Abstract
The main hosts and sites of oviposition for the two bugs, Trigonotylus caelestialium (Kirkaldy) (Hemiptera: Miridae) and Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae), that cause pecky rice were investigated in 24 poaceous plants. Nymphs of T. caelestialium emerged from both spikelets and leaf sheaths, while nymphs of S. rubrovittatus emerged almost exclusively from spikelets. Suitable plants for oviposition by T. caelestialium are Lolium multiflorum, Digitaria violascens and Hordeum murinum, while Poa annua, Anthoxanthum odoratum, Alopecurus aequalis and D. violascens were preferentially used by S. rubrovittatus. There was a greater difference in the number of nymphs emerging from different plants for S. rubrovittatus than for T. caelestialium. This difference may be because T. caelestialium can oviposit on leaf sheaths if the spikelets are not suitable for oviposition, whereas S. rubrovittatus only oviposits on spikelets. Although both bugs oviposited on spikelets, the internal oviposition sites were different. In D. ciliaris, T. caelestialium laid all eggs between the lemma of the first floret and the second floret, whereas S. rubrovittatus laid eggs almost exclusively inside the second floret. In contrast, in P. annua, T. caelestialium laid all eggs inside the florets, whereas S. rubrovittatus laid eggs both between and inside the florets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pecky rice, which is caused by heteropteran bugs, is a major problem that affects rice farming in Japan. The rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Hemiptera: Miridae), and the sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae), have recently received particular attention as major species that cause pecky rice (Watanabe and Higuchi 2006). Brown rice grains may also serve as hosts to both bugs; however, development is poor on the leaves of rice plants (Ishimoto 2008). Therefore, rice is not a major host for these bugs. Hence, rice grains are thought to be damaged by rice bugs that emerge and grow on other poaceous plants adjacent to paddy fields. Because the existence of host plants around paddy fields may affect the abundance of pecky rice bugs, it is important to identify the host plant species. Poaceous plants have been previously reported as a food source for rice bugs, with the removal of grass weeds leading to reduction in rice bug populations (Kikuchi and Kobayashi 2001; Niiyama and Itoyama 2006; Ono et al. 2007; Yokota and Suzuki 2008). However, there have been few reports on the suitability of poaceous plants as hosts for rice bugs.
Trigonotylus caelestialium deposit their eggs into the tight space between the stem and the leaf sheath, whereas S. rubrovittatus oviposit into the spikelet (Hayashi 1986; Kato and Hasegawa 1950; Okuyama and Inoue 1975). In one study, when several grass plants were collected from the field, nymphs of T. caelestialium emerged from foliage and spikes, whereas nymphs of S. rubrovittatus emerged from the spikes only. This observation further indicated that T. caelestialium deposits eggs within the leaf sheath and spikelet, whereas S. rubrovittatus deposits eggs into the spikelet only (Nagasawa 2007). Nymphal or larval mobility is generally inferior to adult mobility. Thus, host use by nymphs or larvae is considered to be greatly affected by adult oviposition preferences (Bernays and Chapman 1994; Schoonhoven et al. 2005; Wennström et al. 2010). Therefore, it is important to study the selection of site oviposition to understand host use by phytophagous insects. Several studies have investigated the number of eggs or emerging nymphs on plants collected from fields (Higuchi et al. 2001; Iimura 1992; Nagasawa 2007). However, plant sampling from fields does not show the exact performance of the plants, because the sampling time or site may influence the occurrence of bugs. Oviposition tests have been conducted in some studies (Hayashi 1986; Iimura 1992; Kikuchi and Kobayashi 2004). However, only a few species were studied, and the testing conditions were not comparable. In this study, we investigated oviposition by two rice bug species on various poaceous plants to estimate their potential as host plants.
Materials and methods
Insects
Trigonotylus caelestialium and S. rubrovittatus were collected from the experimental field at Hokuriku Research Center, National Agricultural Research Center, Niigata Prefecture, Japan. The insects were reared on young wheat seedlings under laboratory conditions (16L:8D photoperiod, 25 °C), as described previously (Higuchi and Takahashi 2000; Nagasawa and Higuchi 2008, 2010). Adult females of T. caelestialium and S. rubrovittatus were used for oviposition tests 4–7 and 7–12 days after eclosion, respectively. The preoviposition period of T. caelestialium is 3.1 days at 27 °C and 4.4 days at 23 °C (Takahashi and Higuchi 2001). Females of T. caelestialium start to attract males within 24 h after eclosion (Higuchi and Takahashi 2002). Therefore, T. caelestialium should have mated by the 4th day after eclosion. Mating tests indicated that females of S. rubrovittatus started mating with males at 26 h after eclosion and that all females had mated 5 days after eclosion in mating tests (Okutani-Akamatsu et al. 2009). Therefore, almost all females used in this study were considered to have been mated.
Plants
The following 24 poaceous plant species were used in this study (in the order of time of spikes emergence from spring to autumn): Poa annua L., Alopecurus aequalis Sobol. var. amurensis (Komar.) Ohwi, Anthoxanthum odoratum L., Poa sphondylodes Trin., Hordeum murinum L., Dactylis glomerata L., Festuca arundinacea Schreb., Vulpia myuros (L.) C. C. Gmel., Agrostis clavata Trin. subsp. matsumurae (Hack. ex Honda) T. Tateoka, Lolium multiflorum Lam., Elymus tsukushiensis Honda var. transiens (Hack.) Osada, Agrostis gigantea Roth, Digitaria ciliaris (Retz.) Koel., Eragrostis multicaulis Steud., Setaria faberi Herrm., Echinochloa crus-galli (L.) Beauv., Eleusine indica (L.) Gaertn., Echinochloa crus-galli (L.) Beauv. var. aristata S. F. Gray, Oryza sativa L., Panicum dichotomiflorum Michx., Setaria glauca (L.) Beauv., Eragrostis ferruginea (Thunb.) Beauv., Digitaria violascens Link and Pennisetum alopecuroides (L.) Spreng (Table 1). All plants, except for the rice O. sativa (cultivated in the paddy fields at Hokuriku Research Center), were found growing wild in or around the experimental fields. Because eggs could be laid on these plants by wild females, the collected plants were placed in conical flasks for 2 days at 25 °C before the oviposition tests to distinguish between nymphs derived from eggs laid by test females and those from eggs laid by wild females.
No-choice oviposition test
Twenty-four poaceous plants were collected from the field when the flowering spikes appeared. Sections of stem with 6–7 leaf sheaths and 2–3 spikes were placed in glass sample tubes (10 ml) filled with water. The plant and sample tube were then placed into a cylindrical plastic container (8 cm in diameter × 30 cm in height) with two mesh-covered openings (8 cm in diameter) at the top and around the side. Five adult females were released into the plastic container and then removed after 48 h. Twelve replications of oviposition tests were conducted for each plant at 25 °C, under 16L:8D light conditions. After removing the females, the plants were divided into the stem and the spikes. These plant parts were placed on moist filter paper in a plastic petri dish at 25 °C. At 25 °C, nymphs of T. caelestialium and S. rubrovittatus are known to emerge at 7–9 and 8–10 days after oviposition, respectively (Nagasawa 2007). Therefore, nymphs that emerged from the plants within 5 days (T. caelestialium) and 6 days (S. rubrovittatus) after the test were considered to be derived from the wild population and were not counted. Trigonotylus caelestialium and S. rubrovittatus nymphs that emerged from the plant parts at 6–9 and 7–10 days, respectively, were counted.
Multiple-choice oviposition tests
Ten plant species, which grow in the experimental field at Hokuriku Research Center, were used for multiple-choice oviposition tests. Since the growing seasons of the plants differed among species, the tests were conducted during three different seasons (i.e., spring, summer and autumn). Poa annua, A. aequalis and V. myuros were tested in spring (May–June); D. ciliaris, E. indica, S. faberi and E. crus-galli were tested in summer (August–September); and D. ciliaris, E. indica, E. crus-galli var. aristata, P. dichotomiflorum and S. glauca were tested in autumn (October). These plants were placed in sample tubes filled with water, which were then placed in a plastic rearing cage (34 × 25 × 34 cm). Ten adult females were released into the rearing cage and then removed after 24 h. Sixteen replications of oviposition tests were conducted for each seasonal test at 25 °C, under 16L:8D light conditions. Test plants were divided into the stem and the spike. These plant parts were placed on moist filter paper in a plastic petri dish at 25 °C. Trigonotylus caelestialium and S. rubrovittatus nymphs that emerged 7–9 and 8–10 days after the test, respectively, were counted.
Ovipositional site preference in spikelets
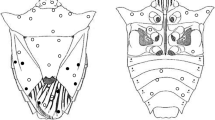
To identify where the insects deposited their eggs in the spikelets of poaceous plants, 8–10 spikelets from D. ciliaris and 4–5 spikelets from P. annua were placed on moist filter paper in a petri dish (5.5 cm in diameter, 1.5 cm in height). An adult female was released into the petri dish and allowed to oviposit for 6 h. After removing the female, the spikelets were dissected to identify the location of the eggs. Ten females were used for each plant in this test. The structure of the spikelet in D. ciliaris and P. annua is shown in Fig. 1.
Results
No-choice oviposition test
Relatively large numbers of T. caelestialium nymphs emerged from L. multiflorum, D. violascens and H. murinum. Relatively small numbers of T. caelestialium nymphs emerged from F. arundinacea and D. glomerata. However, no significant differences were observed among most plants (Tukey’s HSD test after root square transformation, P > 0.05; Fig. 2). Relatively large numbers of S. rubrovittatus nymphs emerged from P. annua, A. odoratum L., A. aequalis and D. violascens (Fig. 3). In particular, more S. rubrovittatus nymphs emerged from P. annua than from the other plants, except A. aequalis, A. odoratum and D. violascens (Tukey’s HSD test after root square transformation, P < 0.05). In contrast, relatively small numbers of S. rubrovittatus nymphs emerged from O. sativa, S. faberi and S. glauca (Fig. 3). In particular, fewer S. rubrovittatus nymphs emerged from O. sativa than from the other plants, except S. faberi and S. glauca (Tukey’s HSD test after root square transformation, P < 0.05). Nymphs of T. caelestialium emerged from eggs deposited in both the spikelets and the leaf sheaths, whereas S. rubrovittatus nymphs emerged almost exclusively from eggs deposited in the spikelets (Figs. 2, 3). Only a small number of S. rubrovittatus nymphs emerged from the leaf sheaths of P. annua, A. aequalis, H. murinum, V. myuros, D. ciliaris, E. multicaulis and P. alopecuroides. The relative proportions of T. caelestialium nymphs emerging from spikelets and leaf sheaths varied among plant species. The nymphs emerged from just the leaf sheaths of H. murinum, E. tsukushiensis var. transiens, O. sativa and S. glauca; however, nymphs emerged from just the spikelets of E. indica. More nymphs emerged from the leaf sheaths than from the spikelets of A. aequalis, F. arundinacea, V. myuros, A. clavata subsp. matsumurae, A. gigantea, S. faberi, E. crus-galli, E. crus-galli var. aristata and P. alopecuroides; however, more nymphs emerged from the spikelets than from the leaf sheaths of P. sphondylodes, D. ciliaris, P. dichotomiflorum and S. faberi (Wilcoxon’s signed rank test, P < 0.05). The number of emerging nymphs was not significantly different between the spikelets and leaf sheaths of P. annua, A. odoratum, L. multiflorum, E. multicaulis and E. ferruginea (Wilcoxon’s signed rank test, P > 0.05).
No-choice oviposition tests for Trigonotylus caelestialium. Adult females were allowed to oviposit on each of 24 poaceous plants for 48 h (12 replications for each plant). Oviposition preference was evaluated from the number of emerging nymphs (not from the number of eggs). Black bars with white vertical lines indicate the mean and standard error of the number of nymphs emerging from spikelets (mean − SE). White bars with lower vertical lines indicate the mean and standard error of the number of nymphs emerging from leaf sheaths (mean − SE). Upper vertical lines on bars indicate the standard error of the mean number of nymphs emerging from whole plants. Significant differences in the means for spikelets and leaf sheaths are indicated as *P < 0.05, **P < 0.01 (Wilcoxon’s signed rank test). Means of emerging nymphs from whole plants with the same alphabetical letters are not significantly different (Tukey’s HSD test after root square transformation, P > 0.05)
No-choice oviposition tests for Stenotus rubrovittatus. Adult females were allowed to oviposit on each of 24 poaceous plants for 48 h (12 replications for each plant). Oviposition preference was evaluated from the number of emerging nymphs (not from the number of eggs). Black bars with white vertical lines indicate the mean and standard error of the number of nymphs emerging from spikelets (mean−SE). White bars with lower vertical lines indicate the mean and standard error of the number of nymphs emerging from leaf sheaths (mean − SE). Upper vertical lines on bars indicate the standard error of the mean number of nymphs emerging from whole plants. Means of emerging nymphs from whole plants with same alphabetical letters are not significantly different (Tukey’s HSD test after root square transformation, P > 0.05)
Multiple-choice oviposition test
Among the spring plants, more T. caelestialium nymphs emerged from P. annua than A. aequalis (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P < 0.05; Fig. 4). In contrast, more nymphs of S. rubrovittatus emerged from A. aequalis than from Vulpia myuros (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P < 0.05; Fig. 5). Among the summer plants, the most nymphs of T. caelestialium emerged from E. indica, followed by D. ciliaris. Fewer nymphs of T. caelestialium emerged from E. crus-galli and S. faberi than from the other plants (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P < 0.05; Fig. 4). More nymphs of S. rubrovittatus emerged from E. indica and D. ciliaris than from S. faberi and E. crus-galli (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P < 0.05; Fig. 5). Among the autumn plants, the largest number of T. caelestialium nymphs emerged from E. indica followed by D. ciliaris, E. crus-galli var. aristata and P. dichotomiflorum. The lowest number of T. caelestialium nymphs emerged from S. glauca (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P < 0.05; Fig. 4). More nymphs of S. rubrovittatus emerged from D. ciliaris than from E. crus-galli var. aristata, P. dichotomiflorum and S. glauca (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P < 0.05; Fig. 5).
Multiple-choice oviposition preference tests for Trigonotylus caelestialium. Ten adult females were allowed to oviposit on different poaceous plants for 24 h (16 replications for each test). The tests were conducted during three different seasons, i.e., spring (May–June), summer (August–September) and autumn (October). Black bars with white vertical lines indicate the mean and standard error of the number of nymphs emerging from spikelets (mean−SE). White bars with lower vertical lines indicate the mean and standard error of the number of nymphs emerging from leaf sheaths (mean−SE). Upper vertical lines on bars indicate the standard error of the number of nymphs emerging from whole plants. Means followed by the same letter are not significantly different (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P > 0.05)
Multiple-choice oviposition preference tests for Stenotus rubrovittatus. Ten adult females were allowed to oviposit on different poaceous plants for 24 h (16 replications for each test). The tests were conducted during three different seasons, i.e., spring (May–June), summer (August–September) and autumn (October). Black bars with white vertical lines indicate the mean and standard error of the number of nymphs emerging from spikelets (mean−SE). White bars with lower vertical lines indicate the mean and standard error of the number of nymphs emerging from leaf sheaths (mean−SE). Upper vertical lines on bars indicate the standard error of the mean number of nymphs emerging from whole plants. Means followed by the same letter are not significantly different (Wilcoxon signed rank test with Holm’s correction for multiple comparisons, P > 0.05)
Ovipositional site preference in spikelets
Trigonotylus caelestialium deposited all eggs between the lemma of the first floret and the second floret of D. ciliaris. Stenotus rubrovittatus deposited most eggs inside the second floret (between the lemma and the palea) of D. ciliaris. In contrast, T. caelestialium deposited all eggs inside the florets of P. annua. Although S. rubrovittatus deposited more eggs inside the florets of P. annua, individuals also deposited some eggs between the florets (Table 2). Egg location was significantly different between T. caelestialium and S. rubrovittatus on both D. ciliaris and P. annua (Fisher’s exact test, P < 0.001).
Discussion
It has been previously reported that T. caelestialium deposits eggs into the tight space between the stem and the leaf sheath of poaceous plants, whereas S. rubrovittatus oviposits into the spikelet (Hayashi 1986; Kato and Hasegawa 1950; Okuyama and Inoue 1975). Moreover, the emergence of nymphs from poaceous plants collected in the field indicated that T. caelestialium oviposits into both the leaf sheath and the spikelet, whereas S. rubrovittatus oviposits in only the spikelet (Nagasawa 2007). In this study, the rice bugs were given the opportunity to oviposit on 24 poaceous plant species. Almost all S. rubrovittatus nymphs emerged from the spikelets of all the plants. This observation indicates that S. rubrovittatus oviposits in spikelets only. However, T. caelestialium emerged from both the foliage and spikes of most plants. This observation indicates that T. caelestialium oviposits into both the leaf sheath and the spikelet. This study confirmed that this species definitely oviposits in the leaf sheaths because oviposition was directly observed. However, since eggs were not directly observed inside the spikelets (as this requires considerable effort), one reason for nymphs not emerging from some spikelets is that some eggs did not hatch. However, since the emergence of nymphs from the spikelets was observed in some plants, both T. caelestialium and S. rubrovittatus are clearly able to oviposit in the spikelets. In addition, our investigation using P. annua and D. ciliaris showed that both rice bugs are able to oviposit in the tight space inside the spikelet. Mirid bugs, like Macrolophus caliginosus and Lygus rugulipennis, oviposit by drilling into the spaces of plant tissues (Ferran et al. 1996; Romani et al. 2005). In contrast, T. caelestialium and S. rubrovittatus oviposit into the natural narrow spaces of plant tissues, such as the leaf sheaths or spikelets. Thus, the presence of a suitable space for oviposition is considered as a factor that affects oviposition site selection in both rice bug species. Although both T. caelestialium and S. rubrovittatus oviposited in the spikelet, the exact sites of oviposition slightly differed. For instance, T. caelestialium deposited eggs between the lemma of first floret (sterile) and the palea side of the second floret (fertile) of D. ciliaris, whereas S. rubrovittatus deposited eggs inside the second floret (between the lemma and the palea). Furthermore, T. caelestialium deposited all eggs inside the florets of P. annua, whereas S. rubrovittatus deposited eggs between adjacent florets and inside florets. These differences may indicate that oviposition cues are different for T. caelestialium and S. rubrovittatus. It is not clear whether this difference is attributable to the structure of the spikelet or chemical components. However, these factors might contribute to the difference in selection of leaf sheaths for oviposition between T. caelestialium and S. rubrovittatus.
In the no-choice tests, many T. caelestialium nymphs emerged from A. aequalis, A. odoratum, L. multiflorum and D. violascens. These plants are suitable for oviposition by T. caelestialium. In contrast, only a few T. caelestialium nymphs emerged from D. glomerata and F. arundinacea. These plants may be unsuitable for oviposition by T. caelestialium. However, only a small difference in the number of T. caelestialium nymphs that emerged from different plants was observed. In comparison, many S. rubrovittatus nymphs emerged from P. annua, A. aequalis, A. odoratum and D. violascens. These plants are therefore suitable for oviposition by S. rubrovittatus. In contrast, only a few S. rubrovittatus nymphs emerged from O. sativa, S. faberi and S. glauca. These plants may be unsuitable for oviposition by S. rubrovittatus. Compared to T. caelestialium, a greater difference in the number of S. rubrovittatus nymphs that emerged from different plants was observed. For O. sativa, S. faberi and S. glauca, it should be noted that T. caelestialium deposited a few eggs into the spikelets; however, many eggs were deposited into the leaf sheaths. Since T. caelestialium oviposits into both the leaf sheath and the spikelet, this species might preferentially select the leaf sheath when the spikelets are not suitable for oviposition. In comparison, since S. rubrovittatus only oviposits into the spikelets, the number of eggs deposited on a given plant might decrease because of the absence of an alternative oviposition site when spikelets are not suitable for oviposition. Differences in the choice range of sites between T. caelestialium, which can oviposit on both spikelets and leaf sheaths, and S. rubrovittatus, which only oviposits on spikelets, may lead to differences in suitable plant ranges for oviposition.
Because the no-choice oviposition tests were only conducted for 48 h, the number of emerging nymphs was probably affected by both the oviposition site selection and egg production derived from plant nutrition. In addition to these factors, we cannot exclude the possibility that the number of emerging nymphs was affected by other factors, such as differences in hatching rates. Therefore, our results may not exclusively reflect the oviposition preference of these bugs. However, the results of this study are important toward evaluating the potential of each plant as hosts for bugs that cause pecky rice. The plants from which more nymphs emerged should be recognized as the sources of these bugs. These results support previous studies that identified P. annua, D. ciliaris and L. multiflorum as major sources of these two bugs (Hachiya 1999; Hayashi 1986; Kakizaki 2004). While the two mirid bugs laid many eggs on Italian ryegrass, L. multiflorum, they laid only a few eggs on orchard grass, D. glomerata, and tall fescue, F. arundinacea. Therefore, differences in the characteristics of pasture grasses may contain important information for controlling the occurrence of these mirid bugs.
In this study, the multiple-choice tests were conducted for 24 h. However, it is unclear how nutrient intake affects the length of egg production by the two rice bug species. It is likely that the fecundity of individual bugs is at the same level during the 24-h test because a decrease in the number of deposited eggs only occurred after 24 h in a continuous oviposition test in which plants were examined every 24 h (Nagasawa, unpublished data). Moreover, there was no drastic difference in egg numbers in the sampling survey. Therefore, the results of the multiple-choice tests provide a reliable indication of the oviposition preferences of these bugs. Trigonotylus caelestialium preferred P. annua in spring and E. indica in summer and autumn. Stenotus rubrovittatus preferred A. aequalis in spring and D. ciliaris and E. indica in summer and autumn. These mirid bugs showed different oviposition preferences among Poaceae plants. However, the factor that influences the oviposition preference of these bugs remains unclear; hence, it is important to investigate the correlation between oviposition preference and nymphal growth, to study host selection of phytophagous insects and to identify the host plants on which these mirid bugs develop.
The existence of grass spikes is essential for the survival of S. rubrovittatus and for improving the potential for T. caelestialium nymphal growth (Nagasawa and Higuchi, unpublished data). Thus, few nymphs of S. rubrovittatus occur in paddy fields, especially before the heading time, because of their low oviposition preference for rice, in addition to the poor growth of nymphs of this species on rice. Moreover, oviposition preference for rice remains low after the heading time. These results indicate that generally S. rubrovittatus should rarely occur in paddy fields. However, if grass weeds, such as a barnyard grass, grow in a paddy field, these weeds may act as hosts for S. rubrovittatus. Emerging nymphs of S. rubrovittatus from the paddy weeds would damage rice in a similar way to T. caelestialium nymphs, which are considered to be a major cause of pecky rice (Ishimoto 2004; Ishimoto and Nagase 2005). In contrast, larger populations of T. caelestialium are likely to be found in paddy fields because T. caelestialium is able to oviposit sufficiently into the leaf sheaths of rice. Plants that have suitable oviposition sites, namely spikelets and leaf sheaths, are important for the occurrence of both rice bug species. Therefore, the closer these plants grow to paddy fields, the more attention they should receive by agricultural management practices, since it is predicted that the rice bugs that emerge from these plants directly cause pecky rice.
References
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman & Hall, New York
Ferran A, Rortais A, Malausa JC, Gambier J (1996) Ovipositional behaviour of Macrolophus caliginosus (Heteroptera: Miridae) on tobacco leaves. Bull Entomol Res 86:123–128
Hachiya K (1999) Migration of rice leaf bug, Trigonotylus caelestialium (Kirkaldy) into rice fields and forecasting of the abundance. Plant Prot 53:268–272 (in Japanese)
Hayashi H (1986) Ecology and control of the sorghum plant bug (Stenotus rubrovittatus Matsumura) causing the pecky rice. Plant Prot 40:321–326 (in Japanese)
Higuchi H, Takahashi A (2000) Method of rearing the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) with wheat seedlings. Proc Assoc Plant Prot Hokuriku 48:23–25 (in Japanese)
Higuchi H, Takahashi A (2002) Influence of physiological conditions on sex pheromone production by females of rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Proc Assoc Plant Prot Hokuriku 51:7–9 (in Japanese)
Higuchi H, Takahashi A, Mima J-i (2001) Grasses used as host plants on oviposition by rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae), in autumn. Proc Assoc Plant Prot Hokuriku 49:15–17 (in Japanese)
Iimura S (1992) Host plants of the sorghum plant bug, Stenotus rubrovittatus Matsumura (Hemiptera: Miridae). Tohoku Agric Res 45:101–102
Ishimoto M (2004) Seasonal prevalence of occurrence of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) on paddy rice plants. Jpn J Appl Entomol Zool 48:79–85 (in Japanese with English summary)
Ishimoto M (2008) Development of sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae) nymphs reared on hulled rice. Jpn J Appl Entomol Zool 52:139–141. doi:10.1303/jjaez.2008.139 (in Japanese with English summary)
Ishimoto M, Nagase A (2005) Proper application timing of insecticide to the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Proc Assoc Plant Prot Hokuriku 54:29–38 (in Japanese)
Kakizaki M (2004) Investigation on the occurrences of the sorghum plant bug, Stenotus rubrovittatus (Matsumura), in the gramineous forage grass fields of southern Hokkaido in 2003. Annu Rep Plant Prot North Japan 55:110–112 (in Japanese with English summary)
Kato S, Hasegawa H (1950) Calocoris rubrovittata Mats., a new pest of Sorghum sudanense. Oyo-Kontyu 6:149 (in Japanese)
Kikuchi A, Kobayashi T (2001) Effect of weed mowing on the abundance of Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Annu Rep Plant Prot North Japan 52:143–145 (in Japanese)
Kikuchi A, Kobayashi T (2004) Growth and oviposition of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae), on gramineous weeds and Italian rye grass. Annu Rep Plant Prot North Japan 55:149–154 (in Japanese)
Nagasawa A (2007) Gramineous plants used as oviposition sites by pecky rice bugs, Trigonotylus caelestialium (Kirkaldy) and Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae). Proc Assoc Plant Prot Hokuriku 56:29–31 (in Japanese)
Nagasawa A, Higuchi H (2008) A method for rearing Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae) by using the spikelets of gramineous weeds as oviposition sites and wheat seedlings for rearing nymphs. Jpn J Appl Entomol Zool 52:1–6. doi:10.1303/jjaez.2008.1 (in Japanese with English summary)
Nagasawa A, Higuchi H (2010) Storage of stem cuttings of wheat seedlings under high-humidity conditions to improve larval emergence after oviposition by Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae). Jpn J Appl Entomol Zool 54:197–203. doi:101.1303/jjaez.2010.197 (in Japanese with English summary)
Niiyama T, Itoyama K (2006) Control of rice leaf bug, Trigonotylus caelestialium (Heteroptera: Miridae) by application of herbicide to their source of immigration. Annu Rep Plant Prot North Japan 57:129–133 (in Japanese)
Okutani-Akamatsu Y, Watanabe T, Azuma M (2009) Mating behavior and oviposition of the sorghum plant bug, Stenotus rubrovittatus (Matsumura), (Heteroptera: Miridae) under laboratory conditions. Jpn J Appl Entomol Zool 53:13–20
Okuyama S, Inoue H (1975) Effect of temperature and humidity on the oviposition and the development of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy). Bull Hokkaido Prefect Agric Exp Stn 32:45–52 (in Japanese)
Ono T, Kashin J, Kidokoro T (2007) Control of sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae), in rice paddy field by grass mowing in relation to migration source. Annu Rep Plant Prot North Japan 58:75–79 (in Japanese)
Romani R, Salerno G, Frati F, Conti E, Isidoro N, Bin F (2005) Oviposition behaviour in Lygus rugulipennis: a morpho-functional study. Entomol Exp Appl 115:17–25
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology. Chapman & Hall, London
Takahashi A, Higuchi H (2001) Effect of temperature on the development of rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Proc Assoc Plant Prot Hokuriku 49:19–22 (in Japanese)
Watanabe T, Higuchi H (2006) Recent occurrence and problem of rice bugs. Plant Prot 60:201–203 (in Japanese)
Wennström A, Hjulström LN, Hjältén J, Julkunen-Tiitto R (2010) Mother really knows best: host choice of adult phytophagous insect females reflects a within-host variation in suitability as larval food. Chemoecology 20:35–42. doi:10.1007/s00049-010-0040-8
Yokota H, Suzuki T (2008) Most effective timing of mowing on the population density of the nymphs of the overwintering generation of Stenotus rubrovittatus in the footpath between paddy fields. Annu Rep Plant Prot North Japan 59:116–119 (in Japanese)
Acknowledgments
We thank Dr. Ishimoto of Niigata Agricultural Research Institute for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagasawa, A., Takahashi, A. & Higuchi, H. Host plant use for oviposition by Trigonotylus caelestialium (Hemiptera: Miridae) and Stenotus rubrovittatus (Hemiptera: Miridae). Appl Entomol Zool 47, 331–339 (2012). https://doi.org/10.1007/s13355-012-0123-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-012-0123-9