Abstract
In mass-spectrometry based peptide sequencing, formation of b- and y-type fragments by cleavage of the amide C–N bond constitutes the main dissociation pathway of protonated peptides under low-energy collision induced dissociation (CID). The structure of the b 2 fragment ion from peptides containing glutamine (Gln) and asparagine (Asn) residues is investigated here by infrared ion spectroscopy using the free electron laser FELIX. The spectra are compared with theoretical spectra calculated using density functional theory for different possible isomeric structures as well as to experimental spectra of synthesized model systems. The spectra unambiguously show that the b2-ions do not possess the common oxazolone structure, nor do they possess the alternative diketopiperazine structure. Instead, cyclic imide structures are formed through nucleophilic attack by the amide nitrogen atom of the Gln and Asn side chains. The alternative pathway involving nucleophilic attack from the side-chain amide oxygen atom leading to cyclic isoimide structures, which had been suggested by several authors, can clearly be excluded based on the present IR spectra. This mechanism is perhaps surprising as the amide oxygen atom is considered to be the better nucleophile; however, computations show that the products formed via attack by the amide nitrogen are considerably lower in energy. Hence, b2-ions with Asn or Gln in the second position form structures with a five-membered succinimide or a six-membered glutarimide ring, respectively. b2-Ions formed from peptides with Asn in the first position are spectroscopically shown to possess the classical oxazolone structure.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Current proteomics research relies heavily on mass spectrometry (MS) based peptide sequencing methods [1, 2]. Practical applications make use of the differences in the m/z values of peptide fragments generated by collision-, electron-, surface-, or photon-induced dissociation of protonated peptides. In most applications, protein identification is then effected by database matching. Along with the practical application of MS-based peptide sequencing, much effort, both experimental and theoretical, has been devoted to a fundamental understanding of the dissociation reactions occurring in the mass spectrometer. Such experimental and computational studies generally address the energetics and dynamics of the dissociation reactions including the minimum and transition-state structures along the reaction path [3–12].
Under low-energy collision conditions, protonated peptides fragment predominantly by cleavage of the backbone amide bond generating so-called b- and y-type fragments [2], corresponding to the charged N- and C-terminal products, respectively, of the dissociation reaction. The mobile proton model [3, 4, 13] predicts that upon collisional activation, the added proton migrates from the most basic site in the peptide to one of the amide linkages (either the amide nitrogen or oxygen atom), weakening the amide bond and making the amide carbonyl C-atom a strong electrophile (see Scheme 1) [6, 12]. This carbonyl C-atom becomes subject of nucleophilic attack leading to the reactive configuration. The oxygen atom of the adjacent amide carbonyl is generally assumed to act as the nucleophile in this process, leading to the formation of an oxazolone, five-membered ring structure as shown in Scheme 1 [10, 14, 15]. However, the peptide generally contains several other nucleophiles that could possibly attack the electrophilic carbon atom as well, leading to isomeric, non-classical, b-type fragments. Thus, while y-type fragments are generally assumed to possess the structure of a truncated peptide, the structure of the N-terminal b-fragment has been a subject of much debate [10, 14, 16].
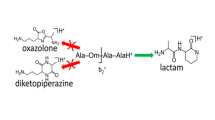
Possible dissociation/rearrangement reactions based on the ‘mobile proton’ model, leading to different b2-ion structures. In addition to the classic oxazolone and diketopiperazine structures, a Gln residue in the second position allows for the formation of glutarimide (cyclic imide) and imino-δ-valerolactone (cyclic isoimide) structures. Observation of the c1 ion in MS3 experiments can be interpreted as evidence for Gln side-chain induced cyclization reactions, although it cannot distinguish between the imide and isoimide structures
When unprotonated, the N-terminal amine nitrogen atom is a strong nucleophile. For oxazolone b-type fragments containing more than four or five residues, this nitrogen atom has been suggested to attack the carbonyl C-atom of the oxazolone ring forming a cyclic peptide [17, 18]. Experimental evidence for the formation of these cyclic structures, a potential source of non-sequence ions, has been provided by multistage CID MS [17–20], H/D exchange [21, 22], electron capture dissociation (ECD) [23] and IR spectroscopic [21, 22, 24–26] measurements.
The structure of shorter b-type fragments, in particular the b 2 fragment ion, has also been under much debate [16]. A statistical analysis of a large set of tryptic peptide tandem MS spectra revealed that the occurrence of this fragment ion is anomalously high as compared to longer b-fragments, which was suggested to be due to bifurcating reaction pathways partly leading to a different molecular structure [27]. In particular, the formation of a cyclic dipeptide (i.e., a diketopiperazine structure was suggested. Analogous to the formation of macrocyclic structures, diketopiperazines form when the N-terminal nitrogen atom, instead of the amide carbonyl O-atom, acts as the nucleophile attacking the electrophilic amide carbonyl C-atom (see Scheme 1). It is well-known from theoretical studies that the diketopiperazine structure is in fact lower in energy than the oxazolone motif, although its formation is also generally assumed to be kinetically disfavored [6, 15, 28–30].
IR photo-dissociation spectroscopy [31–33] of mass-isolated peptide fragment ions has proven to be a diagnostic tool to establish the molecular structures of these gas-phase reaction products [34, 35]. Isomeric oxazolone and diketopiperazine b2 structures are easily distinguished based on their IR spectra. The first b2-ions investigated with IR spectroscopy contained only Ala and Gly residues and were all found to possess oxazolone structures [21, 36–38]. Moreover, ion spectroscopy identified oxazolone b2-ion structures to be formed from three doubly charged tryptic peptides [39, 40] as well as from non-oxazolone b3 ions [41]. Spectroscopic evidence for a diketopiperazine structure was first found for the b2 ion of protonated His(Ala)4 [42]. It was suggested that since the added proton in the precursor peptide resides on the His side chain, the N-atom of the N-terminal amino group has greater freedom to carry out the nucleophilic attack leading to the diketopiperazine product ion. This scenario is supported by recent spectroscopic studies on b2-ions containing Arg, which are also predominantly diketopiperazines [43]. Further support is provided by a study on b2-ions containing histidine analogues, which suggest that the protonated side chain may aid in the trans-to-cis isomerization required for diketopiperazine formation [44].
Thus, while the side-chain identity has been shown to influence product ion structures indirectly by sequestering the proton [42, 43], virtually no spectroscopic evidence has thus far been found for direct involvement of the side chain in the rearrangement reaction. As the rearrangement reactions are largely driven by nucleophilic attack [45], side chains of high nucleophilicity yet relatively poor proton affinity are expected to be most prone to become directly involved in the rearrangement reaction. In this study, we therefore focus on glutamine (Gln) and asparagine (Asn) residues, which feature an amide group in their side chain. The amide nitrogen and oxygen atoms form strong nucleophiles, while the proton affinity of the amide group is expected to be lower than that of the N-terminal amine of the peptide.
In their computational survey of possible alternative b2-ion structures, O’Hair and coworkers indeed suggest cyclization rearrangements induced by the side-chain amide oxygen or nitrogen atom of Gln and Asn residues [16]. Structures formed by such amide N- or O-atom induced nucleophilic attack are however higher in energy than the isomeric diketopiperazine structure, although the energetic ordering of N- and O-atom induced cyclization remains somewhat unclear Footnote 1. Cyclization reactions involving the Gln side-chain N- or O-atom were suggested to occur in analogy to biological degradation of Gln-containing peptides [46]. Further evidence for such processes was found in multistage tandem MS CID experiments of b2-ions with Gln in the second position, where the unusual formation of a c1-ion was observed [47–50], which is of interest in de novo peptide sequencing strategies [50]. Mechanistically, formation of the c1-ion was interpreted as evidence for the b2-ion having a non-oxazolone structure; in particular, it was suggested that the b2-ions having Gln as the second residue possess an imino-δ-valerolactone structure (i.e., a cyclic isoimide, see Scheme 1), formed via nucleophilic attack from the side-chain amide oxygen atom [47, 48]. However, nucleophilic attack from the Gln side-chain nitrogen atom forming an isomeric glutarimide structure (i.e., a cyclic imide, see Scheme 1) is evenly consistent with the observation of the c1-ion in the MS3 spectrum and can thus not be excluded [46]. Studies on longer b-type ions containing Gln have encountered the same isomeric question, which remained unresolvable based on MSn data [20].
For peptides containing Asn, analogous side-chain induced reactions could lead to non-oxazolone structures, in particular to species containing a five-membered cyclic isoimide (imino-γ-butyrolactone) or imide (succinimide) structure (Scheme 2), resulting from side-chain amide oxygen or nitrogen attack, respectively. While both structures were indeed proposed in reference [16], MS3 data reported on Asn containing peptides is ambiguous as to the formation of c-type ions [48, 49]. Energetics of imide and isoimide structures relative to the more common oxazolone and diketopiperazine motifs are significantly different for b2-ions produced from Asn- and Gln-containing peptides [48].
Here, we present IR spectra of b2-ions containing Gln and Asn residues. The b2-ion structures are determined by comparison of the experimental spectra with spectra calculated for candidate structures as well as with an experimental spectrum of a synthesized model compound. These data suggest that it is indeed the amide side chain that acts as the nucleophile, although not through its oxygen atom as previously suggested, but through its nitrogen atom forming cyclic imide structures: glutarimide for Gln and succinimide for Asn.
2 Experimental and Computational Methods
2.1 IRMPD Spectroscopy
Infrared spectra of precursor and fragment ions were obtained via IR multiple photon dissociation (IRMPD) spectroscopy using the Fourier transform ion cyclotron resonance (FTICR) mass spectrometer coupled to the beamline of the infrared free electron laser FELIX [51, 52]. Protonated ions were generated by electrospray ionization (ESI) using a Micromass Z-Spray source and peptide solutions of approximately 0.5 mM in acetonitrile/water with about 0.2 % acetic acid added. This study focuses on the Gln and Asn containing peptides PheGlnAla, AlaAsnAla, and AsnAlaAla possessing an amide moiety in their side chains. The sample peptides were obtained from GeneCust (Dudelange, Luxemburg) and used without further purification. (S)-2-amino-N-({S}-2,6-dioxopiperidin-3-yl)-3-phenylpropanamide, which contains a glutarimide ring and serves as a model for the b2 fragment of PheGlnAla (vide infra), was synthesized at the University of Amsterdam following the method reported by Fox et al.[53].
The experimental procedure is described in detail elsewhere [54]. Briefly, after generation by ESI, the protonated peptides are accumulated in a linear hexapole trap before being injected into the home-built FTICR mass spectrometer [52], equipped with a 4.7 T actively-shielded superconducting magnet. In the ICR cell, the ion of interest is mass isolated by a stored waveform inverse Fourier transform (SWIFT) excitation pulse [55]. Ions are then irradiated with FELIX, which produces 5 μs long IR pulses with energies of 30–40 mJ inside the FTICR cell and a bandwidth of ≈ 0.5 % of the central wavelength. IR spectra were recorded from 5 to 12.5 μm corresponding to a frequency range of 800–2000 cm–1. A mass spectrum is generated using an excite/detect sequence as implemented in the FTICR control software adapted from that of Heeren and co-workers [56]. Three mass spectra were averaged at each wavelength point.
IRMPD occurs whenever the frequency of the photon is in resonance with the frequency of a molecular vibration; tens to hundreds of photons are absorbed leading to unimolecular dissociation [31, 33, 57]. From fragment and parent ion intensities observed in the mass spectrum, the fragmentation yield is derived as ΣI(fragments)/ΣI(all ions). Plotting this yield as a function of the wavenumber of the radiation generates the infrared spectrum. The yield is corrected for variations in the laser pulse energy over the scanned wavelength range assuming a linear power dependence.
The b 2 fragment ions from PheGlnAla and AsnAlaAla are generated by nozzle–skimmer dissociation in the high-pressure region at the inlet of the mass spectrometer. Dissociation of AlaAsnAla is induced in the ICR cell by 0.1 s irradiation with a 35-W CO2 laser (ULR-25; Universal Laser Systems, Scottsdale, AZ, USA,), which was found to be more efficient than CID for this system. It was checked experimentally that fragment ions generated by either method exhibit the same IR spectrum.
2.2 Density Functional Theory Calculations
Experimental spectra are compared to spectra computed for candidate structures at the density functional theory (DFT) level in order to identify the molecular structure of the ions generated. Optimized ion structures and their vibrational spectra were computed using the B3LYP functional and the 6-31++G(d,p) basis set, as implemented in Gaussian03, rev. C.02. Input structures were generated based on chemical intuition, where structures exhibiting resonance stabilization by conjugation, stabilization by hydrogen bonding, and avoiding syn steric interactions were particularly considered. A molecular dynamics search of the potential energy surfaces was not deemed necessary as the systems under study (particularly the b 2 fragment ions) are fairly small; moreover, the main goal of this study was to establish the isomeric structure and protonation site rather than the precise conformeric structure. Computed vibrational frequencies were scaled by 0.97 and convoluted with a 25 cm–1 full-width-at-half-maximum Gaussian line shape function to facilitate comparison with experimental spectra.
3 Results and Discussion
3.1 Structure and Protonation Site of the PheGlnAla Precursor Peptide
Recent spectroscopic investigations of His, Arg, and Lys containing peptides have revealed their high propensity of forming diketopiperazine b2-ions [42–44]. Protonation at the side chain nucleophile instead of at the N-terminal amine has been suggested to enhance the trans-to-cis isomerization of the peptide bond required for the formation of the diketopiperazine structure [44]. In other words, deviation from the formation of the classical oxazolone b2-ion may thus be related to the protonation site in the precursor peptide. Since Gln and Asn containing peptides have been suggested to not form oxazolone b2-ions [6, 16, 47–49], we first address the question of where the proton resides in the precursor PheGlnAla peptide. Although peptides without basic residues are generally assumed to be protonated on the N-terminus, protonation of backbone amide oxygen atoms has been suggested to be competitive [58, 59]. Moreover, the Gln side-chain amine group may sequester the proton.
The main IRMPD fragment ions of protonated PheGlnAla are b2 (m/z 276), b2–NH3 (m/z 259), c1 (m/z 165), z2 (m/z 201), and a1 (m/z 120). The rather unusual observation of c1 and z2 fragments was also reported in CID studies and interpreted as evidence for non-oxazolone structures of the b-fragment [48, 49]. Figure 1 compares the experimental IRMPD spectrum with computed spectra for PheGlnAla protonated at the N-terminus, at the glutamine side chain oxygen and at the glutamine side chain nitrogen (panels a, b, and c, respectively). It is seen immediately that the match between experiment and theory is much better for protonation at the N-terminus. Moreover, the computed relative energies strongly favor protonation at the N-terminus.
Comparison of the IRMPD spectrum of the protonated precursor peptide PheGlnAla (black trace) with DFT-calculated spectra (blue dashed lines) for structures protonated at the N-terminal nitrogen (a), Gln side-chain oxygen (b) and the Gln side-chain nitrogen (c). Calculated relative free energies are given for each structure. The arrow indicates the protonation site
We can thus safely assume that prior to activation, the peptide is protonated on the N-terminal amino group rather than on the Gln side chain, similar to what is observed for the majority of small protonated peptides and markedly different from peptides containing His, Arg, or Lys.
3.2 b2-Ion of Protonated PheGlnAla
The b2-ion of protonated PheGlnAla was produced by CID in the front end of the instrument and subsequently mass isolated in the FTICR-MS. Upon IRMPD, the ion undergoes dissociation forming charged products at m/z 120 (a1) and m/z 165 (c1), with the former being most abundant. This dissociation pathway is markedly different from that observed for typical oxazolone b2-ions, which are known to generate mainly the a2 ion via the loss of a carbon monoxide unit. It also differs from the most abundant IRMPD channels observed for diketopiperazine b2 structures (i.e., cyclic dipeptides), which are mainly loss of CO, loss of CO + NH3, and generation of the a1 fragment [36–38].
Figure 2 compares the experimental IRMPD spectrum of the PheGln b2-ion at m/z 276 with calculated spectra for selected candidate structures. Ball-and-stick structures revealing the conformeric structures of these systems can be found in the Supplementary Material (Figure S2). The diketopiperazine structure protonated on the carbonyl oxygen adjacent to the α-carbon of the Phe residue (panel a) is the global minimum structure. The structure with a six-membered glutarimide ring resulting from a nucleophilic attack by the Gln side chain N-atom (panel b) is 28 kJ/mol higher in free energy, but still lower than the common oxazolone motif, protonated at the oxazolone nitrogen, at 44 kJ/mol (panel c). The alternative oxazolone structure, where the proton is at the N-terminus (panel d) is nearly iso-energetic. The imino-δ-valerolactone structure (panel e) resulting from nucleophilic attack by the Gln side chain O-atom, as suggested based on MS3 data [47, 48], lies at more than 100 kJ/mol. Protonation at the glutarimide or imino-valerolactone ring nitrogen atoms leads to structures that are substantially higher in free energy, such as the glutarimide protonated structure in panel f. Further protonation sites for each of these four main isomeric motifs were also investigated, but in all cases resulted in calculated vibrational spectra that deviate strongly from the experimental spectrum (not shown).
IRMPD spectrum of the b2-ion of protonated PheGlnAla (black in all panels) compared to DFT-calculated spectra (blue dashed lines) for isomeric candidate structures: diketopiperazine protonated on carbonyl oxygen (a), glutarimide protonated at N-terminus (b), oxazolone protonated on oxazolone nitrogen (c), oxazolone protonated on N-terminus (d), 6-imino-δ-valerolactone protonated on N-terminus (e), and glutarimide protonated on glutarimide nitrogen (f). Calculated relative free energies are given for each of the structures. Three-dimensional ball-and-stick structures can be found in the Supplementary Material (Figure S2)
Inspection of the six calculated spectra in Figure 2 and comparison with the experimental spectrum leads quite readily to the conclusion that only the glutarimide structure in panel b, resulting from nucleophilic attack by the glutamine side chain nitrogen, can reasonably explain the experimental spectrum. An assignment of the most intense peaks in the experimental IR spectrum is presented in Table 1. The carbonyl stretching modes found roughly between 1600 and 1800 cm–1 are, as in many cases, very characteristic and most diagnostic for the structural assignment. Three strong bands appear to be observed in this range, although two of them are not resolved. Of the computed spectra in Figure 2, only those of the glutarimide (b) and the imino-δ-valerolactone (e) structures reproduce the positions of the three bands. All other structures display strong bands in this region which are not observed experimentally, so that oxazolone as well as diketopiperazine structures can be confidently excluded. Based mainly on the comparison of experimental and theoretical spectra in the 1100–1300 cm–1 range, it is then concluded that the glutarimide structure matches the experimental spectrum substantially better than the isomeric imino-δ-valerolactone structure. However, some discrepancies between experimental and theoretical spectra are apparent in Figure 2b, particularly for the bands at 1391 and 1180 cm–1. The band at 1391 cm–1 is assigned to the NH3 umbrella vibration (computed at 1408 cm–1), which is often found to be hard to reproduce accurately by computation, likely due the anharmonic shape of the potential well for these type of vibrations [60–62]. The feature at 1180 cm–1 is probably due to a convolution of vibrational modes calculated at 1158 cm–1 (C-C stretching coupled with CH2 wagging modes) and 1203 cm–1 (NH3 wagging coupled to CH wagging).
While the glutarimide structure of Figure 2b clearly provides the best spectral match with the experiment, the deviations in the 1100–1400 cm–1 range make a further experimental confirmation for this structural assignment desirable. First, we investigate the structure of the m/z 165 fragment ion, which has been assumed to be a c1 ion [47–49]. Appearance of c-type ions is uncommon in CID mass spectra and has been interpreted as evidence for Gln side-chain-induced rearrangement[47, 48] (although it cannot distinguish between cyclic imide and isoimide structures; see Scheme 1). Although c-type ions are rarely observed in CID of protonated peptides, they are well-known fragmentation products in electron capture and electron transfer dissociation (ECD/ETD) [5, 63–66] and their structures have been extensively discussed [7, 8, 67, 68]. In CID as well as in ECD/ETD, c-type ions result from cleavage of the N–Cα bond and are assumed to be linear N-terminal peptide fragments possessing either an amide or an enol-imine moiety at the C-terminus. Here, the IRMPD spectrum of the m/z 165 fragment ion of protonated PheGlnAla was obtained by recording the wavelength-dependent fragmentation into m/z 120, presumably the a1 ion. Figure 3 compares the experimental IRMPD spectrum with computed spectra for the c1 ion having either an amide (panel a) or an enol-imine (panel b) moiety at the C-terminus. Apart from the relative band intensities of a few of the minor bands, the experimental spectrum matches favorably with that calculated for the c1-ion having a C-terminal amide, which is also substantially lower in energy than the isomeric enol-imine structure. The comparison in Figure 3a further suggests that the system is protonated on the N-terminus. The conclusion that the m/z 165 fragment ion is indeed the c1 ion as previously suggested [16, 47, 48] thus lends support to the b2-ion having either the glutarimide or the imino-δ-valerolactone structure.
Comparison of the IRMPD spectrum of the m/z 165 fragment ion of protonated PheGlnAla (black trace) with DFT-calculated spectra for amide (a) and enol-imine (b) structures of the c1 ion, both protonated at the N-terminal amino group (blue dashed lines). Relative free energy values are given. Three-dimensional structures are available in the Supplementary Material (Figure S3)
Secondly, to provide further support for our assignment as a glutarimide structure versus the imino-δ-valerolactone structure, an IRMPD spectrum was recorded for the conjugate acid of (S)-2-amino-N-({S}-2,6-dioxopiperidin-3-yl)-3-phenylpropanamide (see Figure 4), to serve as a reference for the assumed glutarimide b2-ion structure. The compound was synthesized following the method of Fox et al.[53] and introduced into the FTICR-MS as a protonated ion by ESI. The experimental IRMPD spectrum is displayed in Figure 4, overlaid on the experimental spectrum of the b2 fragment ion of protonated PheGlnAla. Within experimental accuracy, the two spectra are identical and moreover, the same dissociation channels are observed upon IRMPD. The excellent match in Figure 4 identifies the glutarimide structure as the sole contributor to the spectrum of the PheGlnAla b2-ion, thus suggesting that the nucleophilic attack leading to the formation of the b2-ion occurs through the Gln side chain nitrogen atom and not through its oxygen atom as suggested based on MS3 data [47, 48].
Comparison of the experimental IRMPD spectra of the protonated glutarimide containing reference compound [protonated (S)-2-amino-N-({S}-2,6-dioxopiperidin-3-yl)-3-phenylpropanamide, black] with that of the b2 fragment ion from protonated PheGlnAla (red). All bands are seen to be reproduced exactly; the slight deviation in relative intensities is probably due to a slow decline of laser power during the scan of the b2-ion, which is not accounted for in the power profile used for normalization
Based on the experimental evidence, we can thus safely conclude that the PheGln b2-ion has a glutarimide structure, which suggests that it is the side chain amide nitrogen atom that attacks the backbone carbonyl carbon. This is quite surprising as the side chain amide oxygen is considered to be a stronger nucleophile, since conjugation in the amide moiety places a partial negative charge on the O-atom and a partial positive charge on the N-atom. Furthermore, protonation at the O-atom of acetamide (CH3-C{=O}-NH2) is computed to be 54 kJ mol–1 more favorable than protonation at the N-atom Footnote 2. In addition, the oxygen atoms in the amide linkages of peptides are known to be more basic than the nitrogen atoms [58]. Based on these considerations, it is perhaps not surprising that observation of the c1-ion in MS3 studies was interpreted as evidence for the formation of cyclic isoimides rather than cyclic imides by several authors [6, 47–49].
To address the question why it is then the amide nitrogen that acts as the nucleophile and not the amide oxygen, we first consider the relative thermodynamics of the resulting product structures. Cyclization through the nitrogen atom leads to the glutarimide structure, which is about 80 kJ mol–1 (see Figure 2) more stable than the isomeric imino-δ-valerolactone structure resulting from cyclization through the oxygen atom. A reaction under thermodynamic rather than kinetic control could have explained the observed product [69], if the diketopiperazine structure had not been lower in energy still. Note that the energy difference between the imide and isoimide structures is due to the isomerism of the six-membered ring, and not to the conformational structure of the peptide chain; at the B3LYP/6-31++G** level of theory, the glutarimide isomer of C5H7NO2 lies 91 kJ mol-1 lower in free energy than the imino-δ-valerolactone isomer (see Scheme 3). We note that O’Hair and coworkers [16] also investigated the relative thermodynamics of the two forms of the b2-ion, although their peptides were N-acetylated. Therefore, only structures protonated at the (iso)imide ring were considered, so that their results are not entirely comparable to ours, where the proton resides on the N-terminal amino group of the b2-fragment. For the b2-fragments with a free N-terminus, protonation at the N-terminal amino group is computed to be substantially lower in energy (see Figure 2).
Understanding the mechanistic details of the dissociation/rearrangement reaction requires extensive transition-state calculations, which are beyond the scope of this study. Speculatively, the reaction may not proceed via a mobile proton scenario (Scheme S1 in the Supplementary Material) but instead follow a charge-remote pathway, such as sketched in Scheme S2, similar to what has been suggested for glutamic acid (Glu) containing peptides [70] and similar to degradation pathways of Gln containing peptides in biological samples [46]. In the lowest-energy conformers of the precursor peptide (see Figure 1 and Figure S1), the protonated N-terminus is hydrogen-bonded to the side-chain amide O-atom of the Gln residue. This reduces the nucleophilicity of the side-chain O-atom and simultaneously sequesters the proton to some extent, possibly allowing for such charge-remote reactions to occur.
3.3 b2-Ion of Protonated AlaAsnAla
Asparagine (Asn) differs from glutamine only in the length of the alkyl chain connecting the α-carbon and the amide moiety, which consists of one methylene unit for Asn versus two for Gln. O’Hair and coworkers suggested similar side-chain-induced reactions could take place upon formation of b-type fragment ions for Asn containing peptides [16], forming in this case five-membered ring species as shown in Scheme 2. However, Lee and Lee report that no c1-ion is observed from peptides with Asn in the second position, suggesting that the Asn side chain does not interfere with amide bond cleavage [48]. In sharp contrast, Lehmann and coworkers report formation of c1-ions from various peptides with Asn in the second position, though at lower abundance than for the Gln-containing peptides [49]. Relative thermodynamics of the side-chain rearranged species compared with regular oxazolone species were suggested to be different for Gln and Asn [48]. Moreover, one could imagine the kinetics of the reaction to be different, for instance due to the shorter alkyl chain in Asn possibly restricting the accessibility of the amide heteroatoms to the adjacent carbonyl carbon. We therefore investigate here the structure of the b2-ion from protonated AlaAsnAla to address the question whether the side chain of Asn becomes involved in the rearrangement reaction as observed for Gln or whether peptide dissociation proceeds via the more common oxazolone (or diketopiperazine) pathway.
In addition to the formation of oxazolone and diketopiperazine b2-structures analogous to those depicted in Scheme 1, the Asn side chain may become involved in the rearrangement reaction via one of the reactions outlined in Scheme 2, leading to two possible five-membered ring structures [16]. Nucleophilic attack by the side-chain amide nitrogen leads to the formation of a succinimide ring species, analogous to the glutarimide species observed for the Gln containing peptide. Alternatively, the b2-ion may adopt a structure containing a 5-imino-γ-butyrolactone ring, if the nucleophile is the side chain amide oxygen atom. Depending on the protonation site, diketopiperazine and succinimide structures are iso-energetic within a few kJ mol–1 (see Figure 5). Oxazolone structures are typically around 30 kJ mol–1 higher in free energy, while structures containing a 5-imino-γ-butyrolactone ring are more than 80 kJ mol–1 higher, in accordance with values reported previously [48]. Note that this energetic ordering is significantly different from that for the analogous PheGln b2-ion isomers in Figure 2.
Comparison of the IRMPD spectrum for the b2-ion of protonated AlaAsnAla (black trace in all panels) and DFT-calculated spectra (blue dashed line) for candidate structures: succinimide protonated at N-terminus (a) and (c), diketopiperazine protonated at carbonyl oxygen (b), oxazolone protonated on N-terminus (d), 5-imino-γ-butyrolactone protonated on imino-nitrogen (e), and 5-imino-γ-butyrolactone protonated on N-terminal nitrogen (f).The succinimide structures in (a) and (c) are isomerically identical but conformationally different, as shown by the ball-and-stick models. Calculated relative free energy values are indicated for each of the structures. Three-dimensional structures are available in the Supporting Information (Figure S4)
Upon CO2 laser induced multiple-photon dissociation, protonated AlaAsnAla was mainly observed to yield fragments at m/z 186 (b2), m/z 258 (loss of ammonia), and m/z 257 (loss of water). At longer irradiation times, the c1-ion becomes clearly observable, suggesting that the fragmentation pathways are (at least partly) analogous to those seen for the Gln containing peptide. However, direct formation of the c1-ion (i.e., not traversing the b2-ion, may also occur as suggested in references [49, 50]. An IRMPD-based MS3 experiment on the b2 fragment does not produce the a2-ion as would have been expected for oxazolone or diketopiperazine b2-ion structures, but instead produces mainly the a1 fragment ion (m/z 44). While the c1-ion is not as abundantly observed as for PheGln b2-ion, formation of the a1-ion is similar.
The experimental IRMPD spectrum of the AlaAsn b2 fragment ion is compared with theoretical spectra for six representative structural isomers in Figure 5. Further structures differing in protonation site or conformation not shown in Figure 5 have higher relative energies and spectra poorly matching experiment. The figure displays calculated spectra for the succinimide ring structure protonated at the N-terminus (two different conformers in panels a and c), the diketopiperazine ring protonated at one of the carbonyls (panel b), the oxazolone structure protonated at the N-terminus (panel d), the structure having a 5-imino-γ-butyrolactone “isoimide” ring protonated at the imino nitrogen (panel e) and the same butyrolactone structure protonated on the N-terminal amino group (panel f). Panel a shows in addition a scan taken at reduced laser power. 3-dimensional structures revealing the conformations are shown in the Supporting Information (Figure S4).
Again, the carbonyl stretching region between 1600 and 1900 cm–1 is very diagnostic. The trident peak shape observed in the experimental spectrum is only reproduced by the succinimide and butyrolactone structures. Diketopiperazine (panel b) and oxazolone (panel d) structures can be confidently excluded, since they utterly fail to match with the experiment in this diagnostic region of the spectrum. Protonation at the succinimide or imino-butyrolactone nitrogen atoms (e.g., panel e) can also be excluded based on these grounds, which is in line with the computed relative energies: protonation at the N-terminus is substantially favored over protonation at the imide or isoimide ring. Calculated spectra for both the succinimide (panels a and c) and 5-imino-γ-butyrolactone (panel f) structures, protonated at the N-terminus, show reasonable overlap in the 1600–1900 cm–1 spectral range. However, peak positions in the 800–1200 cm–1 spectral range match much better with the succinimide structure than with the imino-butyrolactone structure; combined with the much higher relative free energy and analogous to the PheGln b2-ion structure, we therefore discard the imino-butyrolactone isomer as possible structure for the b2-ion of AlaAsnAla.
The computed spectra for the two conformers (panels a and c) of the succinimide-based structure match the experimental spectrum reasonably well except for the region between 1200 and 1500 cm–1. A comparison between experimentally observed frequencies and those calculated for the conformer in panel c is given in Table 2. It is of interest to note here that the same spectral region was also poorly reproduced by the calculations for the glutarimide structure for the PheGln b2 fragment ion (vide supra). The largest deviation between experiment and theory is found for the NH3 umbrella mode calculated at 1398 cm–1 and tentatively assigned to the strong band observed at 1432 cm–1. Following the potential energy along this normal coordinate yields the curve plotted in Figure 6, exhibiting the anharmonic nature of the potential, which is likely also due to the strong hydrogen bond to the peptide carbonyl O-atom. Note that the C = O stretching mode of this peptide bond is also miscalculated substantially at 1695 cm–1 versus an observed frequency of 1725 cm–1.
Potential energy scan (solid black) following the normal mode corresponding to the 1432 cm–1 vibration in the succinimide structure of the b2-ion of protonated AlaAsnAla. The dotted line represents a perfect harmonic curve. The normal mode is seen to involve mainly NH bending of the strongly hydrogen-bonded H-atom
Despite the mismatch between experimental and DFT-calculated spectra in the 1200–1500 cm–1 range, we conclude that the succinimide isomer is the major contributor to spectrum observed for the AlaAsn b2 fragment ion. This suggests that the Asn side-chain nitrogen atom acts as the nucleophile on the reaction pathway towards this fragment, analogous to what is observed for the Gln residue. This observation appears somewhat in contrast with that of reference [48], where peptides with Gln and Asn in the second position were suggested to behave differently in the formation of the b2-ion.
3.4 b2-Ion of Protonated AsnAlaAla
It has been suggested that b n -ions of longer protonated peptides with Gln or Asn in the n-th position may give rise to structures similar to those observed for the b2-ions [48]. While such longer peptide fragments are beyond the scope of this investigation, it is of interest to investigate the structure of a b2-ion from a peptide with Gln or Asn in the first position. Will the side chain amide nitrogen continue to be the nucleophile leading to cyclic imide structures, or will the b2-ions adopt one of the more common motifs? MS3 experiments report b2-ions from peptides with Asn or Gln in the first position to yield predominantly the a2-ion [49], presumably formed by loss of carbon monoxide from a classical oxazolone b2-ion. Here we investigate the influence of an amide containing residue in the first position by recording the IRMPD spectrum for the b2-ion of protonated AsnAlaAla.
Upon CID, this peptide (m/z 275) was found to undergo dissociation mainly into m/z 257 (loss of water), m/z 258 (loss of ammonia), b2 (m/z 186), and a2 (m/z 158). No c- or z-type ions were observed in accordance with previous CID experiments [49], The b2-ion was mass isolated and underwent fragmentation into m/z 44 (NH3-CH = CH2 +) and the a2-ion at m/z 169. This breakdown pathway resembles that observed for the oxazolone AlaAla, AlaGly, and GlyGly b2 species.
The IRMPD spectrum of the b2-ion of protonated AsnAlaAla indeed confirms an oxazolone structure as shown in Figure 7. Along with the experimental spectrum, calculated spectra are shown for oxazolone structures protonated either at the N-terminal nitrogen or at the oxazolone nitrogen, which are computed to be close in energy (5 kJ mol–1). The diagnostic C = O stretching band of the oxazolone carbonyl at frequencies near 1900 cm–1 is prominently present and is seen to be split into two components. As seen from the comparison with the theoretical spectra, the splitting of the oxazolone band suggests the coexistence of both oxazolone structures. All bands in the spectrum are seen to be reproduced by the bands calculated for the two oxazolone structures, strongly suggesting that any contribution from alternative structures can be ruled out for this b2-ion. Nucleophilic attack from either the amide side chain oxygen or nitrogen atom would give rise to cyclic 8-membered imide or isoimide structures. Their computed spectra indeed do not match with the experimental spectrum, as shown in the Supporting Information (Figure S5).
Observation of the N-terminally protonated oxazolone structure is somewhat in contrast with the AlaAla [37], AlaGly [36], and GlyGly [21] oxazolone b2-ion structures, which were spectroscopically found to be protonated exclusively on the oxazolone nitrogen atom. On the other hand, coexistence of both protonation structures was also observed for the b2-ion from protonated Leu-enkephaline [22, 34, 35], which was explained by additional stabilization of the higher-energy N-terminally protonated isomer by interactions with the Tyr π-cloud. For the AsnAla b2-ion studied here, additional stabilization of the N-terminally protonated structure is provided by hydrogen bonding to the amide oxygen of the Asn residue.
4 Conclusions
Infrared ion spectroscopy was applied in combination with electronic structure calculations to resolve the molecular structure of b2 peptide fragment ions containing a Gln or Asn residue. Previous MSn based studies on peptides with Gln or Asn as the second residue had suggested that the amide side chain becomes involved in the dissociation reaction and that the b2-ion forms a cyclic isoimide structure via nucleophilic attack by the side chain amide oxygen atom [47–49]. Our spectroscopic results presented herein show, however, that the ring formation occurs through the side chain amide nitrogen instead, forming b2 fragments with glutarimide and succinimide structures for Gln and Asn, respectively. Although the match between experimental and computed harmonic IR spectra shows some deviations in the 1200–1500 cm–1 range, an experimental IR spectrum of a synthesized glutarimide reference compound agrees perfectly with that of the PheGln b2-ion. The cyclic imide structures resulting from amide nitrogen nucleophilic attack are substantially lower in energy than the isomeric isoimide structures. Further computational investigation of the potential energy surface of the reaction are required to resolve the mechanistic details of the reaction, but is well beyond the scope of the present study. The structures determined here are expected to provide important guides in finding the correct trajectories.
Compared with the previously suggested isoimide structures, formation of imide b2-ion structures is evenly consistent with available MS3 data, in particular with the formation of a c1-ion upon dissociation of the b2-ion. In addition, IRMPD spectroscopy was applied here to confirm that the dissociation product indeed has the structure of a c1-ion, with a C-terminal amide moiety and protonation at the N-terminus.
The protonation site of the PheGlnAla precursor peptide is determined to be the N-terminal amino group rather than the side chain amide moiety. The fact that no diketopiperazine structure is found here for the b2-ion lends further support to the dissociation mechanism recently proposed for peptides with His, Arg, and Lys residues [42–44].
IR spectroscopy of the b2-ion of protonated AsnAlaAla confirms that side-chain involvement in the dissociation reaction can be clearly ruled out in this case and that this Asn-containing fragment possesses purely an oxazolone structure. This conclusion was also inferred from MS3 data [49], although no distinction between oxazolone and diketopiperazine structures could be made. Detailed inspection of the IR spectrum recorded here reveals that structures protonated both at the oxazolone nitrogen as well as at the N-terminus coexist.
Notes
In reference 16, the text and the values in Table 3 are ambiguous about the energetic ordering of the structures resulting from side-chain nitrogen and side-chain oxygen attack, referred to as Gln G1 and Gln G2, respectively.
Basicity and nucleophilicity are of course different scales, but usually run parallel.
References
Aebersold, R., Goodlett, D.R.: Mass spectrometry in proteomics. Chem Rev 101, 269–295 (2001)
Steen, H., Mann, M.: The ABC's (and XYZ's) of peptide sequencing. Nat Rev Mol Cell Biol 5, 699–711 (2004)
Burlet, O., Orkiszewski, R.S., Ballard, K.D., Gaskell, S.: Charge promotion of low-energy fragmentations of peptide ions. Rapid Commun Mass Spectrom 6, 658–662 (1992)
Wysocki, V.H., Tsaprailis, G., Smith, L.L., Breci, L.A.: Mobile and localized protons: a framework for understanding peptide dissociation. J Mass Spectrom 35, 1399 (2000)
Zubarev, R.A., Kelleher, N.L., McLafferty, F.W.: Electron capture dissociation of multiply charged protein cations. A nonergodic process. J Am Chem Soc 120, 3265–3266 (1998)
Paizs, B., Suhai, S.: Fragmentation pathways of protonated peptides. Mass Spectrom Rev 24, 508–548 (2004)
Turecek, F.: N–Ca bond dissociation energies and kinetics in amide and peptide radicals. Is the dissociation a non-ergodic process? J Am Chem Soc 125, 5954–5963 (2003)
Syrstad, E.A., Turecek, F.: Toward a general mechanism of electron capture dissociation. J Am Soc Mass Spectrom 16, 208–224 (2004)
Barlow, C.K., O'Hair, R.A.J.: Gas-phase peptide fragmentation: how understanding the fundamentals provides a springboard to developing new chemistry and novel proteomics tools. J Mass Spectrom 43, 1301–1319 (2008)
Harrison, A.G.: To b or not to b: the ongoing saga of peptide b ions. Mass Spectrom Rev 28, 640–654 (2009)
Bythell, B.J., Maitre, P., Paizs, B.: Cyclization and rearrangement reactions of a n fragment ions of protonated peptides. J Am Chem Soc 132, 14766–14779 (2010)
Polce, M.J., Ren, D., Wesdemiotis, C.: Dissociation of the peptide bond in protonated peptides. J Mass Spectrom 35, 1391–1398 (2000)
Boyd, R., Somogyi, A.: The mobile proton hypothesis in fragmentation of protonated peptides: a perspective. J Am Soc Mass Spectrom 21, 1275–1278 (2010)
Yalcin, T., Khouw, C., Csizmadia, I.G., Peterson, M.R., Harrison, A.G.: Why are b ions stable species in peptide spectra? J Am Soc Mass Spectrom 6, 1164–1174 (1995)
Paizs, B., Lendvay, G., Vekey, K., Suhai, S.: Formation of b2 + ions from protonated peptides: an ab initio study. Rapid Commun Mass Spectrom 13, 525–533 (1999)
Farrugia, J.M., O'Hair, R.A.J., Reid, G.E.: Do all b2 ions have oxazolone structures? Multistage mass spectrometry and ab initio studies on protonated N-acyl amino acid methyl ester model systems. Int J Mass Spectrom 210/211, 71–87 (2001)
Bleiholder, C., Osburn, S., Williams, T.D., Suhai, S., Van Stipdonk, M., Harrison, A.G., Paizs, B.: Sequence-scrambling fragmentation pathways of protonated peptides. J Am Chem Soc 130, 17774–17789 (2008)
Harrison, A.G.: Peptide Sequence scrambling through cyclization of b5 ions. J Am Soc Mass Spectrom 19, 1776–1780 (2008)
Molesworth, S., Osburn, S., Van Stipdonk, M.: Influence of size on apparent scrambling of sequence during CID of b-type ions. J Am Soc Mass Spectrom 20, 2174–2181 (2009)
Molesworth, S., Osburn, S., Van Stipdonk, M.: Influence of amino acid side chains on apparent selective opening of cyclic b5 ions. J Am Soc Mass Spectrom 21, 1028–1036 (2010)
Chen, X., Yu, L., Steill, J.D., Oomens, J., Polfer, N.C.: Effect of peptide fragment size on the propensity of cyclization in collision-induced dissociation: oligoglycine b2−b8. J Am Chem Soc 131, 18272–18282 (2009)
Chen, X., Steill, J.D., Oomens, J., Polfer, N.C.: Oxazolone versus macrocycle structures for Leu-enkephalin b2–b4: insights from infrared multiple-photon dissociation spectroscopy and gas-phase hydrogen/deuterium exchange. J Am Soc Mass Spectrom 21, 1313–1321 (2010)
Li, X., Huang, Y., O'Connor, P.B., Lin, C.: Structural heterogeneity of doubly-charged peptide b-ions. J Am Soc Mass Spectrom 22, 245–254 (2011)
Erlekam, U., Bythell, B.J., Scuderi, D., Van Stipdonk, M., Paizs, B., Maitre, P.: Infrared spectroscopy of fragments of protonated peptides: direct evidence for macrocyclic structures of b5 ions. J Am Chem Soc 131, 11503–11508 (2009)
Tirado, M., Rutters, J., Chen, X., Yeung, A., van Maarseveen, J., Eyler, J.R., Berden, G., Oomens, J., Polfer, N.C.: Disfavoring macrocycle b fragments by constraining torsional freedom: the “twisted” case of QWFGLM b6. J Am Soc Mass Spectrom 23, 475–482 (2012)
Chen, X., Tirado, M., Steill, J.D., Oomens, J., Polfer, N.C.: Cyclic peptide as reference system for b ion structural analysis in the gas phase. J Mass Spectrom 46, 1011–1015 (2011)
Savitski, M.M., Falth, M., Fung, Y.M.E., Adams, C.M., Zubarev, R.A.: Bifurcating fragmentation behavior of gas-phase tryptic peptide dications in collisional activation. J Am Soc Mass Spectrom 19, 1755–1763 (2008)
Paizs, B., Suhai, S.: Combined quantum chemical and RRKM modeling of the main fragmentation pathways of protonated GGG. I. Cis-trans isomerization around protonated amide bonds. Rapid Commun Mass Spectrom 15, 2307–2323 (2001)
Balta, B., Aviyente, V., Lifshitz, C.: Elimination of water from the carboxyl group of GlyGlyH+. J Am Soc Mass Spectrom 14, 1192–1203 (2003)
Armentrout, P.B., Heaton, A.L.: Thermodynamics and mechanisms of Protonated diglycine decomposition: a computational study. J Am Soc Mass Spectrom 23, 621–631 (2012)
Oomens, J., Sartakov, B.G., Meijer, G., von Helden, G.: Gas-phase infrared multiple photon dissociation spectroscopy of mass-selected molecular ions. Int J Mass Spectrom 254, 1–19 (2006)
Eyler, J.R.: Infrared multiple photon dissociation spectroscopy of ions in Penning traps. Mass Spectrom Rev 28, 448–467 (2009)
Polfer, N.C.: Infrared multiple photon dissociation spectroscopy of trapped ions. Chem Soc Rev 40, 2211–2221 (2011)
Polfer, N.C., Oomens, J., Suhai, S., Paizs, B.: Spectroscopic and theoretical evidence for oxazolone ring formation in collision-induced dissociation of peptides. J Am Chem Soc 127, 17154–17155 (2005)
Polfer, N.C., Oomens, J., Suhai, S., Paizs, B.: Infrared spectroscopy and theoretical studies on gas-phase protonated Leu-enkephalin and its fragments: Direct experimental evidence for the mobile proton. J Am Chem Soc 129, 5887–5897 (2007)
Yoon, S.H., Chamot-Rooke, J., Perkins, B.R., Hilderbrand, A.E., Poutsma, J.C., Wysocki, V.H.: IRMPD spectroscopy shows that AGG forms an oxazolone b2 + ion. J Am Chem Soc 130, 17644–17645 (2008)
Oomens, J., Young, S., Molesworth, S., van Stipdonk, M.: Spectroscopic evidence for an oxazolone structure of the b2 fragment ion from protonated trialanine. J Am Soc Mass Spectrom 20, 334–339 (2009)
Wang, D., Gulyuz, K., Stedwell, C.N., Polfer, N.C.: Diagnostic NH and OH vibrations for oxazolone and diketopiperazine structures: b2 from protonated triglycine. J Am Soc Mass Spectrom 22, 1197–1203 (2011)
Bythell, B.J., Erlekam, U., Paizs, B., Maitre, P.: Infrared spectroscopy of fragments from doubly protonated tryptic peptides. Chem Phys Chem 10, 883–885 (2009)
Sinha, R.K., Erlekam, U., Bythell, B.J., Paizs, B., Maitre, P.: Diagnosing the protonation site of b2 peptide fragment ions using IRMPD in the X-H (X = O, N, and C) Stretching Region. J Am Soc Mass Spectrom 22, 1645–1650 (2011)
Morrison, L., Somogyi, A., Wysocki, V.H.: The influence of glutamic acid in protonated b3 –> b2 formation from VGEIG and related analogs. Int J Mass Spectrom 325/327, 139–149 (2012)
Perkins, B.R., Chamot-Rooke, J., Yoon, S.H., Gucinski, A.C., Somogyi, A., Wysocki, V.H.: Evidence of diketopiperazine and oxazolone structures for HA b2 + ion. J Am Chem Soc 131, 17528–17529 (2009)
Zou, S., Oomens, J., Polfer, N.C.: Competition between diketopiperazine and oxazolone formation in water loss products from protonated ArgGly and GlyArg. Int J Mass Spectrom 316/318, 12–17 (2012)
Gucinski, A.C., Chamot-Rooke, J., Nicol, E., Somogyi, A., Wysocki, V.H.: Structural influences on preferential oxazolone versus diketopiperazine b2 + ion formation for histidine analogue-containing peptides. J Phys Chem A 116, 4296–4304 (2012)
O'Hair, R.A.J.: The role of nucleophile–electrophile interactions in the unimolecular and bimolecular gas-phase ion chemistry of peptides and related systems. J Mass Spectrom 35, 1377–1381 (2000)
Jonsson, A.P., Bergman, T., Jörnvall, H., Griffiths, W.J., Bratt, P., Strömberg, N.: Gln-Gly cleavage: correlation between collision-induced dissociation and biological degradation. J Am Soc Mass Spectrom 12, 337–342 (2001)
Harrison, A.G.: Fragmentation reactions of protonated peptides containing glutamine or glutamic acid. J Mass Spectrom 38, 174–187 (2003)
Lee, Y.J., Lee, Y.M.: Formation of c1 fragment ions in collision-induced dissociation of glutamine-containing peptide ions: a tip for de novo sequencing. Rapid Commun Mass Spectrom 18, 2069–2076 (2004)
Winter, D., Seidler, J., Hahn, B., Lehmann, W.: Structural and mechanistic information on c1 ion formation in collision-induced fragmentation of peptides. J Am Soc Mass Spectrom 21, 1814–1820 (2010)
Winter, D., Lehmann, W.D.: Sequencing of the 13 structurally isomeric quartets of N-terminal dipeptide motifs in peptides by collision-induced dissociation. Proteomics 9, 2076–2084 (2009)
Oepts, D., van der Meer, A.F.G., van Amersfoort, P.W.: The infrared free-electron laser facility FELIX. Infrared Phys Technol 36, 297–308 (1995)
Valle, J.J., Eyler, J.R., Oomens, J., Moore, D.T., van der Meer, A.F.G., von Helden, G., Meijer, G., Hendrickson, C.L., Marshall, A.G., Blakney, G.T.: Free electron laser-Fourier transform ion cyclotron resonance mass spectrometry facility for obtaining infrared multiphoton dissociation spectra of gaseous ions. Rev Sci Instrum 76, 023103 (2005)
Fox, D.J., Reckless, J., Warren, S.G., Grainger, D.J.: Design, synthesis, and preliminary pharmacological evaluation of N-acyl-3-aminoglutarimides as broad-spectrum chemokine inhibitors in vitro and anti-inflammatory agents in vivo. J Med Chem 45, 360–370 (2002)
Polfer, N.C., Oomens, J.: Reaction products in mass spectrometry elucidated with infrared spectroscopy. Phys Chem Chem Phys 9, 3804–3817 (2007)
Marshall, A.G., Wang, T.C.L., Ricca, T.L.: Tailored excitation for Fourier transform ion cyclotron mass spectrometry. J Am Chem Soc 107, 7893–7897 (1985)
Mize, T.H., Taban, I., Duursma, M., Seynen, M., Konijnenburg, M., Vijftigschild, A., Doornik, C.V., Rooij, G.V., Heeren, R.M.A.: A modular data and control system to improve sensitivity, selectivity, speed of analysis, ease of use, and transient duration in an external source FTICR-MS. Int J Mass Spectrom 235, 243–253 (2004)
Polfer, N.C., Oomens, J.: Vibrational spectroscopy of bare and solvated ionic complexes of biological relevance. Mass Spectrom Rev 28, 468–494 (2009)
Rodriguez, C.F., Cunje, A., Shoeib, T., Chu, I.K., Hopkinson, A.C., Siu, K.W.M.: Proton migration and tautomerism in protonated triglycine. J Am Chem Soc 123, 3006–3012 (2001)
Wu, R., McMahon, T.B.: Infrared multiple photon dissociation spectroscopy as structural confirmation for GlyGlyGlyH+ and AlaAlaAlaH+ in the gas phase. Evidence for Amide oxygen as the protonation site. J Am Chem Soc 129, 11312–11313 (2007)
Sinclair, W.E., Pratt, D.W.: Structure and vibrational dynamics of aniline and aniline–Ar from high resolution electronic spectroscopy in the gas phase. J Chem Phys 105, 7942–7956 (1996)
Piest, H., von Helden, G., Meijer, G.: Infrared spectroscopy of jet-cooled neutral and ionized aniline–Ar. J Chem Phys 110, 2010–2015 (1999)
Luis, J.M., Reis, H., Papadopoulos, M., Kirtman, B.: Treatment of nonlinear optical properties due to large amplitude anharmonic vibrational motions: umbrella motion in NH3. J Chem Phys 131, 034116 (2009)
Zubarev, R.A., Horn, D.M., Fridriksson, E.K., Kelleher, N.L., Kruger, N.A., Lewis, M.A., Carpenter, B.K., McLafferty, F.W.: Electron capture dissociation for structural characterization of multiply charged protein cations. Anal Chem 72, 563–573 (2000)
Cooper, H.J., Hakansson, K., Marshall, A.G.: The role of electron capture dissociation in biomolecular analysis. Mass Spectrom Rev 24, 201–222 (2005)
Zubarev, R.A.: Reactions of polypeptide ions with electrons in the gas phase. Mass Spectrom Rev 22, 57–77 (2003)
Coon, J.J., Shabanowitz, J., Hunt, D.F., Syka, J.E.P.: Electron transfer dissociation of peptide anions. J Am Soc Mass Spectrom 16, 880–882 (2005)
Frison, G., van der Rest, G., Turecek, F., Besson, T., Lemaire, J., Maitre, P., Chamot-Rooke, J.: Structure of electron-capture dissociation fragments from charge-tagged peptides probed by tunable infrared multiple photon dissociation. J Am Chem Soc 130, 14916–14917 (2008)
Skurski, P., Sobczyk, M., Jakowski, A., Simons, J.: Possible mechanisms for protecting N–Ca bonds in helical peptides from electron-capture (or transfer) dissociation. Int J Mass Spectrom 265, 197–212 (2007)
Brodbelt, J.S., Wysocki, V.H., Cooks, R.G.: Thermochemical versus kinetic control of reactions in an ion trap mass spectrometer. Org Mass Spectrom 23, 54–56 (1988)
Tsaprailis, G., Nair, H., Somogyi, A., Wysocki, V.H., Zhong, W., Futrell, J.H., Summerfield, S.G., Gaskell, S.J.: Influence of secondary structure on the fragmentation of protonated peptides. J Am Chem Soc 121, 5142–5154 (1999)
Acknowledgments
The authors gratefully acknowledge Dr. Jan van Maarseveen from the University of Amsterdam for synthesizing the glutarimide model compound. They thank the FELIX staff, in particular Drs. A. F. G. van der Meer, B. Redlich, and G. Berden, for their expert technical support. J.O. acknowledges the Stichting Physica for support. Financial support for this project was provided by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) in the form of VICI grant no. 724.011.002. The authors also thank the SARA Supercomputer Center for providing the computational resources. This work is part of the research program of FOM, which is financially supported by NWO.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Proposed mechanisms for dissociation of protonated PheGlnAla are presented in Scheme S1 and Scheme S2. Three-dimensional ball-and-stick structures as well as atomic coordinates for the ions considered in Figures 1, 2, 3, and 5. Calculated IR spectra for alternative isomers of the b2-ion from protonated AsnAlaAla. (PDF 845 kb)
Rights and permissions
About this article
Cite this article
Grzetic, J., Oomens, J. Spectroscopic Identification of Cyclic Imide b2-Ions from Peptides Containing Gln and Asn Residues. J. Am. Soc. Mass Spectrom. 24, 1228–1241 (2013). https://doi.org/10.1007/s13361-013-0661-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0661-6