Abstract
An understanding of species ecology is vital for effective conservation, particularly if the species forms an important constituent of the lesser mammal guild and regulates small mammal and bird populations. As the ecological role of the leopard cat (Prionailurus bengalensis) in the intricate eastern Himalayan habitats is not known, we assessed the site occupancy, detection probability and activity pattern of leopard cats in Khangchendzonga Biosphere Reserve, India, based on sign surveys and camera trapping. The estimated site occupancy was 0.352 ± 0.061 and detection probability was 0.143 ± 0.0484. Occupancy modelling indicated low elevation, high rodent abundance and tree cover as best predictors for the occupancy of leopard cat. Diet based on analysed scats revealed murids as the most dominant prey (89.2 %). Information based on photographic captures indicated that the leopard cat exhibited a nocturnal activity pattern (peak activity between 0200–0300 hours), which coincided with its principal prey (revealed through diet analysis), but mainly contradicted with other sympatric competitors, hence indicating a temporal partitioning of resources among them. Ecological niche factor analysis indicated that the leopard cat exhibits high global marginality (1.32) and low global tolerance (0.275). The habitat suitability map for leopard cats showed majority of the habitat as unsuitable (1,959.44 km2) and predicted only 164.54 km2 areas of lower temperate forests as moderate to highly suitable. As highly suitable habitats of the leopard cat are in close proximity to villages, conflict issues are a major threat and therefore need to be addressed in conservation program for this felid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The leopard cat (Prionailurus bengalensis) is a small, relatively common wild felid of Southeast Asia having the widest geographical distribution among all Asian lesser cats (Sunquist and Sunquist 2002). The species is adapted to inhabit a broad variety of habitats, from tropical rainforest to temperate broadleaf and marginally coniferous forest, as well as shrub forest and successional grasslands (Nowell and Jackson 1996). It also inhabits human-modified agricultural landscapes, logged forests, and rubber and oil palm plantations (Rajaratnam et al. 2007) and can extend into rhododendron–oak–maple forests in Himalayas up to an altitude of 3,254 m a.s.l. (Ghimirey and Ghimire 2010). Despite its high adaptability to thrive even in altered and harsh habitats, its population seems to be overall stable, yet declining in some parts of its range (Sanderson et al. 2008) due to increasing threats from habitat destruction, hunting, trapping and live animal trade (Johnson and Jinping 1996). There have been a number of studies focusing on the different ecological aspects of the leopard cat throughout its geographical range (Inoue 1972; Rabinowitz 1990; Izawa et al. 1991; Grassman 2000; Rajaratnam 2000; Austin 2002; Khan 2004; Grassman et al. 2005; Rajaratnam et al. 2007; Watanabe 2009), but no such information is available from the intricate high-altitude habitats of Himalayas.
Effective conservation and management of species requires sound understanding of its ecology and interactions with its environment. But gathering such information on elusive species in remote and intricate Himalayan habitats has always been challenging since conventional sampling protocols have been proven inadequate in such areas (Sathyakumar et al. 2011). Camera trapping has emerged as an efficient noninvasive monitoring tool with wide applicability in ecological studies in varied habitat conditions and proved very effective in this study.
Habitat use by carnivores in general is known to vary primarily with the availability, distribution and abundance of prey and competitive species than other habitat features (Bothma et al. 1984; Johnson et al. 1996; Sunquist et al. 1989). In high-altitude landscapes, both biological and geographical factors equally affect the use of an area (Jackson and Ahlborn 1989; Chen et al. 2009). Generating information on the habitat ecology of carnivores in intricate and inaccessible areas is difficult, and also challenging to meet many assumptions of conventional sampling methods (Singh and Milner-Gulland 2011). Resource selection probability functions and occupancy models are powerful methods of identifying areas within a landscape that are highly used by a species (MacKenzie 2005, 2006; MacKenzie and Royle 2005). Occupancy models estimate the probability of a species occupying a sampling site whilst correcting for imperfect species detection based on repeated detection/non-detection data (MacKenzie et al. 2006). Camera trapping has been used to generate such data in mountainous areas (Chen et al. 2009; Davis et al. 2011) as a surrogate to conventional radiotelemetry-based assessment of habitat use by carnivores since it can yield information on a larger part of the population (MacKay et al. 2008).
Camera trapping has also been proven to be a useful tool for providing information on the activity pattern of several species, including leopard cats, based on the date and time stamps on each photograph captured (Azlan and Sharma 2006; Cheyne and Macdonald 2011). There are various ecological factors that contribute in shaping the activity patters of carnivores, but in general, they adapt their activities for efficient predation by coinciding them with periods when the prey is most vulnerable, whereas their prey must contend with the conflicting demands of acquiring resources and avoiding predators (Zielinski 1988; Harmsen et al. 2011) by changing their daily activity patterns (Eccard et al. 2008; Gliwicz and Dabrowski 2008). Using camera trap data, one can deduce the activity pattern of the predator and also find a relationship with the activity pattern of its principal prey. Considering the fact that the diet of the leopard cat comprises small mammals (murids) as their major prey species (Grassman 2000; Grassman et al. 2005; Inoue 1972; Khan 2004; Rabinowitz 1990; Rajaratnam et al. 2007), we expected to find a correspondence between the activity patterns of the cat and its major prey in the photo-trapping data.
Ecological information on a species can aid in predicting its geographical distribution over a larger area, which is crucial for its conservation and management (Margules and Pressey 2000). Yet, for most regions and taxa, detailed information on species distribution is usually not available, and collecting such data is costly and labour-intensive (Prendergast et al. 1999; Bowker 2000). Spatially explicit habitat models (Guisan and Zimmermann 2000) and multivariate models prepared with the help of geographic information system (GIS) are very helpful in understanding the habitat associations of a particular species and also in making predictive habitat suitability maps (Lenton et al. 2000; Hirzel et al. 2002; Marzluff et al. 2004).
In this study, we determined site and sampling covariates influencing the occupancy and detection of leopard cats in mountainous habitats. We also provide some insights into its activity patterns and food habits and also tested for any synchronisation in activity pattern with its principal prey. We prepared a presence-only habitat suitability model and predictive map for a larger landscape. Such ecological information on leopard cat from a high-altitude eastern Himalayan landscape is rare and hence generates important fundamental knowledge for its conservation and management in this intricate landscape in the future.
Materials and methods
Study area
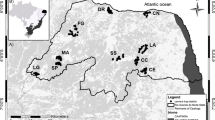
The study was carried out in the Prek Chu [river] catchment of the Khangchendzonga Biosphere Reserve [2,620 km2 (national park = 1,784 km2, buffer = 836 km2)] located in Sikkim, India (27°30′–27°55′ N, 88°02′–88°37′ E; Fig. 1). The area is included in the eastern Himalayan global biodiversity hotspot (Myers et al. 2000) and also listed among the important Global 200 Ecoregions (Olson and Dinerstein 1998). The Khangchendzonga Biosphere Reserve (BR) has a sharp elevation gradient of 1,220–8,586 m, varying within an aerial distance of just 42 km (Tambe 2007). The area of Khangchendzonga BR is divided into seven catchments or river subsystems, viz., Lhonak, Zemu, Lachen, Rangyong, Rangit, Prek and Churong. Among these, Prek catchment (27°21′–27°37′ N, 88°12′–88°17′ E; 182 km2) was selected for intensive studies due to its similarity in habitat characteristics with the entire Khangchendzonga BR (Sathyakumar et al. 2011). The altitudinal range of Prek catchment varies from 2,200 to 6,691 m, with an average of 3,562 m. The area can be characterized into six habitat classes, viz., mixed subtropical (1 %), mixed temperate (16 %), sub-alpine (36 %), alpine pastures (5 %), rock and snow cover (41 %), and water bodies (1 %), and receives an average annual rainfall of around 2,230 mm (Tambe 2007). The study was conducted from April 2008 to December 2009. The temperature during the study period ranged from 2.5 to 23.6 °C, with an average of 13.34 °C.
Occupancy: survey design and analysis
Reconnaissance surveys were done in 2008 in different catchments of Khangchendzonga BR to generate baseline information on the presence and distribution of leopard cats based on trail sampling and sign surveys (Sathyakumar et al. 2009). Within the entire camera trap sampling duration, i.e. throughout 2009 in the Prek Chu catchment, systematic camera trapping for occupancy surveys was done for a period of 3 months (March to May) covering all accessible habitat classes (from 1,500 to 5,000 m) of the catchment in a 2 × 2-km grid-based design (Fig. 2). This design was followed by considering the mean annual home range size of leopard cat to vary from 1.5 to 14 km2 (Grassman et al. 2005; Rabinowitz 1990; Rajaratnam et al. 2007) and assuming that the occupancy status of grids/sites remains constant during the survey period (MacKenzie et al. 2006). In case the occupancy status of sites change within the survey period and these changes are random, the occupancy-based inference will still be valid provided the site occupancy is interpreted as the probability of site utilization (Bailey et al. 2007). Due to limitations of camera units (n = 27), camera traps were deployed for at least 1 month in each grid/site (n = 41). Habitat characteristics were assessed by laying a 10 × 10-m plot around the camera, and information on GPS location, elevation, aspect, slope, habitat type, per cent tree cover (ocular estimation) and per cent ground cover (ocular estimation) was recorded. Through camera trapping, we also recorded richness (presence) and calculated the photo capture rates of the leopard cat’s main prey species and possible sympatric carnivores in the area and human presence at each site. The photo capture rate was calculated as the number of photographs of a species divided by the number of trap days (24-h period) per site and represented per 100 trap days (Carbone et al. 2001; O’Brien et al. 2003). For occupancy analysis, a detection matrix (0 = non-detection, 1 = detection) was created for each camera site for leopard cat occurrence. In order to avoid numerical optimization of the likelihood (Hines 2006), all numerical variables were z-standardized and categorical covariates were dummy-coded. A logit link function (Mackenzie 2006) was used to model leopard cat presence as dependent on habitat covariates in the program PRESENCE, version 4.0 (Hines 2006). We modelled the probability of occupancy (Ψ) and detection probability (p) as constant and as a function of seven habitat covariates (sampling variables) and one detection variable (Table 1). Model selection was based on minimum Akaike information criterion (AICc) values (for small samples), and all the models with ΔAICc < 2 were considered as equivalent models. The summed model weight of each covariate in these equivalent models was used to determine the most significant variables influencing the occupancy of the species. The sign and standard error of logistic coefficient (β) of each variable (positive or negative) was used to determine the direction of influence of the variable.
Food habits
Scat collection and slide preparation
The diet of leopard cat was investigated through scat analysis (n = 37). Scats were opportunistically collected along trails, ridges, nullahs [streams] and at camera trap locations (2008–2009). Identification of scats was done on the basis of their shape, size and presence of other signs (pugmarks) nearby (Austin et al. 2007; Grassman et al. 2005). Differentiation from the scats of mustelids [narrower, flattened, generally single units without subdivisions (diameter = 0.46–1.32 cm)] and golden jackal [Canis aureus; cylindrical, subdivided with blunt ends (diameter = 1.63–2.18 cm)] was easier compared to that of other felid species. Felid scats (cylindrical, sausage-shaped, subdivided and tapered at one of the extremities) with an average diameter of 1–1.5 cm were classified as leopard cat scats (Grassman 1997; Rabinowitz 1990) and used for diet analysis. Larger felid-like scats (diameter = 1.68–2.55 cm) were classified as of golden cat (Pardofelis temminckii). The presence of jungle cat (Felis chaus) was extremely rare and mostly above the altitudinal limit of leopard cat distribution; hence, we did not consider it to be a potential sympatric competitor of leopard cat. Collected scats were washed through a 0.5-mm fine-wire mesh to separate indigested prey remains such as hairs, bones, mandibles, etc. Contents were sundried and, later, the hair remains were stored in 70 % alcohol (for dehydration) followed by a 30-min xylene treatment (to induce transparency). Hair samples were mounted on a glass slide using a mixture of distyrene, a plasticizer and xylene (used as a synthetic resin mounting media) mountant and examined under a microscope with ×10 and ×40 resolutions for cuticular and medullary characteristics and compared with reference slides (Koppikar and Sabins 1976; Mukherjee et al. 1994; Reynolds and Aebischer 1991).
Diet analysis
As reported in most food habit studies on carnivores, results were expressed as the frequency of occurrence of the different prey items (Ray and Sunquist 2001). Using programme SimStat v2.5.8 (Peladeau 2004), we subjected the results of the scat analysis to resampling using the bootstrap simulation. Subsamples equalling the original sample size of scats were iterated 10,000 times to generate means and bias-corrected 95 % confidence intervals for percentage frequency of prey items in leopard cat scats.
Activity pattern
The activity pattern of leopard cat based on the date and time information on the photographs was assessed by calculating the daily activity index (DAI = number of photographs within a duration × 100/total number of photographs) of 1-h durations. Nocturnal activity was categorized as that which occurred from 1800 to 0500 hours, the approximate times of sunset and sunrise in the area during the study period. The same protocol was followed for deriving the activity pattern of the leopard cat’s main prey, other prey species and other sympatric carnivores, with prior information generated from the diet analysis. Rayleigh uniformity test was applied to determine the uniformity in the activity pattern of the leopard cat. The difference between leopard cat activity and that of its main prey was also tested through Watson’s U 2 test in program Oriana 4.0 (Kovach 2011).
Habitat suitability modelling
Generating the presence/absence data for leopard cat and selection of appropriate habitat suitability modelling procedure were the crucial parts of the process. Absence data in particular are often difficult to obtain accurately for the entire Biosphere Reserve since there is a possibility of incorporating false absences due to imperfect detection (McArdle 1990) and historical absence in suitable habitats that can introduce considerable bias in the analysis (Hirzel et al. 2002). Compared to this, reliable presence-only information is much more available and requires much less collection efforts (Tsoar et al. 2007). Ecological niche factor analysis (ENFA) operates on similar principles and requires only presence data to compute suitability functions (Hirzel et al. 2002) and was used as implemented in the program BioMapper (Hirzel et al. 2002, 2007).
Eco-geographical map preparation
GIS data layers for nine eco-geographical variables (EGVs) believed to possibly influence the distribution of leopard cats (Table 2) were generated. However, due to the occurrence of very high eigenvalues and as suggested by software warnings, two eco-geographic variables were discarded. ArcGIS 9.3 was used to determine the slope, elevation, aspect and ruggedness index for each cell of the study area using a 30-m cell resolution digital elevation model of the study area (ASTER data, version 2). As a surrogate to the vegetation cover, the Normalized Differential Vegetation Index for each cell was calculated from the satellite imagery of the study area (LANDSAT data 1990—no more recent image than 1990 was cloud-free). Distance maps to different land cover classes were prepared using ArcGIS 9.3 from the vegetation class map as reported in Sathyakumar et al. (2009). As depicted in the food habit studies, rodents are the major prey of leopard cats; hence, rodent distribution in the study area was considered as an important ecological variable to develop a habitat suitability model for leopard cats. Using the aforementioned topographical variables and vegetation index, a predictive habitat suitability map of rodents in the study area was prepared in Biomapper 4.0. Presence locations of small mammalian prey mainly rodents (rats, squirrels, porcupine), pikas and shrews as recorded (59 GPS fixes) from all the habitat types and elevation zones of Khangchendzonga BR through trail sampling (25 fixes) and camera trap surveys (34 fixes) were used for the analysis. McArthur’s broken-stick model was followed to select the number of factors to be used in preparing the habitat suitability model (Hirzel et al. 2007). This habitat suitability map for rodents was cross-validated following the k-fold cross-validation process as described by Boyce et al. (2002); a high Boyce index (0.92 ± 0.02) indicated the validity of the model.
Habitat suitability modelling for the leopard cat
The habitat suitability map for rodents was used as an eco-geographical variable along with other eight eco-geographical variables for ecological niche factor analysis and habitat suitability map preparation for the leopard cat following the same procedures of modelling. Presence locations of leopard cats (photo capture, sighting, scats and pugmarks) as recorded during trail sampling (53 fixes) and camera trap (14 fixes) surveys were used to generate the species map for the analysis. The predictive power and accuracy of the habitat suitability (HS) model was evaluated by a Jackknifed 10-fold cross-validation procedure (Fielding and Bell 1997) available in Biomapper 4.0. Absolute validation index (AVI; 0 to 1), contrast validation index (CVI; 0 to AVI) and continuous Boyce index (−1 to +1, 0 indicating a random model; Hirzel et al. 2006; Boyce et al. 2002) were used to provide a more continuous and more reliable measure of the accuracy of the model’s predictions.
Results
Detection probability and occupancy
Leopard cat was detected in 14 out of 41 sites at elevations below 2,750 m. The estimated site occupancy (Ψ = 0.352 ± 0.061) of leopard cat was slightly higher than the naive estimates (Ψnaive = 0.3415), which is the proportion of sites occupied by the species out of the total number of sites and does not include detection probability for its estimation. On the other hand, site occupancy estimation includes the detection probability and, hence, should always be greater than the naive estimate, as in the present case. The estimated detection probability (p) was 0.143 ± 0.0484. The overall photo capture rate of murids (8.81 ± 3.87/100 trap days) was high compared to other prey species and sympatric carnivores (Table 3). Occupancy modelling showed that the detection probability of the leopard cat was not influenced by human presence at the camera site (Table 4). Elevation, rodent abundance and tree cover were determined as best predictors for the occupancy of the leopard cat. Elevation negatively influenced the occupancy of the leopard cat, whilst rodent abundance and per cent tree cover had a positive influence (Table 5).
Diet analysis
Food habit analysis of 37 scats revealed the occurrence of nine prey items (Table 6). Of the scats, 51.35 % contained single prey items, 43.24 % contained two prey items, whilst 5.4 % contained three prey items. Small mammals (rodents and Ochotona sp.) comprised 82 % of the mammalian prey consumed by the leopard cats. Murids were the most dominant prey items, occurring in 89.2 % of the scats, followed by pikas (21.62 %) and birds (10.81 %).
Activity pattern
Information on the activity generated from photo captures (N = 61) inferred that leopard cats exhibited a nocturnal activity pattern (87 % nocturnal activity). The Rayleigh uniformity test indicated that the leopard cat activity pattern was not uniformly distributed (Rayleigh Z = 25.68, p < 0.0001), with peak bout activity occurring between 0200 and 0300 hours (DAI = 26.3; Fig. 3). In addition, the activity pattern of the leopard cat coincided with its principal prey (murids = rats + mice), but not with those of sciurids, ungulates, galliformes and other sympatric carnivores (Figs. 3 and 4). Watson’s U 2 test also showed no significant difference in the activity pattern of the leopard cat with its principal prey (U 2 = 0.15, df = 24, p > 0.05).
Habitat suitability
Ecological niche factor analysis
The leopard cat exhibited high global marginality values (1.32), indicating that it occupied a relatively small portion of the set of habitat conditions (defined by the EGVs) available in Khangchendzonga BR. Global tolerance index was low (0.275), indicating low tolerance towards deviations from the species optimal conditions. Table 7 showed the coefficients of each EGV on the marginality and first specialization factors for the leopard cat. Marginality explained 55 % of the total model variance. The occurrence of leopard cat was highly positively correlated with NDVI and rodent habitat suitability index and negatively correlated with elevation and distance to water (Table 7). The first specialization factor (Table 7) explained 23 % of variation. Leopard cats showed high level of specialization for elevation, aspect and ruggedness as well as NDVI, suggesting their major impact in limiting habitat suitability for leopard cat to mountainous areas such as Khangchendzonga BR.
Habitat suitability map
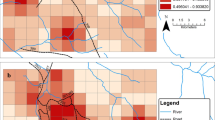
Three ENFA factors were retained after comparison with a broken-stick distribution to construct a HS map (explaining 86.5 % of the total information) for the leopard cat. The HS map (Fig. 5) for the leopard cat showed majority of the habitat as unsuitable (1,959.44 km2) and predicted only 164.54 km2 areas as moderate to highly suitable. Close inspection of the HS map would reveal that all the suitable areas were confined in the lower-elevation temperate forests situated in the southern or southwestern aspects and near to the streams.
Cross-validation
The presence-only evaluators AVI and CVI were 0.53 ± 0.15 and 0.52 ± 0.15, respectively, indicating that the HS model was able to discriminate between suitable and unsuitable habitats and that the set of EGVs allowed distinguishing specific habitats preferred by the leopard cat from the overall habitat available in Khangchendzonga BR. Boyce’s index (0.72 ± 0.22) provided a more continuous assessment of the model and predictive map accuracy. The positive and high value of this index reflected the reliability of the model predictions, and the low standard deviation indicated more robustness.
Discussion
Occupancy modelling suggests that leopard cats prefer areas with high rodent abundance and high tree cover in the intensive study area. Although rodents were distributed widely in all the habitats of the study area, a strong negative influence of elevation suggests that leopard cats are more functional in lower reaches much below the tree line. The species is otherwise capable of utilizing a broad variety of habitat types (Nowell and Jackson 1996; (Sanderson et al. 2008). In the Himalayan context, the species has been reported to extend into rhododendron–oak–maple forests up to an altitude of 3,254 m in Makalu-Barun National Park, Nepal (Ghimirey and Ghimire, 2010) and up to 2,928 m in Annapurna Conservation Area, Nepal (Appel et al. 2012). In Khangchendzonga BR, the leopard cat is restricted up to 2,750 m, indicating its preference for lower elevations (temperate and subtropical habitats) in the wet environments of eastern Himalaya. The insignificant influence of human presence on the detection of leopard cat indicates that the species may be tolerant to human interference, which is similar to the findings of Azlan et al. (2009) in Deramakot Forest Reserve in Sabah, Malaysia, where high photo capture rates were recorded in open areas along roads. This argument can be further supported by the fact that human habitations are largely located in the lower temperate and subtropical habitats where leopard cats occur. There was no significant influence of sympatric carnivores on leopard cats with reference to site occupancy, and these carnivores may be coexisting due to temporal segregation, as revealed by the activity patterns. Future research on the food habits and spatial distribution of other sympatric carnivores in the area can elucidate the results further.
The dietary composition of the leopard cat in our study reveals similar results when compared to the food habit studies conducted elsewhere, with murids contributing a dominant proportion of the prey (Table 8). Since murids dominate the terrestrial small-mammal communities in tropical rainforests (Wells et al. 2004), leopard cats have evolved to efficiently hunt small mammals, particularly murids, as predominant food resources (Kitchener 1991) for rapid energy maximization (Mukherjee et al. 2004). The absence of herpetofauna and insects in the diet of leopard cats also indicates high rodent abundance in the area compared to other species, because of which leopard cats do not require supplementing their main prey.
Information on the activity pattern of the leopard cat revealed from our camera trapping study synchronizes with the findings of Cheyne and Macdonald (2011) (camera trapping) and Rajaratnam (2000) (radiotelemetry), reporting 65 and 85 % nocturnal activity, respectively, but contradicts those of Austin et al. (2007) (radiotelemetry), Azlan and Sharma (2006) (camera trapping), Grassman et al. (2005) (radiotelemetry) and Rabinowitz (1990) (radiotelemetry), which report a diurnal, crepuscular and arrhythmic activity pattern. It has been established that the daily activity of many felids is correlated with the activity pattern of their main prey (Zielinski 1988). This has been observed in leopard cats (Rajaratnam 2000) and Iriomote cats (Prionailurus iriomotensis; Schmidt et al. 2009) preying upon their main prey—nocturnal murids—and in the present study as well. Such synchronicity increases the encounter rates when the prey is active (Curio 1976) and hence explains the absence of sciurids and the negligible presence of other prey species in their diet due to non-synchronicity in their activity patterns in the present study.
Ecological niche factor analysis depicted the leopard cat as a specialist species, at least in the perspective of Khangchendzonga landscape. Though this species is widespread in most of its range in India (Mukherjee et al. 2010), in the Khangchendzonga landscape, its range is confined to the lower limits of the entire landscape. The preference for areas with high probability of rodent distribution was quite explainable with respect to the results of food habit analysis. Proximity to perennial water bodies was also regarded as a crucial factor for the distribution of this cat (Sunquist and Sunquist 2002; Mukherjee et al. 2010). However, the preference for lower elevation and cooler western aspects needs to be explained in light of the effect of climatic variables, particularly temperature, on the leopard cat distribution (Mukherjee et al. 2010). Temperature played a great role in subdividing the range of leopard cats in India between two biogeographic zones, Himalayan and Northeast India and Western Ghat (Mukherjee et al. 2010). The present global distribution map for leopard cats as recognized by IUCN depicted the entire Sikkim (including Tibetan marginal mountains) to be inhabited by them. Contrary to this, a sharp decline in habitat suitability beyond temperate habitat indicates that the lower part of Khangchendzonga BR may represent the upper edge of leopard cat distribution in Sikkim. However, the results of this study are correlational and hypothetical, and there are likely to be other ecological variables such as competition with other lesser carnivores and habitat alterations that could explain the leopard cat distribution in a more comprehensive manner. Similar studies on the spatial distribution of sympatric small carnivores are very essential to describe the reason behind this special pattern of leopard cat distribution inside Khangchendzonga BR.
Conclusion
Leopard cats are mainly distributed and abundant in temperate habitats (Sathyakumar et al. 2011) and, as deduced from the results of occupancy and habitat suitability models, exhibit a temporal partitioning of resources to overcome competition with sympatric carnivores. The preferred temperate habitats are situated mostly in the Biosphere Reserve part of the protected area. Consequently, temperate habitats are in proximity to the villages situated outside the PA boundary and are therefore most prone to be impacted by human disturbances. During our survey in these parts of the Biosphere Reserve, we encountered evidences of poaching (snares) and also recorded evidence of retaliatory killing of a leopard cat by the villagers at Yuksam. The leopard cat is regarded as least concerned by the IUCN red list category, and this is the most abundant felid of Khangchendzonga BR, but conservation actions in the forms of snare removal and generation of awareness among the villagers are very necessary to maintain the present conservation status of leopard cats in the Khangchendzonga BR landscape. Strict vigil in the temperate zone of the Biosphere Reserve part (buffer zone) is needed to protect the species and other associated lesser carnivores from threats such as poaching for pelt (Sanderson et al. 2008), persecution and habitat destruction.
References
Appel A, Ghimirey Y, Acharya R (2012) Status assessment of wild felids with a special focus on clouded leopard and Asian golden cat in the Hugu-Kori forest, Annapurna Conservation Area, Nepal. Report submitted to the Point Defiance Zoo and Aquarium and the Wuppertal Zoo Association
Austin SC (2002) Ecology of sympatric carnivores in Khao Yai National Park, Thailand. PhD dissertation, Texas A and M University, Kingsville
Austin SC, Tewes ME, Grassman LI Jr, Silvy NJ (2007) Ecology and conservation of leopard cat Prionailurus bengalensis and clouded leopard Neofelis Nebulosa in Khao Yai National Park, Thailand. Acta Zool Sin 53:1–14
Azlan JM, Sharma DSK (2006) The diversity and activity patterns of wild felids in a secondary forest in Peninsular Malaysia. Oryx 40:36–41
Azlan M, Samejima H, Wilting A (2009) Records of five Bornean cat species from Deramakot Forest Reserve in Sabah, Malaysia. Cat News 51:12–15
Bailey LL, Hines JE, Nichols JD, MacKenzie DI (2007) Sampling design trade-offs in occupancy studies with imperfect detection: examples and software. Ecol Appl 17:281–290
Bothma JP, Nel JAJ, MacDonald A (1984) Food niche separation between four sympatric Namib Desert carnivores. J Zool 202:327–340
Bowker GC (2000) Mapping biodiversity. Int J Geogr Inf Syst 14:739–754
Boyce MS, Vernier PR, Nielson SE, Schmiegelow FKA (2002) Evaluating resource selection functions. Ecol Model 157:281–300
Carbone C, Christie S, Conforti K, Coulson T, Franklin N, Ginsberg JR, Griffiths M, Holden J, Kawanishi K, Kinnaird M, Laidlaw R, Lynam A, Macdonald DW, Martyr D, McDougal C, Nath L, O’Brien T, Seidensticker J, Smith DJL, Sunquist M, Tilson R, Shahruddin WNW (2001) The use of photographic rates to estimate densities of tigers and other cryptic mammals. Anim Conserv 4:75–79
Chen M-T, Tewes ME, Pei KJ, Grassman LI Jr (2009) Activity patterns and habitat use of sympatric small carnivores in southern Taiwan. Mammalia 73:20–26
Cheyne SM, Macdonald DW (2011) Wild felid diversity and activity patterns in Sabangau peat–swamp forest, Indonesian Borneo. Oryx 45:119–124
Curio E (1976) The ethology of predation. Springer, Berlin
Davis ML, Kelly MJ, Stauffer DF (2011) Carnivore co-existence and habitat use in the Mountain Pine Ridge Forest Reserve, Belize. Anim Conserv 14:56–65
Eccard JA, Pusenius J, Sundell J, Halle S, Ylonen H (2008) Foraging patterns of voles at heterogeneous avian and uniform mustelid predation risk. Oecologia 157:725–734
Fernandez DAP, de Guia APO (2011) Feeding habits of Visayan leopard cats (Prionailurus bengalensis rabori) in sugarcane fields of Negros Occidental, Philippines. Asia Life Sci 20:143–154
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49
Ghimirey Y, Ghimire B (2010) Leopard cat at high altitude in Makalu-Barun National Park, Nepal. Cat News 52:16–17
Gliwicz J, Dabrowski MJ (2008) Ecological factors affecting the diel activity of voles in a multi-species community. Ann Zool Fenn 45:242–247
Grassman LI Jr. (1997) Ecology and behavior of four sympatric carnivore species (Carnivora: Mammalia) in Kaeng Krachan National Park, Thailand. MSc thesis, Kasetsart University
Grassman LI Jr (2000) Movements and prey selection of the leopard cat (Prionailurus bengalensis) in a dry evergreen forest in Thailand. Acta Theriol 45:421–426
Grassman LI Jr, Tewes ME, Silvy NJ, Kreetiyutanont K (2005) Spatial organization and diet of the leopard cat (Prionailurus bengalensis) in north-central Thailand. J Zool 266:45–54
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135:147–186
Harmsen BJ, Foster RJ, Silver SC, Ostro LET, Doncaster CP (2011) Jaguar and puma activity patterns in relation to their main prey. Mamm Biol 76:320–324
Hines JE (2006) PRESENCE v 4.0—software to estimate patch occupancy and related parameters. USGSPWRC. http://www.mbrpwrc.usgs.gov/software/presence.html. Accessed 18 August 2012
Hirzel AH, Hausser J, Chessel D, Perrin N (2002) Ecological niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology 83:2027–2036
Hirzel AH, Le Lay G, Heifer V, Randin C, Guisan A (2006) Evaluating the ability of habitat suitability models to predict species presences. Ecol Model 199:142–152
Hirzel AH, Hausser J, Perrin N (2007) Biomapper 4.0. Laboratory of Conservation Biology, Department of Ecology and Evolution, University of Lausanne, Switzerland. http://www2.unil.ch/biomapper. Accessed 23 May 2012
Inoue T (1972) The food habit of Tsushima leopard cat, Felis bengalensis sp., analyzed from their scats. J Mamm Soci Japan 5:155–169
Izawa M, Doi T, Ono Y (1991) Ecological study on the two species of Felidae in Japan. In: Maruyama N et al (eds) Wildlife conservation: present trends and perspectives for the 21st century. Wildlife Research Centre, Yokohama, Japan, pp 141–144
Jackson R, Ahlborn G (1989) Snow leopards (Panthera uncia) in Nepal—home range and movements. Nat Geogr Res 5:161–175
Johnson KA, Jinping Y (1996) The leopard cat in China: ecology and management for sustainable utilization. Project S-064 1992. Final Report to CITES Secretariat. CITES, Geneva
Johnson WE, Fuller TK, Franklin WL (1996) Sympatry in canids: a review and assessment. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution, vol. 2, Cornell University Press. Ithaca, NY, pp 189–218
Khan MMH (2004) Food habits of leopard cat Prionailurus bengalensis (Kerr, 1792) in the Sundarbans East Wildlife Sanctuary, Bangladesh. Zoo’s Print 19:1475–1476
Kitchener A (1991) The natural history of the wild cats. Cornell University Press, Ithaca
Koppikar BR, Sabins JH (1976) Identification of hairs of some Indian mammals. J Bombay Nat Hist Soc 73:5–20
Kovach WL (2011) Oriana—circular statistics for Windows, ver. 4. Kovach Computing Services, Pentraeth
Lenton SM, Fa JE, Perez Del Val J (2000) A simple nonparametric GIS model for predicting species distribution: endemic birds in Bioko Island, West Africa. Biodivers Conserv 9:869–885
MacKay P, Zielinski WJ, Long RA, Ray JC (2008) Non-invasive research and carnivore conservation. In: Long RA, MacKay P, Ray J (eds) Non-invasive survey methods for carnivores. Island Press, Washington, pp 1–7
Mackenzie DI (2005) What are the issues with presence–absence data for wildlife managers? J Wildl Manag 69:849–860
Mackenzie DI (2006) Modeling the probability of resource use: the effect of, and dealing with, detecting a species imperfectly. J Wildl Manag 70:367–374
Mackenzie DI, Royle A (2005) Designing occupancy studies: general advice and allocating survey effort. J Appl Ecol 42:1105–1114
MacKenzie DI, Nichols JD, Royle JA, Pollack KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling. Academic, New York
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:243–253
Marzluff JM, Millspaugh JJ, Hurvitz P, Handcock MS (2004) Relating resources to a probabilistic measure of space use: forest fragments and Steller’s jays. Ecology 85:1411–1427
McArdle BH (1990) When are rare species not there? Oikos 57:276–277
Mukherjee S, Goyal SP, Chellam R (1994) Standardization of scat analysis techniques for leopards (Panthera pardus) in Gir National Park, Western India. Mammalia 58:139–143
Mukherjee S, Goyal SP, Johnsingh AJT, Pitman MRPL (2004) The importance of rodents in the diet of jungle cat (Felis chaus), caracal (Caracal caracal) and golden jackal (Canis aureus) in Sariska Tiger Reserve, Rajasthan, India. J Zool 262:405–411
Mukherjee S, Krishnan A, Tamma K, Home C, Navya R, Joseph S, Das A, Ramakrishnan U (2010) Ecology driving genetic variation: a comparative phylogeography of jungle cat (Felis chaus) and leopard cat (Prionailurus bengalensis) in India. PLoS One 5:e13724
Myers N, Mittermier RA, Mittermier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 40:853–858
Nowell K, Jackson P (1996) Wild cats: status survey and conservation action plan. IUCN, Gland, Switzerland
O’Brien TG, Kinnaird MF, Wibisono HT (2003) Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6:131–139
Olson D, Dinerstein E (1998) The Global 200. A representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv Biol 12:502–515
Peladeau N (2004) SimStat, version 2.5, Provalis Research
Prendergast JR, Quinn RM, Lawton JH (1999) The gaps between theory and practice in selecting nature reserves. Conserv Biol 13:484–492
Rabinowitz AR (1990) Notes on the behavior and movements of the leopard cat, Felis bengalensis, in a dry tropical forest mosaic in Thailand. Biotropica 22:397–403
Rajaratnam R (2000) Ecology of the leopard cat (Prionailurus bengalensis) in Tabin Wildlife Reserve, Sabah, Malaysia. PhD thesis, University Kebangsaan
Rajaratnam R, Sunquist M, Rajaratnam L, Ambu L (2007) Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J Trop Ecol 23:209–217
Ray JC, Sunquist M (2001) Trophic relations in a community of African rainforest carnivores. Oecologia 127:395–408
Reynolds JC, Aebischer NJ (1991) Comparison and quantification of carnivore diet by faecal analysis: a critique, with recommendations based on a study of the fox (Vulpes vulpes). Mamm Rev 21:97–122
Sanderson J, Sunarto S, Wilting A, Driscoll C, Lorica R, Ross J, Hearn A, Mujkherjee S, Khan JA, Habib B, Grassman L (2008) Prionailurus bengalensis. In: IUCN 2011. IUCN Red List of Threatened Species, version 2011.2. www.iucnredlist.org. Accessed 21 February 2012
Sathyakumar S, Bhattacharya T, Bashir T, Poudyal K, Lachungpa U (2009) Developing spatial database on the mammal distributions and monitoring programme for large carnivores, prey populations and their habitats in Khangchendzonga Biosphere Reserve, Sikkim. Interim Report (February 2008 to July 2009), Wildlife Institute of India, Dehradun, India
Sathyakumar S, Bashir T, Bhattacharya T, Poudyal K (2011) Assessing mammal distribution and abundance in intricate eastern Himalayan habitats of Khangchendzonga, Sikkim, India. Mammalia 75:257–268
Schmidt K, Nakanishi N, Izawa M, Okamura M, Watanabe S, Tanaka S, Doi T (2009) The reproductive tactics and activity patterns of solitary carnivores: the Iriomote cat. J Ethol 27:165–174
Singh NJ, Milner-Gulland EJ (2011) Monitoring ungulates in Central Asia: current constraints and future potential. Oryx 45:38–49
Sunquist M, Sunquist F (2002) Wildcats of the world. University of Chicago Press, Chicago
Sunquist ME, Sunquist F, Daneke DE (1989) Ecological separation in a Venezuelan llanos carnivore community. In: Redford KH, Eisenberg JF (eds) Advances in neotropical mammology. Sandhill Crane Press, Gainesville, pp 197–232
Tambe S (2007) Ecology and management of the Alpine landscape in the Khangchendzonga National Park, Sikkim Himalaya. PhD thesis, FRI University
Tatara M, Doi T (1994) Comparative analysis on food habits of Japanese marten, Siberian weasel and Leopard cat in Tsushima Islands, Japan. Ecol Res 9:99–107
Tsoar A, Allouche O, Steinitz O, Rotem D, Kadmon R (2007) A comparative evaluation of presence only methods for modelling species distribution. Divers Distrib 13:397–405
Watanabe S (2009) Factors affecting the distribution of the leopard cat Prionailurus bengalensis on East Asian islands. Mamm Stud 34:201–207
Wells K, Pfeiffer M, Lakim MB, Lisenmair KE (2004) Use of arboreal and terrestrial space by a small mammal community in a tropical rain forest in Borneo, Malaysia. J Biogeogr 31:641–652
Zielinski WJ (1988) The influence of daily variation in foraging cost on the activity of small carnivores. Anim Behav 36:239–249
Acknowledgments
We are grateful to the Department of Forests, Environment and Wildlife Management, Government of Sikkim, for granting us permission to work in Sikkim. We thank the Wildlife Institute of India, Dehradun, for providing us the grants and support. We thank our field assistants, Mr. Sukhbahadur and Mr. Sukhraj, for their help during field work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Krzysztof Schmidt
Rights and permissions
About this article
Cite this article
Bashir, T., Bhattacharya, T., Poudyal, K. et al. Integrating aspects of ecology and predictive modelling: implications for the conservation of the leopard cat (Prionailurus bengalensis) in the Eastern Himalaya. Acta Theriol 59, 35–47 (2014). https://doi.org/10.1007/s13364-013-0145-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-013-0145-x