Abstract
The Azuki bean weevil, Callosobruchus chinensis (L.), is a destructive pest of stored mung bean [Vigna radiata (L.) Wilczek] as well as other leguminous seeds. The development of resistant seeds to manage this pest is of current great interest to plant breeders. In this study, we investigated the oviposition preference and development of C. chinensis on two susceptible mung bean cultivars (Seonhwa and Gyeongseon) and one previously reported resistant cultivar (Jangan), compared to the susceptible cowpea (Vigna unguiculata L.), cultivar (Yeonbun) using both multiple-choice and no-choice tests. In addition, the development of C. chinensis was also examined at four constant temperatures (20, 25, 30, and 35 °C). Both tests found cowpea to be the most suitable seed for oviposition. Total developmental time from oviposition to adult emergence ranged from 27.01 to 38.2 days, being shortest on cowpea and longest on the mung bean, cv. Jangan. However, no successful development of C. chinensis larvae on mung bean, cv. Jangan, occurred at any temperature. The highest rate of adult emergence and the longest adult longevity both occurred on cowpea and certain mung bean cultivars (Seonhwa and Gyeongseon), with the dramatic exception of cv. Jangan. These results suggest that the higher preference and performance of C. chinensis on cowpea (3.3 egg/seed) and least on mung bean, cv. Jangan (0.4 egg/seed). This information may facilitate the exploration of resistant genetic materials and chemicals associated with seeds for successful breeding. Further studies should examine the chemicals associated with mung bean cultivars and its resistant mechanism to develop a control method against bruchines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mung bean is one of the most important legume crops in many Asian countries, including Korea (Hong et al. 2015; Malik 1994; Somta et al. 2008). At least three species of bruchines are known to attack mung bean (Talekar 1988). Among the bruchines, azuki bean weevil, Callosobruchus chinensis (L.) is a pest of Vigna beans such as mung bean (V. radiata) and cowpeas (V. unguiculata) (Tuda et al. 2005). It is a widely distributed species throughout the tropical and sub-tropical regions of the world (Rees 2004; Southgate 1979). Infestation by C. chinensis starts in the field and can cause severe damage to stored leguminous seeds (Bellows 1982; Southgate 1984). Damage to stored mung bean seeds leads to weight loss, low viability of seeds, and low nutritional quality (de Sa et al. 2014; Talekar 1988). Losses of 35% in Central America (McGuire and Cradall 1967), 7–13% in South America (Schoonhoven 1976), and 73% in Africa have been recorded for this pest (Khamala 1978; Nahdy 1994). Immature stages, especially larva, of the beetle spend their entire time inside the seeds (Credland and Wright 1990), and reduce nutritional value of seeds (Tohiuddin et al. 1993).
Callosobruchus maculatus (F.) beetles commonly mate and oviposit immediately after adult emergence and spend adulthood without water and food (Chiu and Messina 1994). Adults of C. chinensis lay numerous eggs on seeds and can complete many overlapping generations (6–7) during seed storage (Chandra 2006). Callosobruchus chinensis have a wide range of leguminous hosts on which they successfully develop (Fujii 1968; Nakamura 1969). Host selection for oviposition by adult C. chinensis has important fitness consequences, because offspring survival is influenced by physical and biochemical factors of seed coats (Nwanze et al. 1975), seed size (Horber 1983), thickness of seed coat (Gupta and Mishra 1970), geographical differences among host plants (Horton et al. 1988; Scriber 1986), host quality (Bernays and Chapman 1994; Coyle et al. 2011; Heisswolf et al. 2005; Hoffman and Rao 2011; Mangel 1989), and plant chemical defense compounds (Dobie 1981; Janzen et al. 1976) as well as abiotic factors such as temperature (Howe and Currie 1964; Maharjan et al. 2017). Female seed beetles have the ability to discriminate quality of host seeds, and do distribute their eggs according to host seed size in a manner that maximizes the amount of resources and secures the offspring survival (Cope and Fox 2003). Prior experience with particular hosts can also alter beetle orientation and host acceptance for oviposition (Howard and Bernays 1991; Papaj and Prokopy 1989; Szentesi and Jermy 1990). Natural selection shapes the oviposition behavior of bruchine seed beetles so as to maximize individual fitness because immature life stages of beetles have limited mobility and beetle larvae must feed on the seed on which oviposition occurred (Cope and Fox 2003; Wijeratne 1998).
Callosobruchus chinensis has until recently been managed with chemical pesticides and fumigants, but insecticide residues can remain on treated seeds, reducing their quality for both human consumption and planting. Other effective, low-cost control methods have therefore been sought, such as plants with natural resistance to bruchine beetles. Several studies have reported resistance of the wild mung bean [V. radiata var. sublobata (Roxb.) Verdc.], to bruchines, whose use in plant breeding programs has led to a number of newly developed mung bean cultivars (TC1966, V2709 and V2802, ACC23, and ACC41) (Fujii and Miyazaki 1987; Fujii et al. 1989; Lambrides and Imrie 2000; Talekar and Lin 1992). In Korea, three mung bean cultivars are widely grown: cv. Seonhwa, cv. Gyeongseon, and cv. Jangan. Among these, cv. Jangan is reported to be particularly resistant to bruchines and stink bugs, a trait developed by back crossing with the V2709 resistant donor (Bae et al. 2009; Hong et al. 2015). Previous studies have reported cowpea seeds to be the most preferred host for C. chinensis oviposition (Mainali et al. 2015a, b). However, information on the oviposition behavior and development of bruchines on these cultivars is limited and is needed to help breeders enhance the development of resistant lines for use in Korea. In this study, we evaluated the oviposition preference and development of the C. chinensis on three mung bean varieties Seonhwa, Gyeongseon, and Jangan, and compared this to cowpea, cv. Yeonbun. Studies have reported temperature is a critical factor, and it has been an important source of variation in bruchine development and oviposition choices (Maharjan et al. 2017; Mainali et al. 2015a, b); therefore, our experiments were also conducted in four constant temperatures.
Materials and methods
Insect rearing
Azuki bean weevil adults were collected from a field of azuki bean, Vigna angularis (Willd.) Ohwi & Ohashi, of NICS, RDA in Miryang (Gyeongsangnam-do; 35° 49′ N, 128° 74′ E), Korea in 2011. This field-collected population was maintained in the laboratory on azuki bean seeds, cv. Hongeon, for over a year before being used in this study. Collected adult weevils were held in a square shaped, transparent petri-plate (24 length × 2.5 height cm with lid ventilation) and provided with azuki bean seeds. All petri-plates were held at 28 ± 1 °C, 50 ± 5% RH and a 16:8 h L:D photoperiod. Adults were collected with the help of a glass funnel.
Azuki bean weevil adults used in this study were collected from a location where average high temperature and relative humidity during the azuki bean cultivation season are around 31 °C and 72%, respectively. However, average daily temperature for each month during cultivation season ranged from 18.5 to 28.9 °C. The experimental conditions we chose were, therefore, similar to the average of the environmental conditions that the adult weevils would experience in the field. We thus believe this minimizes the possible variations in results due to the environmental conditions experienced by C. chinensis.
Sources of seeds
One bruchine -resistant mung bean cultivar [cv. Jangan: Geumseong Nokdu (Korean cultivar) × AVI-3-1 (AVRDC pedigree)], two susceptible cultivars [cv. Seonhwa (AVRDC line) and cv. Gyeongseon: Gyeonggi Nokdu (Korean cultivar) × VC3738A (AVRDC pedigree)], and cowpea [cv. Yeonbun (pure line selection from Yecheon landrace, Korea)] were used in this study. Mung bean and cowpea seeds were dried to a consistent moisture level (< 12%) before being tested. To determine the seed size of each legume species, digital photographs of seeds were taken with a Nikon D300 digital SLR camera. The camera was fixed pointing down on the frame at a height of 40 cm. The length of seeds was measured from looking at these photographs on a Leica M205C stereomicroscope (Leica, Wetzlar, Germany), and the net length of seeds of each legume species was analyzed. Previous studies reported that an image analysis system has been widely adopted and used to measure the size and shape, and even physical properties of a wide range of crop seeds and fruits (Boydas et al. 2012; Ercisli et al. 2012; Firatligil-Durmus et al. 2010; Kara et al. 2013; Sayinci et al. 2012; Venora et al. 2007; Yurtlu and Yesiloglu 2011). However, moisture content, variation in carbon, and the carbon/nitrogen relation can also influence seed hardness and seed size (Amin et al. 2004; Kanawde et al. 1990; McGinley and Charnov 1988). A total of 100 seeds were measured for each species.
No-choice and choice experiments
Observations on the oviposition preference, developmental time, adult emergence, and adult weight of C. chinensis on different mung bean and cowpea seeds were carried out at 28 ± 1 °C, 50 ± 5% RH and a 16:8 h L:D photoperiod. Twenty seeds of mung bean (cvs. Seonhwa and Gyeongseon [susceptible], Jangan [resistant], or cowpea (cv. Yeonbun) were placed in a petri-dish (5 cm dia. × 1.5 cm height), and held inside a fine mesh ventilated breeding dish (14.5 cm dia. × 2.5 cm height) for a no-choice experiment in which a pair of newly emerged adult C. chinensis was introduced to the legumes.
In a separate choice experiment, another newly emerged pair of C. chinensis was introduced to the legumes, with each legume (n = 20) presented separately in petri-dishes (5 cm dia. × 1.5 cm height), which themselves were placed together inside a fine mesh ventilated breeding dish (14.5 cm dia. × 2.5 cm height) with random distribution.
In both experiments, the introduced weevils were allowed to lay eggs for 72 h. After every 24 h, seeds with eggs were removed and replaced with new seeds, and the numbers of eggs per seed were counted. The seeds with eggs were held under the same conditions until adult emergence. Two days after adult emergence, unmated adults were counted and weighed with a digital scale (Sartorius, CP124S, Sartorius AG Gottingen, Germany). Their sex was then determined, and weevils were placed individually in micro-tubes (1.3 cm dia., 3.8 cm height, vol. 1.7 ml; Scientific Specialties Inc. USA) without water or food to determine the longevity of each male and female. A week after adult emergence, seeds were also dissected to determine immature mortality. After death, adults were sexed based on the morphology of antenna (Hu et al. 2009). Each experiment was replicated 15 times.
Temperature experiment
Developmental time, adult longevity, and sex ratio of C. chinensis was examined at four constant temperatures [20, 25, 30, and 35 °C (± 0.5 °C)], 75 ± 5% RH, and a 16:8 h L:D photoperiod. The RH level within the chambers was maintained by using saturated salt solutions (Duksan Pure Chemicals, Korea) as described in Winston and Bates (1960). The saturated salt solution used was NaCl to maintain 75% RH. To monitor these conditions (temperature and humidity), data loggers [Huato Log-USB, Huato Electronic (Shenzhen) Co. Ltd., China] were used to record the actual temperature inside the environmental chambers. To allow oviposition, seeds of each legume (100 seeds/legume variety) were exposed for 24 h to a laboratory colony C. chinensis (a mixed sex population, 1–2 days old) in a square, transparent petri-plate (25 cm L × 15.5 cm W × 15 cm H) at 28 ± 1 °C, 50 ± 5% RH and a 16: 8 h L: D photoperiod. Since oviposition could only take place during this period, the age of the eggs and therefore of the subsequent larvae were uniform. After this 24 -h exposure, adult C. chinensis were removed, and eggs were tracked and counted using a stereomicroscope (4.4:1, 35X, Leica EZ4, Wetzlar, Germany). Any seeds bearing eggs were placed individually into a centrifuge-tube case (13.5 cm L × 13.5 cm W × 5 cm H with 100 individual wells; Daihan Scientific, Korea), and held inside humidity chambers (27 cm L × 20 cm W × 17 cm H). Humidity chambers were then placed inside incubators (Eyela, model-MTI-202B, Japan) set at 20, 25, 30, and 35 °C. The larva and pupa stages of C. chinensis were separated according to Campbell et al. (1976). To measure the duration of each developmental stage, some seeds were dissected every 12 h to identify the developmental stage, and observations were made until adult emergence or death of all insects in the experiment. Once adults emerged, their sex was determined, and they were kept individually in micro-tubes (1.3 cm dia., 3.8 cm height, vol. 1.7 ml; Scientific Specialties Inc. USA) without food or water until death to determine the adult longevity providing respective temperatures used during their development.
Seed weight loss
Seed weight was measured with a digital balance (Sartorius, CP124S, Sartorius AG Gottingen, Germany) before and after use in the study. Weight loss of seeds was estimated by subtracting weight of seeds after being exposed to C. chinensis from the initial weight.
Statistical analysis
Seed size and number of eggs laid, for both no-choice and choice tests, were analyzed using one-way analysis of variance (ANOVA), PROC GLM in SAS (SAS Institute Inc 2000), and Tukey’s test was used for post hoc analysis. Developmental time and adult longevity were analyzed using a two-way ANOVA, and the Tukey’s test was used for post hoc analysis for sex, varieties, and their interactions (SAS Institute Inc 2000). Adult emergence rates were analyzed with a Chi-square test using a contingency table and a post hoc multiple comparison test analogous to the Tukey’s test (Zar 2010). Developmental time of life stages on different leguminous seeds at different temperatures were analyzed using one-way analysis of variance (ANOVA), PROC GLM in SAS (SAS Institute Inc 2000) and the Tukey’s test was used for post hoc analysis. Longevity was compared between females and males with t-tests.
Results
Seed size
The leguminous seeds from different species or varieties used in the study were significantly different in size (F = 2094.34, df = 3, 396, p < 0.0001). Among the cultivars, the mung bean seed, cv. Jangan was the smallest in size.
No-choice and choice experiments
Oviposition
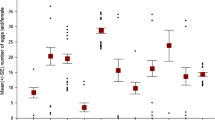
The ovipositional response of female C. chinensis varied with different leguminous seed sources. The number of eggs laid was significantly higher on the cowpea seeds (cv. Yeonbun) in both no-choice (F = 529.27, df = 3, 76, p < 0.0001) and choice tests (F = 1230.9, df = 3, 76, p < 0.0001), followed by mung bean, cvs. Seonhwa and Gyeongseon (Fig. 1). Mung bean cv. Jangan had the fewest eggs in both tests (no-choice: 0.7 per seed and choice: 0.4 per seed).
Developmental time
The developmental time [egg- adult (oviposition date to adult emergence)] of C. chinensis also varied significantly on legume seeds of different species of varieties tested in both no-choice (F = 407.15, df = 7, 110, p < 0.0001), and choice tests (F = 266.48, df = 7, 56, p < 0.0001). The developmental time was found to be significantly shorter on cowpea, cv. Yeonbun in both no-choice (F = 635.91, df = 3, 110, p < 0.0001), and choice (F = 441.77, df = 3, 56, p < 0.0001) tests compared to values on mung bean cultivars. The longest developmental time was found on mung bean, cv. Jangan (Fig. 2). A significant difference in developmental time between weevil sexes was found in both no-choice (F = 933.03, df = 1, 110, p < 0.0001), and choice (F = 489.86, df = 1, 56, p < 0.0001) tests. Also, significant differences in the interaction between seed types (species or varieties) and sexes were detected in both no-choice (F = 3.11, df = 3, 110, p = 0.02), and choice (F = 16.72, df = 3, 56, p < 0.0001) tests (Fig. 2).
Developmental time (day, mean ± SE) of Callosobruchus chinensis on different legume seeds in no-choice and choice tests. Capital and small letters above bars denote differences among cultivars for female and male, respectively (Tukey’s test, p < 0.05). Asterisk denotes the significant differences between sexes (t test, p < 0.05)
Adult emergence
We found significant differences in the number of adult emergences among legume seeds types (species or varieties). Higher adult emergence was recorded on the cowpea, cv. Yeonbun in the no-choice (χ 2 = 13.73, df = 3, p = 0.003), while adult emergence rates were not different between mung bean cvs. Seonhwa and Gyeongseon. Higher adult emergence rates were recorded on cowpea, cv. Yeonbun and mung bean, cv. Seonhwa than mung bean cv. Jangan in the choice (χ 2 = 12.36, df = 3, p = 0.006) tests (Fig. 3).
Adult longevity
Adult longevity of C. chinensis under conditions of starvation and lack of water was found to differ among leguminous seeds in both no-choice (F = 111.36, df = 7, 109, p < 0.0001), and choice (F = 73.32, df = 7, 96, p < 0.0001) tests. Adults that emerged from cowpea seeds, cv. Yeonbun were found to be longer-lived than those emerging from mung bean cultivars, while the shortest longevity was found on the mung bean, cv. Jangan in both no-choice (F = 200.55, df = 3, 109, p < 0.0001), and choice (F = 111.00, df = 3, 96, p < 0.0001) tests. A significant difference in longevity between sexes was detected in both no-choice (F = 165.24, df = 1, 109, p < 0.0001), and choice (F = 160.69, df = 1, 96, p < 0.0001) tests. Also, significant differences in the interaction between seed type and beetles sex were detected in both no-choice (F = 4.20, df = 3, 109, p = 0.007), and choice (F = 6.53, df = 3, 96, p = 0.0005) tests (Fig. 4).
Adult longevity (day, mean ± SE) of female and male Callosobruchus chinensis that emerged from different legume seeds from no-choice and choice tests. Capital and small letters above bars denote differences among cultivars for female and male, respectively (Tukey’s test, p < 0.05). Asterisk denotes the significant differences between sexes (t test, p < 0.05)
Adult weight
Adults of C. chinensis that emerged from the mung bean, cv. Seonhwa were significantly heavier than adults from other seed types, in both no-choice (F = 42.57, df = 3, 252, p < 0.0001) and choice (F = 17.00, df = 3, 217, p < 0.0001) tests (Fig. 5).
Temperature experiment
Developmental time
The developmental time (oviposition date to adult emergence) of C. chinensis varied significantly with temperatures on different legume seed types: 20 °C (F = 88.92, df = 2, 93, p < 0.0001), 25 °C (F = 2.71, df = 2, 227, p = 0.04). 30 °C (F = 11.20, df = 2, 163, p < 0.0001), and 35 °C (F = 8.13, df = 2125, p = 0.0005) (Table 1). Developmental time was significantly shorter on cowpea, cv. Yeonbun at all tested temperatures. Meanwhile, larva could not survive on the mung bean, cv. Jangan at any temperature.
Adult longevity
Adult longevity of C. chinensis under conditions of starvation and lack of water, was found to differ with temperature among legume seed types: 20 °C (female: F = 7.66, df = 2, 118, p = 0.0007 and male: F = 4.32, df = 2, 104, p = 0.01), 25 °C (female: F = 5.33, df = 2, 125, p = 0.006 and male: F = 3.98, df = 2, 98, p = 0.02), and 30 °C (female: F = 4.32, df = 2, 78, p = 0.01 and male: F = 2.94, df = 2, 82, p = 0.05). Adults that emerged from the cowpea seeds, cv. Yeonbun, lived longer than those that emerged from the mung bean cultivars at 20, 25 and 30 °C, and females lived longer than males at 20 °C (cowpea, cv. Yeonbun: t = 2.01, p = 0.04), 25 °C (cowpea, cv. Yeonbun: t = 3.43, p = 0.001, mung bean, cvs. Seonhwa: t = 6.63, p < 0.0001 and Gyeongseon: t = 2.53, p = 0.01), and 30 °C (cowpea, cv. Yeonbun: t = 2.79, p = 0.007, mung bean, cv. Seonhwa: t = 3.79, p = 0.0004), and 35 °C (mung bean, cvs. Seonhwa: t = 2.23, p = 0.02 and Gyeongseon: t = 2.32, p = 0.02) (Table 2).
Sex ratio
Callosobruchus chinensis sex ratio (proportion of female) was not different among legume cultivars (p > 0.05) (Table 1). The female proportion of individuals reaching adulthood was almost a 1:1 ratio.
Seed weight loss
Seed weight loss was found to differ with temperature among legume seed types: 20 °C (F = 61.44, df = 3, 284, p < 0.0001), 25 °C (F = 30.64, df = 3, 348, p < 0.0001), and 30 °C (F = 19.74, df = 3, 348, p < 0.0001), and 35 °C (F = 43.65, df = 3, 348, p < 0.0001) (Table 3). The least seed weight loss was recorded for mung bean, cv. Jangan.
Discussion
In this study, Callosobruchus chinensis showed a consistent oviposition preference to one legume varieties among the tested legume varieties. Previous studies have suggested that beetles varieties preference could be related to physical characteristics of seed such as seed size, surface area of seed, seed texture and so on (Cope and Fox 2003; de Sa et al. 2014; Huignard et al. 1985; Mitchell 1990). Instead, females must either use olfactory cues other than physical parameters of seeds for host location and continued through oviposition (Cope and Fox 2003). Credland and Wright (1988) reported that chemical cues from the seeds play a vital role in oviposition, and C. maculatus discriminate among hosts based on odors, which response is further mediated by taste receptors on the maxillary palps (Messina et al. 1987), demonstrating that olfaction is used by beetles in host location. Moreover, chemical compounds such as phenol concentration in seed (Bhattacharya and Banerjee 2001), and seed morphology and texture can also influence the oviposition choice of female Callosobruchus spp. (Brewer and Horber 1983; de Sa et al. 2014; Huignard et al. 1985; Janzen 1977; Nwanze and Horber 1976).
In this study, we found a higher number of eggs laid by C. chinensis on cowpea, cv. Yeonbun in both no-choice and choice tests. This might be due to the size of seeds. The seeds of cowpea had a larger surface than the mung bean seeds, findings that are in line with that of Seddiqi (1972), and Bhattacharya and Banerjee (2001), who reported more eggs were laid by Callosobruchus spp. on larger seeds. Very few eggs were laid by C. chinensis on mung bean, cv. Jangan in either no-choice or choice tests. This low preference on mung bean, cv. Jangan might be associated with its rough seed coat and small size. Previous studies have shown that both C. chinensis and C. maculatus choose leguminous seeds with smooth seed coat for oviposition (Bhattacharya and Banerjee 2001; de Sa et al. 2014; Girish et al. 1974; Huignard et al. 1985; Nwanze et al. 1975; Southgate 1979). Further, a small seed size functions as a protection mechanism against bruchines through countermechanisms to defensive traits against bruchines such as gum production by pods, dehiscence, smaller size of seeds, indehiscence, and flaking of pod surface (Center and Johnson 1974).
In this study, we noticed scraped markings (2–3 short lines) made by C. chinensis on the seeds with rough seed coat such as mung bean, cv. Jangan (personal observation), suggesting the beetles prefer suitable seed hosts on which their offspring will have the best chance to survive and develop. Study has shown that both larvae and adults of C. maculatus altered their host preference behavior based on past experience (Wasserman 1981). In addition, the test insect used in this study (C. chinensis) was originally collected from an azuki bean field and reared on the azuki bean seed for successive generations, precisely so that host preferences would not be influenced by previous feeding experience. The results presented here suggest that C. chinensis can complete its development on cowpea, cv. Yeonbun, and mung bean, cvs. Seonhwa and Gyeongseon, but not on mung bean, cv. Jangan. This phenomenon, similar to the ovipositional preference of C. chinensis, might be mediated by nutrient and the morphology/physical characteristics and chemicals associated with the seeds (Brewer and Horber 1983; Janzen 1977; Panizzi 1987). In this study, none of C. chinensis were able to develop on mung bean, cv. Jangan in constant temperature experiments. Microscopic observation showed that internal damage occurred by larvae from mung bean cv. Jangan and all larva of C. chinensis feeding on the cv. Jangan died in the first and second instar due to a failure to penetrate through the seed coat to the cotyledons. Similarly, Somta et al. (2008) reported that first and second instar larvae failed to develop because of plant defense compounds in the embryo and/or cotyledons and that such insecticidal chemicals play a vital role in making a seed coat resistant to bruchines. Several studies related to biochemical defense in legume seeds (see Kashiwaba et al. 2003; Somta et al. 2006; Sugawara et al. 1996; Talekar and Lin 1992 for examples) have been reviewed by Gatehouse et al. (1990). Seed genotypes can also influence resistance characteristics against bruchines (Kitamura et al. 1988).
In this study, we found the shortest developmental time of C. chinensis was on cowpea seeds, in both no-choice and choice tests, and in the constant temperature experiments. These findings are in line with that of Mainali et al. 2015a, b, who reported the shortest developmental time on cowpea seeds, and stand in contrast to the result of Kim and Choi (1987) who reported that the shortest developmental time was on the azuki bean seed. In our study, females took longer to develop than males. Tuda and Shimada (1995) also reported longer female development. The results from our constant temperature experiments also suggests that ovipositional behavior and development of C. chinensis are influenced first by host seeds but then by environmental conditions.
Higher adult emergence was recorded from mung bean, cv. Seonhwa and cowpea, cv. Yeonbun in choice tests. Similarly, adults that emerged from the cowpea, cv. Yeonbun lived longer in both no-choice and choice tests, and in the constant temperature experiments; meanwhile, males lived for shorter periods than females, similar to findings by Seddiqi (1972) and Mainali et al. 2015a, b, who reported higher adult emergence from mung bean and cowpea seeds, and contrary to the findings of Tuda and Shimada (1995), who found no difference in longevity of males and females. This discrepancy might be due to differences in the genetic makeup of each insect population. Adults of C. chinensis that emerged from the mung bean, cv. Jangan were weighed significantly less than adults that emerged from other seed types tested here. This difference might be due to a longer exposure to the defensive biochemicals and proteins associated with seeds such as cyclopeptide alkaloid (Sugawara et al. 1996), trypsin (Dobie 1981), peptide compound ‘GIF-5′ (Kaga et al. 2000), and cysteine-rich protein (Chan et al. 2005; Chen et al. 2002).
The ovipositional behavior preference and developmental time trend of C. chinensis in both no-choice and choice tests, and in the constant temperature experiments were similar. From both experiments, mung bean, cv. Jangan is found to be the least attractive seed host for oviposition, development, adult emergence, and shortest adult longevity. It also had the lowest seed weight losses. The behavioral preference of C. chinensis and its performance on different seeds is likely mediated by the biochemicals and toxicity associated with the seeds as discussed earlier. Our findings suggest that mung bean cv. Jangan could be a model seed source for exploring resistance related to seed chemicals to better understand resistance mechanisms against bruchines. Further, this information could be a base to enhance the resistant breeding program of mung beans in Korea.
References
Amin MN, Hossain MA, Roy KC (2004) Effects of moisture content on some physical properties of lentil seeds. J Food Eng 65:83–87
Bae SD, Kim HJ, Yoon YN, Park ST, Choi BR, Jung JK (2009) Effects of a mung bean cultivar, Jangannogdu on nymphal development, adult longevity and oviposition of soybean stinkbugs. Kor J Appl Entomol 48:311–318
Bellows TS Jr (1982) Analytical models for laboratory populations of Callosobruchus chinensis and C. maculatus (Coleoptera: Bruchidae). J Anim Ecol 51:263–287
Bernays EA, Chapman RF (1994) Host–plant selection by phytophagous insects. Chapman and Hall, New York
Bhattacharya B, Banerjee TC (2001) Factors affecting egg-laying behavior and fecundity of Callosobruchus chinensis (L.) (Coleoptera: Bruchidae) infesting stored pulses. Orient Insects 35:373–386
Boydas MG, Sayinci B, Gozlekci S, Oztürk I, Ercisli S (2012) Basic physical properties of fruits in loquat (Eriobotrya japonica (Thunb. Lindl.) cultivars and genotypes determined by both classical method and digital image processing. Afr J Agric Res 7:4171–4181
Brewer IN, Horber E (1983) Evaluating resistance to Callosobruchus chinensis L. in different seed legumes. In: Proceedings of 3rd international working conference on stored products entomology, K.S.U., Manhattan, Kansas, Oct 23–28, 1983, pp 435–443
Campbell A, Singh NB, Singh RN (1976) Bioenergetics of the granary weevil, Sitophilus granaries (L.) (Coleoptera: Curculionidae). Can J Zool 54:786–798
Center TD, Johnson CD (1974) Coevolution of some seed beetles (Coleoptera: Bruchidae) and their hosts. Ecology 55:1096–1103
Chan L, Chen CS, Horng SB (2005) Characterization of resistance to Callosobruchus maculatus (Coleoptera: Bruchidae) in mungbean variety VC6089A and its resistance-associated protein VrD1. J Econ Entomol 98:1369–1373
Chandra G (2006) Callosobruchus chinensis the pulse beetle cowpea bruchid. http://www.iaszoology.com/callosobruchus-chinensis/. Accessed Oct 2016
Chen KC, Lin CY, Kuan CC, Sung HY, Chen CS (2002) A novel defensin encoded by a mungbean cDNA exhibits insecticidal activity against bruchid. J Agric Food Chem 50:7258–7263
Chiu YJ, Messina FJ (1994) Effect of experience on host preference in Callosobruchus maculatus (Coleoptera: Bruchidae): Variability among populations. J Insect Behav 7:503–515
Cope JM, Fox CW (2003) Oviposition decisions in the seed beetle, Callosobruchus maculatus (Coleoptera: Bruchidae): effects of seed size on superparasitism. J Stored Prod Res 39:355–365
Coyle DR, Clark KE, Raffa KF, Johnson SN (2011) Prior host feeding experience influences ovipositional but not feeding preference in a polyphagous insect herbivore. Entomol Exp Appl 138:137–145
Credland PF, Wright AW (1988) The effect of artificial substrates and host extracts on oviposition by Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J Stored Prod Res 24:157–164
Credland PE, Wright AW (1990) Oviposition deterrents of Callosobruchus maculatus (Coleoptera: Bruchidae). Physiol Entomol 15:285–298
de Sa LF, Wermelinger TT, da Ribeiro ES, de Gravina GA, Fernandes KV, Xavier-Filho J, Venancio TM, Rezende GL, Oliveira AEA (2014) Effects of Phaseolus vulgaris (Fabaceae) seed coat on the embryonic and larval development of the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae). J Insect Physiol 60:50–57
Dobie P (1981) The use of resistant varieties of cowpeas (Vigna unguiculata) to reduce losses due to post-harvest attack by Callosobruchus maculatus. In: Labeyrie V (ed) Series Entomologica, vol 19. Junk Publishers, The Hague, Dr. W, pp 182–192
Ercisli S, Sayinci B, Kara M, Yildiz C, Ozturk I (2012) Determination of size and shape features of walnut (Juglans regia L.) cultivars using ımage processing. Scientia Horti 133:47–55
Fıratlıgil-Durmuş E, Šárka E, Bubník Z, Schejbal M, Kadlec P (2010) Size properties of legume seeds of different varieties using image analysis. J Food Eng 99:445–451
Fujii K (1968) Studies on interspecies competition between the azuki bean weevil and the southern cowpea weevil. III. Some characteristics of strains of two species. Res Popul Ecol 10:87–98
Fujii K, Miyazaki S (1987) Infestation resistance of wild legumes (Vigna sublobata) to azuki bean weevil, Callosobruchus chinensis (L.) (Coleoptera: Bruchidae) and its relationship with cytogenetic classification. Appl Entomol Zool 22:229–230
Fujii K, Ishimoto M, Kitamura K (1989) Patterns of resistance to bean weevils (Bruchidae) in Vigna radiata-mungo-sublobata complex inform the breeding of new resistant varieties. Appl Entomol Zool 24:126–132
Gatehouse AMR, Minney BH, Dobie P, Hilder V (1990) Biochemical resistance to bruchid attack in legume seed; investigation and exploitation. In: Fujii K, Gatehouse AMR, Johnson CD, Mitchel R, Yoshida T (eds) Bruchids and legumes: economics, ecology and coevolution. Kluwer Academic Publishers, Dordrecht, pp 241–256
Girish GK, Singh K, Krishnamurthy K (1974) Studies on the oviposition and development of Callosobruchus maculatus (Feb.) on various stored pulses. Bull Grain Tech 12:113–115
Gupta DS, Mishra RC (1970) Further studies on the relative resistance of some important varieties of Bengal gram, Cicer arietinum L. to pulse beetle, Callosobruchus chinensis (L.). Bull Grain Tech 8:14–17
Heisswolf A, Obermaier E, Poethke HJ (2005) Selection of large host plants for oviposition by a monophagous leaf beetle: nutritional quality or enemy-free space? Ecol Entomol 30:299–306
Hoffman GD, Rao S (2011) Oviposition site selection on oats: the effect of plant architecture, plant and leaf age, tissue toughness, and hardness on cereal leaf beetle, Oulema melanopus. Entomol Exp Appl 141:232–244
Hong MG, Kim KH, Ku JH, Jeong JK, Seo MJ, Park CH, Kim YH, Kim HS, Kim YK, Baek SH, Kim DY, Park SK, Kim SL, Kim SL, Moon JK (2015) Inheritance and quantitative trait loci analysis of resistance genes to bruchid and bean bug in mungbean (Vigna radiata L. Wilczek). Plant Breed Biotech 3:39–46
Horber E (1983) Principles, problems, progress and potential inn host resistance to stored grain insects. In: Proceedings of the 3rd international working conference on stored products entomology, K.S.U., Manhattan, Kansas, Oct 2328, 1983, 391–417
Horton DR, Capinera JL, Chapman PL (1988) Local differences in host use between two populations of the Colorado potato beetle. Ecology 69:823–831
Howard JJJ, Bernays EA (1991) Effects of experience on palatability hierarchies of novel plants in the polyphagous grasshopper Schistocerca Americana. Oecologia 87:424–428
Howe RW, Currie JE (1964) Some laboratory observations on the rates of development, mortality, and oviposition of several species of Bruchidae breeding in stored pulses. Bull Entomol Res 55:437–477
Hu F, Zhang GN, Wang JJ (2009) Scanning electron microscope studies of antennal sensilla of bruchid beetles, Callosobruchus chinensis (L.) and Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Micron 40:320–326
Huignard J, Leroi B, Alzouma I, Germain JF (1985) Oviposition and development of Bruchidius atrolineatus and Callosobruchus maculatus in Vigna unguiculata cultures in Niger. Insect Sci Appl 6:691–699
Janzen DH (1977) How southern cowpea weevil larvae (Bruchidae: Callosobruchus maculatus) die on nonhost seeds. Ecology 58:921–927
Janzen DH, Juster HB, Liener IE (1976) Insecticidal action of the phytohemagglutinin in black beans on a bruchid beetle. Science 192:795–796
Kaga A, Teraishi M, Iijima N, Sugawara F, Ishimoto M (2000) Progresses in identification of the bruchid resistance gene in mungbean (Vigna radiata (L.). In: Abstract in plant Animal genome VIII Conference, Town and Country Hotel, San Diego, CA
Kanawde LR, Bhosale BW, Kadam MS (1990) Effects of moisture content on certain selected physical properties of pulse seeds. J Maharastra Agric Univ 15:60–62
Kara M, Saynci B, Elkoca E, Ozturk I, Ozmen TB (2013) Seed size and shape analysis of registered common bean (Phaseolus vulgaris L.) cultivars in Turkey using digital photography. J Agric Sci 19:219–234
Kashiwaba K, Tomooka N, Kaga A, Han OK, Vaughan DA (2003) Characterization of resistance to three bruchid species (Callosobruchus spp., Coleoptera: Bruchidae) in cultivated rice bean (Vigna umbellata). J Econ Entomol 96:207–213
Khamala CPM (1978) Pests of grain legumes and their control in Kenya. In: Singh SR, van Emden HF, Taylor TA (eds) Pests of grain legumes: ecology and control. Academic Press, London, pp 124–134
Kim KC, Choi HS (1987) Effects of temperature on the oviposition, feeding and emergence of the azuki bean weevil (Callosobruchus chinensis L.) in the stored beans. Kor J Plant Prot 26:71–81
Kitamura K, Ishimoto M, Sawa M (1988) Inheritance of resistance to infestation with azuki bean weevil in Vigna sublobata and successful incorporation to V. radiata. Jpn J Breed 38:459–464
Lambrides CJ, Imrie BC (2000) Susceptibility of mungbean varieties to the bruchid species Callosobruchus maculatus (F.), C. phasroli (Gyll.), C. chinensis (L.), and Acabthoscelides obtectus (Say.) (Coleoptera: Chrysomelidae). Aust J Agric Res 51:85–89
Maharjan R, Ahn JJ, Park CG, Yoon YN, Jang Y, Kang HW, Bae SD (2017) Effects of temperature on development of the azuki bean weevil, Callosobruchus chinensis (Coleoptera: Bruchidae) on two leguminous seeds. J Stored Prod Res 72:90–99
Mainali BP, Kim HJ, Park CG, Kim JH, Yoon YN, Oh IS, Bae SD (2015a) Oviposition preference and development of azuki bean weevil, Callosobruchus chinensis, on five different leguminous seeds. J Stored Prod Res 61:97–101
Mainali BP, Kim HJ, Park CG, Yoon YN, Lee YH, Park IH, Kang HW, Bae SD (2015b) Interactive effects of temperature and relative humidity on oviposition and development of Callosobruchus chinensis (L.) on azuki bean. J Stored Prod Res 63:47–50
Malik BA (1994) Grain legumes. National Book Foundation, Islamabad, pp 227–326
Mangel M (1989) Evolution of host selection in parasitoids: does the state of the parasitoids matter? Am Nat 133:688–705
McGinley MA, Charnov EL (1988) Multiple resources and the optimal balance between size and number of offspring. Evol Ecol 2:77–84
McGuire JU, Crandall BS (1967) Survey of insect pests and plant diseases of selected food crops of Mexico, Central America and Panama. International Agriculture Development Service, Agricultural Research Service, US Department of Agriculture, Washington, DC, USA, p 157
Messina FJ, Barmore JL, Renwick JAA (1987) Oviposition deterrent from eggs of Callosobruchus maculatus: spacing mechanism or artifact? J Chem Ecol 13:219–226
Mitchell R (1990) Behavioral ecology of Callosobruchus maculatus. In: Fujii K, Gatehouse MR, Johnson CD, Mitchell R, Yoshida T (eds) Bruchids and legumes: economics, ecology and coevolution. Kluwer Academic Publishers, Dordrecht, pp 317–330
Nahdy MS (1994) Bean sieving, a possible control measure for the dried bean beetles, Acabthoscelides obtectus (Say.) (Coleoptera: Bruchidae). J Stored Prod Res 30:65–69
Nakamura H (1969) The effect of density on populations in Callosobruchus chinensis L. from different localities. Jpn J Ecol 19:92–97
Nwanze KF, Horber E (1976) Seed coats of cowpeas affect oviposition and larval development of Callosobruchus maculatus. Environ Entomol 5:213–218
Nwanze KF, Horber E, Pitts CW (1975) Evidence for ovipositional preference of Callosobruchus maculatus for cowpea varieties. Environ Entomol 4:409–412
Panizzi AR (1987) Nutritional ecology of seed-sucking insects of soybean and their management. Mem Ins Oswaldo Cruz Intern Symp Insects 82:161–175
Papaj DR, Prokopy RJ (1989) Ecological and evolutionary aspects of learning in phytophagous insects. Annu Rev Entomol 34:315–350
Rees D (2004) Insects of stored products. CSIRO Publishing, Canberra
SAS Institute Inc (2000) SAS user’s guide: statistics. Cary, N.C.
Sayinci B, Ercisli S, Ozturk I, Eryilmaz Z, Demir B (2012) Determination of size and shape in the ‘Moro’ blood orange and ‘Valencia’ sweet orange cultivar and its mutants using image processing. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 40:234–242
Schoonhoven AV (1976) Pests of stored beans and their economic importance in Latin America. In: Proc Symp On Trop. Stored Products Entomology, 15th Int Cong Entomol. Entomol Sot Am, College Park, MD, USA, pp 691–689
Scriber JM (1986) Origins of the regional feeding abilities in the tiger swallowtail butterfly: ecological monophagy and the Papilio glaucus australis subspecies in Florida. Oecologia 74:94–103
Seddiqi PM (1972) Studies on Longevity, oviposition, fecundity and development of Callosobruchus chinensis L. (Coleoptera; Bruchidae). J Appl Entomol 72:66–72
Somta P, Talekar NS, Srinives P (2006) Characterization of Callosobruchus chinensis (Coleoptera: Bruchidae) resistance in rice bean (Vigna umbellata (Thunb.) Ohwi and Ohashi). J Stored Prod Res 42:313–327
Somta C, Somta P, Tomooka N, Ooi PAC, Vaughan DA, Srinives P (2008) Characterization of new sources of mungbean (Vigna radiata (L.) Wilczek) resistance to bruchids, Callosobruchus Spp. (Coleoptera: Bruchidae). J Stored Prod Res 44:316–321
Southgate BJ (1979) Biology of the Bruchidae. Annu Rev Entomol 24:449–473
Southgate BJ (1984) Observations on the larval emergence in Callosobruchus chinensis (Coleoptera: Bruchidae). Entomol Gen 9:177–180
Sugawara F, Ishimoto M, Le-Van N, Koshino H, Uzawa J, Yoshida S, Kitamura K (1996) Insecticidal peptide from mungbean: a resistant factor against infestation with azuki bean weevil. J Agric Food Chem 44:3360–3364
Szentesi A, Jermy T (1990) The role of experience in host plant choice by phytophagous insects. In: Bernays EA (ed) Insect-plant interactions, vol 2. CRC, PressBoca Raton, pp 39–74
Talekar NS (1988) Biology, damage and control of bruchid pests of mungbean. In: Shanmugasundaram S, McLean BT (eds) Mungbean: proceeding of the second international symposium. AVRDC, Tainan, Taiwan, pp 329–342
Talekar NS, Lin CP (1992) Characterization of Callosobruchus chinensis (Coleoptera: Bruchidae) resistance in mungbean. J Econ Entomol 85:1150–1153
Tohiuddin G, Banerjee D, Bhattacharya TC (1993) Energy reserves of adult pulse beetle (Callosobruchus chinensis) (Coleoptera: Bruchidae) reared on seeds of gram (Cicer arietinum). Indian J Agric Sci 63:181–185
Tuda M, Shimada M (1995) Developmental schedules and persistence of experimental host-parasitoid systems at two different temperatures. Oecologia 103:283–291
Tuda M, Chou LY, Niyomdham C, Buranapanichpan S, Tateishi Y (2005) Ecological factors associated with pest status in Callosobruchus (Coleoptera: Bruchidae): high host specificity of non-pest to Cajaninae (Fabaceae). J Stored Prod Res 41:31–45
Venora G, Grillo O, Shahin MA, Symons SJ (2007) Identification of Sicilian landraces and Canadian cultivars of lentil using an image analysis system. Food Res Int 40:161–166
Wasserman SS (1981) Host induced oviposition preferences and oviposition markers in the cowpea weevil, Callosobruchus maculatus. Ann Entomol Soc Am 74:242–245
Wijeratne PM (1998) Variation in egg and adult production of Callosobruchus chinensis (L.) and the effect of egg density and oviposition site limitation. Trop Agric Res Ext 1:136–142
Winston PW, Bates DH (1960) Saturated Solutions for the control of humidity in biological research. Ecology 41:232–237
Yurtlu YB, Yeşiloğlu E (2011) Mechanical behaviour and split resistance of chestnut under compressive loading. Tarım Bilimleri Dergisi J Agric Sci 17:337–346
Zar JH (2010) Bio-statistical analysis, 5th edn. Prentice Hall, Upper Saddle River
Acknowledgements
This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ011291012016), Rural Development Administration, Republic of Korea. For reviews of this manuscript, we thank anonymous reviewers. English editing by Van Driesche Scientific Editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maharjan, R., Yi, H., Kim, H. et al. Mung bean (Vigna radiata) cultivars mediated oviposition preference and development of Callosobruchus chinensis (Coleoptera: Chrysomelidae: Bruchinae). Appl Entomol Zool 53, 55–66 (2018). https://doi.org/10.1007/s13355-017-0524-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-017-0524-x