Abstract
Large polarizable n-alkanes (approximately C18 and larger), alcohols, and other nonpolar compounds can be detected as negative ions when sample solutions are injected directly into the sampling orifice of the atmospheric pressure interface of the time-of-flight mass spectrometer with the direct analysis in real time (DART) ion source operating in negative-ion mode. The mass spectra are dominated by peaks corresponding to [M + O2]‾•. No fragmentation is observed, making this a very soft ionization technique for samples that are otherwise difficult to analyze by DART. Detection limits for cholesterol were determined to be in the low nanogram range.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Alkanes are typically analyzed by gas chromatography (GC) combined with electron ionization (EI) mass spectrometry or by field ionization (FI) or field desorption (FD) mass spectrometry. Although chemical ionization (CI) has been applied to the analysis of alkanes, it is not widely used because of the complexity of the resulting CI mass spectra or the difficulties in generating suitable reagent ions [1]. However, chemical ionization combined with laser-induced acoustic desorption has been shown to be effective for the analysis of alkanes [2].
Direct analysis in real time (DART) mass spectrometry [3–8] is not often used for the analysis of alkanes. Under normal DART conditions, oxidation can occur because of reactions with background NO+ [6], although the complexity of the mass spectra limits the analytical utility of this method. Similar discharge-induced oxidation is also observed for the analysis of saturated hydrocarbons by desorption electrospray ionization (DESI) [9]. Yang and Attygalle used a helium plasma ionization source to detect saturated hydrocarbons by hydride abstraction as [M – H]+ [10].
Molecular ions M+• can be observed with DART in positive-ion mode if atmospheric moisture is reduced [6]. Ionization is proposed to occur by direct Penning ionization or by charge exchange with O2 +•. Although Cl– attachment has been reported for atmospheric pressure chemical ionization (APCI) of chlorinated paraffins [11], a chemical ionization scheme for forming negative ions from unsubstituted alkanes has not been previously reported. The electron affinities of unsubstituted saturated alkanes have negative values [12] and so alkanes do not undergo electron capture under typical chemical ionization conditions. Thermochemical data for anion attachment to saturated alkanes are not available, with the exception of methane, which has an enthalpy of reaction for Cl– attachment listed as 15.9 kJ mol–1 [13]. However, values for anion attachment for aromatic hydrocarbons are listed in the database. Benzene and naphthalene have enthalpies of reaction for O2 –• attachment listed as 59.0 ± 9.6 kJ mol–1 and 92.50 ± 9.6 kJ mol–1 and electron affinities listed as 1.06 ± 0.10 and 1.41 ± 0.10 eV, respectively. The free energies of reaction of formation for Cl– adducts of benzene, toluene, and ethylbenzene are listed as 15.9 kJ mol–1, 16.7 kJ mol–1 , and 20.9 kJ mol–1, respectively.
Alcohols, including cholesterol, are also problematic for DART analysis. Protonation usually occurs for cholesterol with dehydration to produce [M + H – H2O]+. Molecular ions can be observed for cholesterol with DART under the same conditions that favor molecular ion formation for alkanes, but it is difficult to avoid dehydration [6]. While small alcohols such as methanol, ethanol, and 2-propanol can be analyzed with DART, larger alcohols generally require derivatization [8, 14, 15]. With the exception of aromatic alcohols and oligosaccharides that can be observed as [M – H]–, alcohols do not generally produce negative ions by negative-ion DART when samples are introduced directly into the DART gas stream. However, we observe O2 –• adducts for alcohols as small as methanol. The enthalpy of reaction for O2 –• attachment to methanol can be found in the literature with a value of 79.9 kJ mol–1 [16].
We report here the observation of nondissociative anion attachment to saturated alkanes and alcohols by DART. To our knowledge, this is the first report of negative ion formation for large unsubstituted saturated alkanes. The method is also applicable to other nonpolar compounds.
2 Experimental
The DART ion source is an atmospheric pressure chemical ionization source where the initial ionization step results from interactions between excited-state neutral species and the analyte or atmospheric gases. The DART source has been previously described in the literature [3, 7, 8], and we will not offer a detailed description here.
All mass spectra were acquired with a JEOL (Tokyo, Japan) AccuTOF-DART time-of-flight (TOF) mass spectrometer equipped with an IonSense (Saugus, MA, USA) DART-SVP ion source. The AccuTOF mass spectrometer has an atmospheric pressure ionization (API) interface consisting of two off-axis skimmers, a ring lens, and a bent rf ion guide [17]. The outer skimmer has a 400 μm i.d. sampling orifice (orifice 1) through which ions are introduced from atmospheric pressure into the first pumping region. The off-axis skimmer design coupled with a bent rf ion guide is highly resistant to contamination when liquid solutions are introduced directly into orifice 1.
The mass spectrometer was operated in negative-ion mode with an rf ion guide voltage (referred to as “Peaks Voltage” in the software) of 800 V. Alkane mass spectra were acquired for the mass range 80–800 with a spectral storage rate of one spectrum per s. To examine the low-mass background ions, the rf ion guide voltage was set to 100 V, the mass spectra were acquired for the mass range 10–300, and summed mass spectra were stored at a rate of 1 spectrum s–1.
The mass spectrometer resolving power was 6000 (FWHM). Polyethylene glycol (average molecular weight 600) and/or perfluorotributylamine were used as external mass reference standards for exact mass determination. The API interface potentials were set to relatively low values (orifice 1 = –10 V, ring lens = orifice 2 = –3 V) to minimize in-source collision-induced dissociation of the weakly bound oxygen adducts of the alkanes. The orifice 1 temperature was 120 °C, the standard value used for DART experiments in our laboratory.
JEOL Mass Center software was used to control the mass spectrometer and acquire data, which were subsequently calibrated and processed by using TSSPro3 software (Shrader Analytical, Detroit, MI, USA). Elemental composition assignments given in this article were supported by accurate mass measurements and isotope matching. Mass Spec Tools software (ChemSW Inc., Fairfield, CA, USA) was used to examine and interpret the mass spectra.
Alkanes and alcohols were commercially available samples selected from our chemical storeroom. Hexachlorodecane samples were provided by Cambridge Isotope Labs. The olive oil sample was purchased at a local grocery store and diesel fuel was purchased from a local gas station.
The DART-SVP ion source was operated with an exit grid voltage of –530 V to maximize the relative abundance of O2 –• in the negative-ion background mass spectrum. Helium was used as the DART gas at the factory-preset flow rate for the DART-SVP. Unless otherwise noted, the gas heater was set to 350 °C. The DART exit grid was aimed directly at the mass spectrometer sampling orifice positioned at 4.2 cm on the DART SVP railing guide. This corresponds to a distance of approximately 1.5 cm from the mass spectrometer sampling orifice. The IonSense Vapur gas transfer interface was not installed.
Initially, the sealed end of a 2 mm o.d. glass melting point tube was used to sample solid or liquid alkane standards. The melting point tube was then placed in the DART gas stream for analysis. Although negative ions could be observed with the melting point tube positioned anywhere in the DART gas stream, the most abundant and longest-lasting adduct signals were observed if the melting point tube was touched briefly to the mass spectrometer heated sampling orifice. This led us to try injecting sample solutions directly into the sampling orifice (Orifice 1). A 1–5 μL calibrated microcapillary PCR pipette (Drummond Scientific Company, Broomall, PA, USA) was used to inject 1–3 μL of solutions containing alkanes and cholesterol in hexane or isooctane directly into the orifice while the DART was operated in negative-ion mode. After loading, samples were introduced by placing the capillary tip directly against the mass spectrometer sampling orifice. The sample was aspirated directly into the instrument by the vacuum from the mass spectrometer API interface.
Strong signals were reproducibly observed for the anion adducts when sample solutions were injected directly. No signals were detected if the DART glow discharge was shut off, confirming the role of the DART source in producing the O2 –• reagent ions and eliminating sonic spray [18] and inlet ionization [19] as explanations for the ionization mechanism.
The optimum temperature for the DART gas and orifice 1 temperature was dependent on analyte volatility. Triacylglycerols (TAGs) in vegetable and olive oils were not detected as O2 –• adducts when injected directly into the API interface even when the orifice 1 and DART gas heater temperatures were set to their maximum values. However, O2 –• adducts were formed when the oils were deposited onto the sealed end of melting point tubes and positioned directly in front of the DART exit with the DART gas heater set to 400 °C. The relatively higher stability of TAG O2 –• adducts may be attributable to their higher polarity compared with saturated alkanes and smaller alcohols.
For the chloride attachment experiment, methylene chloride dopant was added to the DART gas stream at a rate of 1 μL min–1 by using a syringe pump and deactivated fused silica tubing. The tip of the fused silica tubing was placed directly in front of the DART exit.
3 Results and Discussion
The DART negative-ion low-mass background measured under the typical operating conditions described in the experimental section is shown in Figure 1. The most abundant species is O2 –•, although lesser amounts of other species are observed. The formation of O2 –• in DART is attributed to the capture of Penning electrons by atmospheric oxygen [3].
Figure 2a and b show the negative-ion DART mass spectra of hexane solutions of hexatriacontane (n-C36H74) and hexacosane (n-C26H54). The mass spectra show abundant peaks at m/z 398.4133 and 538.5688 respectively, assigned as [M + O2]–•. These measured m/z values are consistent with the calculated m/z values for C26H34O2 –•and C36H75O2 –• with errors of 0.9 and 0.1 mmu, respectively. No significant fragmentation is observed. A sample of paraffin film (Parafilm; Pechiney Plastic Packaging Company, Chicago, IL, USA) touched to orifice 1 showed a distribution of peaks corresponding to [M + O2]–• for alkanes ranging from C28 to C37 (Figure 2c).
When we applied this method to the analysis of hydrocarbons from insect puparial cases (Moore et al., in preparation), we observed a strong peak at m/z 418.3447 that we attributed to cholesterol. Examination of cholesterol standards confirmed that cholesterol does form O2 –• adducts under these conditions. We then tested this method for other alcohols including methanol, 1-butanol, 2-methyl-2-butanol, 1-hexanol, menthol, and cedrol. Alcohols with more than three or four carbons do not produce easily interpretable mass spectra under positive-ion DART conditions [20], although menthol does produce [M – H]+. All six alcohols showed simple negative-ion mass spectra consisting solely of [M + O2]–• when introduced directly into the mass spectrometer sampling orifice as hexane solutions. The negative-ion DART mass spectra for 2-methanol-2-butanol, cedrol, and cholesterol are shown in Figure 3.
Sensitivity was expected to be poor for this method because of the expected instability of the O2 –• adducts. Successive 10-fold dilutions of stock solutions containing 1 mg/mL each of hexacosane (C26H54), cholesterol, and hexatriacontane (C36H74) in hexane were measured to determine the detection limits and linearity of this method. With a DART gas heater temperature of 300 °C, hexacosane and cholesterol could be detected for a 1 μL injection of the 1 ng/μL dilution. Hexatriacontane signals did not appear unless the gas temperature was increased to 350 °C or higher. Injecting several microliters improved the signal-to-noise ratio for a given concentration. The signal rose rapidly and tailed for several seconds, analogous to a typical flow injection. To compensate for variations in individual injections, three replicate injections were summed for each concentration to determine linearity. Supplementary Figure S1 shows the mass spectra for a 1 ng/μL solution of cholesterol in hexane and a solvent blank. Supplementary Figure S2 shows a working curve for cholesterol measured over the concentration range 1–100 ng/μL.
1,5,5,6,6,10-Hexachlorodecane was also analyzed by this method. The base peak was [M + O2]–•, but additional adducts were observed as shown in Figure 4, corresponding to adducts with some of the other background ions described previously (see Figure 1). The presence of chlorine appears to influence the formation of O2 –• adducts. Some other chlorinated compounds we have studied, including 1,2,3,4,5,6-hexachlorocyclohexane (“lindane”) and 4,4′-dichlorodiphenyltrichloroethane (“DDT”), produce Cl– adducts instead of O2 –• adducts. These compounds fragment upon DART ionization to produce Cl–, which then attaches to other analyte molecules. Electron capture may play a role in the formation of the Cl– fragment.
Esters can also undergo O2 –• attachment. A mass spectrum of blended diesel and biodiesel analyzed by this method showed abundant O2 –• adducts for fatty acid methyl esters (FAMES) and a series of alkane peaks with carbon numbers ranging from 19 to 35 (Supplementary Figure S3).
Mono-, di-, and triacylglycerols were detected as O2 –• adducts in the negative-ion DART mass spectrum of an olive oil sample (Figure 5a), whereas free fatty acids were detected as deprotonated molecules ([M – H]–). For comparison, the positive-ion DART mass spectrum for the same sample measured with ammonium hydroxide headspace vapor present (Figure 5b) shows a more complex pattern. Ammonium adducts for triacylglycerols are more abundant and undergo less fragmentation than the corresponding protonated molecules. Mono- and diacylglycerols and phytosterols undergo dehydration to form [M + H – H2O]+. Triacylglycerols form both [M + H]+ and [M + NH4]+, and fatty acids are not detected. Additional peaks are observed for squalene and other trace components.
In contrast to the behavior of small alcohols, Cl– and O2 –• adducts were not observed for amines such as 1-butylamine and di-N-butylamine. For analytical purposes, these compounds can be detected as protonated molecules in positive-ion mode.
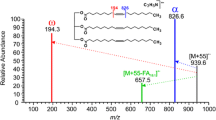
Adduct formation can be directed by adding dopants to the DART gas stream. By adding dichloromethane to the DART gas stream, chloride adducts were observed for hexatriacontane, hexacosane, and paraffin film. Supplementary Figure S4 shows the negative-ion DART mass spectrum of hexatriacontane (C36H74) with the addition of dichloromethane. The base peak is [M + Cl]– at m/z 541.5482. Additional cluster ions [M + Cl + On]– showing the addition of one chloride and up to five oxygens with H2 loss resulted in peaks assigned as [M + Cl + O3 – H2]– and [M + Cl + O5 – H2]–. The origin of these species is not clearly understood, but their formation may be the result of reactions involving radicals in the DART gas stream [3, 21, 22].
The mechanisms responsible for forming O2 –• adducts from alkanes are not clear. However, it is reasonable to postulate that the high polarizability of large alkanes results in longer life times of collision complexes of such molecules, which enables more efficient collisional cooling of the vibrationally hot adducts. The polarizability of n-alkanes increases linearly with size. The polarizabilities of CH4, C2H6, C3H8, n-C4H10, n-C6H14, and n-C7H16 are 2.60, 4,47, 6.29, 8.20, 11.76, and 13.61 Å3, respectively [23]. The predicted polarizability of n-hexacosane according to the method of Miller and Savchik [24] is 48.76 Å3. Collisional cooling in the atmospheric pressure ion source is hypothesized to assist in stabilizing adduct formation. This is not expected to be an efficient process. Further support for this hypothesis is found in the observation that peaks corresponding to abundant [M + O2]–• with minimal fragmentation are only observed for relatively large alkanes. Because we have not observed O2 –• adducts for alkanes smaller than approximately C18, the threshold polarizability may be approximately that of C18H38 or 33.99 Å3 [24].
The polarizability of methanol is only 3.28, yet methanol forms a clear O2 –• adduct. Therefore, a mechanism based on polarizability cannot be applied to the behavior of alcohols. Hydrogen bonding may be a more suitable explanation.
Increasing the orifice 1 potential (also referred to as the “cone voltage”) beyond approximately 20 V resulted in loss of signal. This is attributed to collisional activation of the weakly bound complex resulting in the loss of O2 –•. It was not practical to confirm this by observing an increase in the abundance of O2 –• upon increasing the orifice 1 potential because of the very high relative abundance of O2 –• in the DART negative-ion background.
Although O2 –• adducts were not observed for alkanes with less than approximately 18 carbons, very weak peaks corresponding to [M + O4]–• were seen for the solvents hexane (Supplementary Figure S3) and 2,2,4-trimethylpentane as well as for a hexane solution of an alkylbenzene (sec-butylbenzene). A possible explanation is that reactions with hydroperoxide radicals in the DART gas stream [3, 21, 22] result in oxidation of the smaller alkanes, followed by O2 –• attachment.
4 Conclusion
Ions attributed to anion attachment produced peaks in the negative-ion DART mass spectra of saturated hydrocarbons, alcohols, fatty acids, and esters. Alkanes larger than approximately C18 showed predominantly peaks assigned as [M + O2]–•. The mechanisms responsible for ion formation are not understood, although it is hypothesized that anion attachment is favored for large alkanes because of their relatively high polarizability.
The limitations of the method are its relatively poor sensitivity and the absence of O2 –• adducts for saturated hydrocarbons with less than approximately 18 carbons. However, this does provide a convenient method for detecting alcohols and large alkanes by DART and increases the range of compounds that can be analyzed. An application of this technique to insect speciation will be described in a subsequent publication [20].
References
Harrison, 1.: Chemical Ionization Mass Spectrometry, 2nd edn. CRC Press, Boca Raton, FL (1992)
Campbell, J.L., Crawford, K.E., Kenttämaa, H.I.: Analysis of saturated hydrocarbons by using chemical ionization combined with laser-induced acoustic desorption/Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 76(4), 959–963 (2004)
Cody, R.B., Laramee, J.A., Durst, H.D.: Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 77(8), 2297–2302 (2005)
Song, L.; Gibson, S. C.; Bhandari, D.; Cook, K. D.; Bartmess, J. E.: Ionization mechanism of positive-ion direct analysis in real time: a transient microenvironment concept. Anal. Chem. 81(24), 10080–10088 (2009)
Song, L.; Dykstra, A. B.; Yao, H.; Bartmess, J. E.: Ionization mechanism of negative ion-direct analysis in real time: a comparative study with negative ion-atmospheric pressure photoionization. J. Am. Soc. Mass Spectrom. 20(1), 42–50 (2009)
Cody, R.B.: The observation of molecular ions and analysis of nonpolar compounds with the direct analysis in real time ion source. Anal. Chem. 81(3), 1101–1107 (2009)
Laramee, J.A., Cody, R.B.: Chemi-ionization and Direct Analysis in Real Time (DARTtm) Mass Spectrometry. In: Gross, M.L., Caprioli, R.M. (eds.) The Encyclopedia of Mass Spectrometry. Vol 6: Ionization Methods. Elsevier, Amsterdam, pp 377–387 (2007)
Cody, R.B., Dane, A.J.: Direct Analysis in real-time ion source. In: Encyclopedia of Analytical Chemistry. John Wiley and Sons, Ltd., West Sussex UK, Dec. 15, 2010. doi:10.1002/9780470027318.a9012.
Wu, C.; Qian, K.; Nefliu, M.; Cooks, R.: Ambient analysis of saturated hydrocarbons using discharge-induced oxidation in desorption electrospray ionization. J. Am. Soc. Mass Spectrom. 21(2), 261–267 (2010)
Yang, Z., Attygalle, A.: Aliphatic hydrocarbon spectra by helium ionization mass spectrometry (HIMS) on a modified atmospheric-pressure source designed for electrospray ionization. J. Am. Soc. Mass Spectrom. 22(8), 1395–1402 (2011)
Zencak, Z., Oehme, M.: Chloride-enhanced atmospheric pressure chemical ionization mass spectrometry of polychlorinated n-alkanes. Rapid Commun. Mass Spectrom. 18(19), 2235–2240 (2004)
DePuy, C.H., Bierbaum, V.M., Damrauer, R.: Relative gas-phase acidities of the alkanes. J. Am. Chem. Soc. 106(14), 4051–4053 (1984)
Bartmess, J.E.: NIST Negative Ion Energetics Database, Version 5.12, National Institute of Standards and Technology (2011)
Laramée, J.A., Durst, H.D.N., Michael, J., Connell, T.R.: Alcohols can now be analyzed by a direct analysis in real-time method: applications for chemical warfare agent synthesis. Am. Lab. 41(4), 24–27 (2009)
Laramee, J.A., et al.: An improved protocol for the analysis of alcohols by direct analysis in real time mass spectrometry. Am. Lab. 41(7), 25–27 (2009)
NIST Chemistry Web Book. Available at: http://webbook.nist.gov/chemistry/. Accessed 13 Dec 2012
Tamura, J. and J. Osuga New Generation LC-TOF/MS: "AccuTOF(TM)". JEOL News (2001)
Hirabayashi, A., Sakairi, M., Koizumi, H.: Sonic spray mass spectrometry. Anal. Chem. 67(17), 2878–2882 (1995)
Pagnotti, V. S.; Inutan, E. D.; Marshall, D. D.; McEwen, C. N.; Trimpin, S.: Inlet ionization: a new highly sensitive approach for liquid chromatography/mass spectrometry of small and large molecules. Anal. Chem. 83(20), 7591–7594 (2011)
Laramée, J.A., et al.: Alcohols can now be analyzed by a direct analysis in real-time method: applications for chemical warfare agent synthesis. Am. Lab. 41(4), 24–27 (2009)
Curtis, M.E., Jones, P.R., Sparkman, O.D.: Capturing Radical Species Formed in the Helium Metastable Beam Produced from the Direct Analysis in Real Time Ion Source. Proceedings of the International Mass Spectrometry Conference: Bremen, Germany (2009)
Curtis, M.: Investigation and characterization of the direct analysis in real time helium metastable beam open-air ion source: mechanism of ionization, fluid dynamic visualization, and applications in chemistry. University of the Pacific (2012)
Accuracy of Prediction of Polarizability. Open MOPAC. Available from: http://openmopac.net/Polarizability_table.html. Accessed 13 Dec 2012
Miller, K.J., Savchik, J.: A new empirical method to calculate average molecular polarizabilities. J. Am. Chem. Soc. 101(24), 7206–7213 (1979)
Acknowledgment
The authors thank Dr. Warren Samms of the Harris County Institute for Forensic Sciences for calling attention to the possibility of negative ion formation from alkanes by DART, and Hannah Moore of Keel University, whose research with insect puparial cases during a visit to the authors’ laboratory led us to refine the adduct formation methodology. The authors also acknowledge Professor John Bartmess for helpful discussions about gas-phase ion energetics and anion formation mechanisms.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1250 kb)
Rights and permissions
About this article
Cite this article
Cody, R.B., Dane, A.J. Soft Ionization of Saturated Hydrocarbons, Alcohols and Nonpolar Compounds by Negative-Ion Direct Analysis in Real-Time Mass Spectrometry. J. Am. Soc. Mass Spectrom. 24, 329–334 (2013). https://doi.org/10.1007/s13361-012-0569-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-012-0569-6