HIV and SIV: implications for envelope conformation and cell tropism in vivo. AIDS 17(Suppl 4):S35–S43
Degtyarev M, De MA, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, Davis DP, Stern HM, Murray LJ, Hoeflich KP, Klumperman J, Friedman LS, Lin K (2008) Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol 183:101–116
Dreyer EB, Kaiser PK, Offermann JT, Lipton SA (1990) HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science 248:364–367
Endo H, Nito C, Kamada H, Nishi T, Chan PH (2006) Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab 26:1479–1489
Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E (2002) Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci 21:493–501
Francois F, Klotman ME (2003) Phosphatidylinositol 3-kinase regulates human immunodeficiency virus type 1 replication following viral entry in primary CD4+ T lymphocytes and macrophages. J Virol 77:2539–2549
Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H (2003) PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3:117–130
Gabuzda D, Wang J (2000) Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J Neurovirol 6(Suppl 1):S24–S32
Gao T, Furnari F, Newton AC (2005) PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell 18:13–24
Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D'Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA (2002) Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci 22:4015–4024
Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS (2004) HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J 18:1141–1143
Gonzalez-Scarano F, Martin-Garcia J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81
Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R (1997) CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol 7:112–121
Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R (1998) Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol 8:595–598
Holden CP, Nath A, Haughey NJ, Geiger JD (1999) Involvement of Na+/H+ exchangers, Ca2+ channels, and excitatory amino acid receptors in intracellular Ca2+ responses to HIV-1 gp120 in cultured human fetal brain cells. Neuroscience 91:1369–1378
Hu S, Sheng WS, Lokensgard JR, Peterson PK (2005) Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis 191:886–889
Huang MB, Bond VC (2000) Involvement of protein kinase C in HIV-1 gp120-induced apoptosis in primary endothelium. J Acquir Immune Defic Syndr 25:375–389
Huang MB, Hunter M, Bond VC (1999) Effect of extracellular human immunodeficiency virus type 1 glycoprotein 120 on primary human vascular endothelial cell cultures. AIDS Res Hum Retroviruses 15:1265–1277
Jordan CA, Watkins BA, Kufta C, Dubois-Dalcq M (1991) Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol 65:736–742
Kapasi AA, Fan S, Singhal PC (2001) Role of 14-3-3epsilon, c-Myc/Max, and Akt phosphorylation in HIV-1 gp 120-induced mesangial cell proliferation. Am J Physiol Renal Physiol 280:F333–F342
Kaul M, Lipton SA (1999) Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA 96:8212–8216
Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994
Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA (2007) HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ 14:296–305
Khan MZ, Brandimarti R, Patel JP, Huynh N, Wang J, Huang Z, Fatatis A, Meucci O (2004) Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res Hum Retrovir 20:1063–1071
Kolson DL (2002) Neuropathogenesis of central nervous system HIV-1 infection. Clin Lab Med 22:703–717
Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C (2001) Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol 158:2097–2106
Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C (2002) Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet 32:355–357
Langford D, Hurford R, Hashimoto M, Digicaylioglu M, Masliah E (2005) Signalling crosstalk in FGF2-mediated protection of endothelial cells from HIV-gp120. BMC Neurosci 6(8):8
Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M (1995) HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci 7:2285–2293
Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M (1997) Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol 42:847–856
Lipton SA (1992) Memantine prevents HIV coat protein-induced neuronal injury in vitro. Neurology 42:1403–1405
Lipton SA (1994) HIV-related neuronal injury. Potential therapeutic intervention with calcium channel antagonists and NMDA antagonists. Mol Neurobiol 8:181–196
Lipton SA (1996) Similarity of neuronal cell injury and death in AIDS dementia and focal cerebral ischemia: potential treatment with NMDA open-channel blockers and nitric oxide-related species. Brain Pathol 6:507–517
Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB (1991) Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron 7:111–118
Madani N, Kozak SL, Kavanaugh MP, Kabat D (1998) gp120 envelope glycoproteins of human immunodeficiency viruses competitively antagonize signaling by coreceptors CXCR4 and CCR5. Proc Natl Acad Sci USA 95:8005–8010
Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M (2010) Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol 185:4883–4895
Meucci O, Miller RJ (1996) gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci 16:4080–4088
Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ (1998) Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA 95:14500–14505
Meucci O, Fatatis A, Simen AA, Miller RJ (2000) Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA 97:8075–8080
Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, Zukin RS (2009) The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci 12:618–626
Ning K, Pei L, Liao M, Liu B, Zhang Y, Jiang W, Mielke JG, Li L, Chen Y, El-Hayek YH, Fehlings MG, Zhang X, Liu F, Eubanks J, Wan Q (2004) Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J Neurosci 24:4052–4060
Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D (1999) Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol 73:897–906
Peng F, Dhillon N, Callen S, Yao H, Bokhari S, Zhu X, Baydoun HH, Buch S (2008a) Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol 14:62–72
Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S (2008b) Mechanisms of platelet-derived growth factor-mediated neuroprotection—implications in HIV dementia. Eur J Neurosci 28:1255–1264
Pulliam L, West D, Haigwood N, Swanson RA (1993) HIV-1 envelope gp120 alters astrocytes in human brain cultures. AIDS Res Hum Retroviruses 9:439–444
Robinson B, Li Z, Nath A (2007) Nucleoside reverse transcriptase inhibitors and human immunodeficiency virus proteins cause axonal injury in human dorsal root ganglia cultures. J Neurovirol 13:160–167
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307:1098–1101
Sato S, Fujita N, Tsuruo T (2002) Regulation of kinase activity of 3-phosphoinositide-dependent protein kinase-1 by binding to 14-3-3. J Biol Chem 277:39360–39367
Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF (2004) Apoptotic death of striatal neurons induced by HIV-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol 10:141–151
Singh IN, El-Hage N, Campbell ME, Lutz SE, Knapp PE, Nath A, Hauser KF (2005) Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience 135:781–790
Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW (2001) Regulation of PTEN transcription by p53. Mol Cell 8:317–325
Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM (1999) PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem 274:20693–20703
Trushin SA, Algeciras-Schimnich A, Vlahakis SR, Bren GD, Warren S, Schnepple DJ, Badley AD (2007) Glycoprotein 120 binding to CXCR4 causes p38-dependent primary T cell death that is facilitated by, but does not require cell-associated CD4. J Immunol 178:4846–4853
Wan Q, Douglas SD, Wang X, Kolson DL, O'Donnell LA, Ho WZ (2006) Morphine upregulates functional expression of neurokinin-1 receptor in neurons. J Neurosci Res 84:1588–1596
Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R (1997) Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res 57:4183–4186
Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ (2003) Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology 312:60–73
Yi Y, Lee C, Liu QH, Freedman BD, Collman RG (2004) Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: implications for neuropathogenesis. J Neurovirol 10:91–96, Suppl 1
Yoo LI, Liu DW, Le VS, Bronson RT, Wu H, Yuan J (2006) Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer Res 66:1929–1939
Zhang K, Rana F, Silva C, Ethier J, Wehrly K, Chesebro B, Power C (2003) Human immunodeficiency virus type 1 envelope-mediated neuronal death: uncoupling of viral replication and neurotoxicity. J Virol 77:6899–6912
Zhao TY, Zou SP, Alimova YV, Wang G, Hauser KF, Ghandour MS, Knapp PE (2006) Short interfering RNA-induced gene silencing is transmitted between cells from the mammalian central nervous system. J Neurochem 98:1541–1550
Zhao T, Adams MH, Zou SP, El-Hage N, Hauser KF, Knapp PE (2007) Silencing the PTEN gene is protective against neuronal death induced by human immunodeficiency virus type 1 Tat. J Neurovirol 13:97–106
Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE (1999) Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol 98:185–200
Acknowledgments
The support of NIDA grants R01 DA018633, P01 DA019398, T32 DA007027, and K02 DA027374 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, S., El-Hage, N., Podhaizer, E.M. et al. PTEN gene silencing prevents HIV-1 gp120IIIB-induced degeneration of striatal neurons. J. Neurovirol. 17, 41–49 (2011). https://doi.org/10.1007/s13365-010-0016-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-010-0016-z