Abstract
Sugarcane white leaf disease is a serious problem in many Asian Countries. The leafhoppers Matsumuratettix hiroglyphicus (Matsumura) and Yamatotettix flavovittatus Matsumura are the main vectors of sugarcane white leaf disease phytoplasma. Gaining a better understanding of the dispersal behavior of such insect vectors is essential for both disease epidemiology and vector control. The dispersal distances of M. hiroglyphicus and Y. flavovittatus in a sugarcane field were estimated by means of mark–release–recapture experiments. Adult leafhopper vectors collected from the fields were marked using fluorescent dye powder and released at a central release point in a sugarcane field. The marking method did not have a significant effect on the survival or flight activity of the leafhopper vectors. The overall release–recapture rates of M. hiroglyphicus and Y. flavovittatus within 50 m were 10.1 and 13.4 %, respectively. The estimated natural mean dispersal distances for M. hiroglyphicus and Y. flavovittatus were 162.1 and 387.5 m, respectively. Wind appears to be the main factor influencing the leafhopper dispersal direction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane white leaf (SCWL) is the most destructive sugarcane disease known in Asia, especially in Thailand, Taiwan, Japan, and Sri Lanka (Ling 1962; Nakashima and Murata 1993; Kumarasinghe and Jones 2001; Nakashima et al. 2001). Recently, it was reported that SCWL has spread into Lao PDR and Vietnam (T. Maleerat and T.X. Hoat, pers. commun., December 2, 2011). The disease is caused by plant-pathogenic phytoplasma, and is naturally transmitted by the leafhoppers Matsumuratettix hiroglyphicus (Matsumura) and Yamatotettix flavovittatus Matsumura (Homoptera: Cicadellidae) (Matsumoto et al. 1968; Chen 1974; Hanboonsong et al. 2002, 2006). These leafhoppers are key vectors for spreading the SCWL phytoplasma to healthy plants, and it has been reported that the transmission efficiency of M. hiroglyphicus (55 %) is higher than that of Y. flavovittatus (45 %) (Hanboonsong et al. 2006). These vectors were found in fields during different peak periods, from mid-June to the end of July in the case of M. hiroglyphicus and from mid-July to the beginning of September in the case of Y. flavovittatus (Phisitkul et al. 1989; Hanboonsong et al. 2006). SCWL phytoplasma still cannot be cultured, and there are no effective methods of controlling this disease. It is already known that the insect vector M. hiroglyphicus is one of the pathogen reservoirs (Hanboonsong et al. 2002), but the other host plant’s pathogen reservoir has not yet been identified. Therefore, in the study described in the present paper, we investigated insect vector dispersal. Such studies are important not only for disease epidemiology but also for developing strategies to prevent the spread of disease caused by vector dispersal, which will contribute to disease management. This is the first report on the dispersal behavior of M. hiroglyphicus and Y. flavovittatus in sugarcane fields; however, several studies have reported that mark–release–recapture (MRR) experiments are an effective means of studying the dispersal behavior of leafhoppers (Larsen and Whalon 1988; Whitney and Meyer 1988; Zhou et al. 2003; Blackmer et al. 2006). Therefore, as it is important to quantify the spread of SCWL disease from infected fields through the transmission of insect vectors, the objective of this study was to determine the dispersal distances of M. hiroglyphicus and Y. flavovittatus from a central release point in a sugarcane field using the MRR method.
Materials and methods
Leafhopper capture and marking
The leafhoppers were collected for marking and release from the fields of high-population areas in the districts of Kumphawapi and Non Sa-at, Udon Thani Province, in northeastern Thailand. The collections were performed in the evening during the peak periods of M. hiroglyphicus (June–July) and Y. flavovittatus (July–September) using light traps to attract the insects and insect aspirators to catch the insects. Micronized (3–4 μm particle size) fluorescent dye powder in four colors—pinkish (FZ-2803), purple pink (FZ-2817), green (FZ-2802), and blue (FZ-2808) (Sinloihi Co. Ltd., Kamakura, Japan)—was used for insect marking in this study (Nakata 2008), because it is the most effective means of marking insects that are small in size and large in abundance (Darling 1925). Leafhoppers were aspirated, in groups of 50, into transparent plastic tubes (12 cm in length, 3 cm in diameter) containing 20 mg of fluorescent dye powder for marking. The tubes were then shaken gently by hand for 20 s to coat the leafhoppers with the powder. Different colors of fluorescent dye powder were used to signify different release dates and leafhopper species. The marked leafhoppers were immediately transferred from the plastic tubes to open petri dishes inside transparent plastic mass-rearing cages (80 cm in height, 25 cm in diameter) containing two-month-old healthy sugarcane. Those leafhoppers that flew away from the petri dishes inside the cages were counted as released.
Effects of marking on leafhopper survival and flight activity
Our dispersal studies required the development of an effective marking method that did not affect the survival or flight activity of the SCWL leafhopper vectors. Therefore, we tested the effects of the fluorescent dye powder marking method on leafhopper survival and flight activity under greenhouse conditions. Five sets of 20 adult leafhoppers each of M. hiroglyphicus and Y. flavovittatus were treated with one of the four colors of fluorescent dye powder, or were unmarked (used as controls). The experiment utilized a randomized block design replicated in four cages for each species. The marked leafhoppers were released into cages (70 cm in height, 20 cm in diameter) containing two-month-old sugarcane. After the release of the marked leafhoppers, the cages were placed in a greenhouse with no temperature or light-intensity control. To test the effects of the marking method on leafhopper survival, the living treated leafhoppers in each cage were counted daily (with the dead being removed) for 20 days following release.
To study the effects of the marking method on leafhopper flight activity, five sets of 50 adult leafhoppers each of M. hiroglyphicus and Y. flavovittatus were treated with one of the four colors of fluorescent dye powder, or were unmarked (used as controls). The treated leafhoppers were released together onto two-month-old sugarcane plants in a cage (2 × 1 × 1 m) covered with a nylon screen. Two yellow and two blue sticky traps made from plastic boards (12.5 × 25 cm) were placed in the cage, and trapped leafhoppers were counted daily for 20 days following release (with three replications).
Field experiments using the mark–release–recapture method
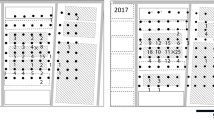
The release–recapture field site was located at Tha Phra, Khon Kaen Province, in northeastern Thailand. The field was 3 ha in size, and contained the Khon Kaen 3 variety of sugarcane, Saccharum officinarum L. (1 m in height). The field was bordered on the east by eucalypt trees, on the west by fallow land, and on the south and north by sugarcane fields. The pattern that sticky recapture traps were placed on the field was modified from Larsen and Whalon (1988). At the release–recapture field site, yellow and blue colored sticky traps made from plastic boards (25 × 40 cm) were fixed on bamboo sticks and placed beside each other at each location, 1.5 m above the ground, at distances of 5, 10, 20, 30, and 50 m, in six directions radiating from the release point (Fig. 1). We used two color sticky traps in this experiment because blue and yellow color sticky traps showed the same efficiency for M. hiroglyphicus, while the blue color sticky trap showed the best efficiency at catching Y. flavovittatus, followed by the yellow color sticky trap (Thein et al. 2011). The plastic boards were wrapped in transparent plastic sheets, and a colorless insect-trapping adhesive (Beetle Glue, Greenplana Co. Ltd., Thailand) was applied onto both surfaces of each board. A total of 60 traps were placed in the field.
Layout of the sticky traps used to recapture marked leafhoppers, M. hiroglyphicus and Y. flavovittatus, from the central release point in the sugarcane field (modified from Larsen and Whalon 1988)
Three releases of marked adult M. hiroglyphicus leafhoppers (during which 1,980, 1,200, and 800 leafhoppers were released at the central release point in the field, respectively; Fig. 1) were performed on different dates in July and August, while two releases of marked adult Y. flavovittatus leafhoppers (during which 2,700 and 2,100 leafhoppers were released, respectively) were performed on different dates in August (Table 2). Different colors of fluorescent dye powder were used to indicate the respective release dates and leafhopper species. All sticky traps were monitored and replaced at two-day intervals until 20 days after release in all of the experiments. Trapped leafhoppers were examined under a microscope to determine their markings. Weather data were obtained from the weather station at Tha Phra, Khon Kaen Province (see the “Appendix”). The data reflecting the effects of fluorescent dye powder marking on the insect vectors’ survival and flight activity were subjected to analysis of variance (one-way ANOVA); and comparison of means was performed using the Tukey–Kramer honestly significant difference (HSD) test. All of the analysis was performed using the JMP 9 statistical software package (SAS Institute 2010).
Estimation of natural dispersal distance
We used the dispersal distance estimation method of Yamamura et al. (2003). First, we calculated the expected number of settled individuals at a distance f(r), assuming that the traveling individuals settle at a position at a rate of λ:
where n 0 is the number of released insects, K 0(·) is a modified Bessel function of the second kind and of order zero; and λ D is λ/D, where D is the diffusion coefficient. λ D is the rate of settlement scaled by the diffusion coefficient.
Then we calculated the expected number of individuals captured by the i-th trap placed at a distance r i as
where c is a constant.
Finally, we calculated the estimated mean dispersal distance of the individuals captured by traps as
where a hat (^) indicates the corresponding estimate.
The standard error (SE) is estimated as
where \( {\text{SE}}\left( {\hat{\lambda }_{\text{D}} } \right) \) indicates the SE of \( \hat{\lambda }_{\text{D}} \) (Seber 1982).
The Microsoft Excel spreadsheet for this estimation is available at http://cse.niaes.affrc.go.jp/yamamura/Yamamura_et_al_2003_estimation.xls.
Results
Effects of the marking method on survival and flight activity
The mean days of survival and days to capture for all treatments (including the control), for both M. hiroglyphicus and Y. flavovittatus, are shown in Table 1. The mean survival duration for M. hiroglyphicus was 11–13 days, and that for Y. flavovittatus was 18–20 days. The survival duration of the leafhoppers marked with the four fluorescent dye colors was not significantly different from that for the unmarked (control) leafhoppers, whether M. hiroglyphicus (one-way ANOVA: F = 1.15, df = 4, 12, P > 0.05) or Y. flavovittatus (one-way ANOVA: F = 3.1, df = 4, 12, P > 0.05). The mean days until capture on the yellow and the blue sticky-board traps as a measure of flight activity was found to be 2–3 days for both M. hiroglyphicus and Y. flavovittatus, which was also not significantly different between the marked and unmarked leafhoppers for either M. hiroglyphicus (one-way ANOVA: F = 0.46, df = 4, 8, P > 0.05) or Y. flavovittatus (one way ANOVA: F = 1.13, df = 4, 8, P > 0.05). Moreover, markings of different fluorescent dye colors were observed on the thoraces or between the heads and thoraces of leafhopper vectors throughout the experimental period of 20 days.
Trap-catch patterns for the mark–release–recapture experiments
A total of 3,980 adult M. hiroglyphicus and 4,800 adult Y. flavovittatus leafhoppers were marked, released, and recaptured between July and September 2011. The recapture rate of marked M. hiroglyphicus leafhoppers was found to range from 5.8 to 12.4 %, and the overall release–recapture rate was 10.1 %. The recapture rate of marked Y. flavovittatus was found to range from 12.3 to 14.2 %, and the overall release–recapture rate was 13.4 % (Table 2). The trap-catch number of individual marked leafhoppers, for both M. hiroglyphicus and Y. flavovittatus, was highest to the southwest (240° from north), followed by to the south (180° from north) and the southeast (120° from north), and lowest to the northeast (60° from north) (Fig. 2).
Total number of marked leafhoppers that were recaptured during 20 days in each direction after being released at the center of release point, for all releases combined: a M. hiroglyphicus, b Y. flavovittatus. The numbers inside the circles indicate the total number of marked leafhoppers recaptured, for all releases combined
The number of marked M. hiroglyphicus and Y. flavovittatus leafhoppers recaptured over the 20 days after release decreased exponentially following their release (Fig. 3). The number of M. hiroglyphicus leafhoppers recaptured (at two-day intervals, for three releases) was 145, 90, 61, 49, 39, 14, and 4, at 2, 4, 6, 8, 10, 12, and 14 days, respectively. No M. hiroglyphicus individuals were recaptured as of 12 days after the third release. The Y. flavovittatus leafhoppers were recaptured (in two releases) at two-day intervals for 18 days after release; 203, 148, 100, 67, 48, 39, 24, 9, and 3 were recaptured at 2, 4, 6, 8, 10, 12, 14, 16, and 18 days, respectively. The number of recaptured vectors decreased as the number of observation days increased. Simple mean dispersal distances of M. hiroglyphicus and Y. flavovittatus were determined by dividing the distance recaptured from the release point (m) by the days of observation. M. hiroglyphicus moved 3.56, 4.44, and 4.77 m per day during the first, second, and third releases, and the mean distance for all releases combined was found to be 4.02 m per day. Y. flavovittatus moved 3.56 and 3.61 m per day during the first and second releases and 3.87 m per day for the two releases combined (Table 3).
Number of marked leafhoppers recaptured at each two-day interval for 20 days after release: a M. hiroglyphicus, b Y. flavovittatus (solid circles first release, solid squares second release, solid triangles third release). The curves were generated by the least squares method, assuming log approximation, combining all of the releases
Estimation of mean dispersal distances
The average dispersal distance was estimated by combining the results for the three releases of M. hiroglyphicus and the two releases of Y. flavovittatus, respectively (Table 3). The total numbers of recaptured M. hiroglyphicus and Y. flavovittatus individuals, in all 60 traps (at 5, 10, 20, 30, and 50 m) over 20 days, were 402 and 641, respectively (Table 2). We used this cumulative number of recaptured leafhoppers to predict the cumulative dispersal distance. The maximum likelihood estimate (±SE) of the rate of settlement of leafhoppers scaled by the diffusion coefficient (obtained using Eq. 2) was \( \hat{\lambda }_{\text{D}} = 9. 4\times 10^{ - 5} \left( { \pm 5. 5\times 10^{ - 5} } \right) \) for M. hiroglyphicus, and \( \hat{\lambda }_{\text{D}} = 1. 6\times 10^{ - 5} \left( { \pm 1. 3\times 10^{ - 5} } \right) \) for Y. flavovittatus. The estimated mean dispersal distance (±SE), using Eqs. 3 and 4, was 162.1 (±47.2) m for M. hiroglyphicus and 387.5 (±156.6) m for Y. flavovittatus. The estimated natural mean dispersal distance was found to be less in the case of M. hiroglyphicus than in that of Y. flavovittatus, and therefore the rate of trapped individuals of M. hiroglyphicus, scaled by the diffusion coefficient, was higher than that of Y. flavovittatus.
Discussion
The present study revealed that the marking method using fluorescent dye powder had no significant effect on the survival or flight activity of leafhoppers under greenhouse conditions (Table 1). The marked insects were observed under a microscope, and the dye was found either around the cervix between the head and thorax or on the thorax. Among the four treatment colors of fluorescent dye, pinkish (FZ-2803) and purple-pink (FZ-2817) were found to be the most easily detectable for tracking leafhoppers. Though the marking method using fluorescent dye powder did not affect the leafhoppers’ survival and flight activity, the mean number of survival days of M. hiroglyphicus was found to be slightly less than that of Y. flavovittatus. This is not surprising, given their respective natural adult longevities. It has been reported in laboratory studies that the adult longevity of M. hiroglyphicus is shorter than that of Y. flavovittatus (Hanboonsong et al. 2002; Hanboonsong 2008). In our MRR experiments in the field, marked Y. flavovittatus were recaptured over a period of 18 days after release, but M. hiroglyphicus were recaptured over 14 days in the case of the first and second releases, and over only ten days in the case of the third release. A high mortality would appear to have occurred in the third release experiment, resulting in the low recapture rate (Table 2). This could be attributable to factors such as unsuitable habitat, or abiotic factors, especially rainfall. Normally, these vectors enter fields during the rainy season of May–October, but the greatest abundance of M. hiroglyphicus was found to occur in June–July, and that of Y. flavovittatus occurred in July–August or at the beginning of September. The number of M. hiroglyphicus vectors decreased after July, but that of Y. flavovittatus decreased slightly through to October (Phisitkul et al. 1989; Hanboonsong et al. 2006). In our MRR experiments, the third release of the M. hiroglyphicus vector was done at the end of August, which may be a period of unstable habitat in the field, leading to changes in fitness for this species; while the greater catch numbers of Y. flavovittatus would suggest a relatively stable field habitat throughout the experiment. Moreover, we noticed that some rain days occurred during the experimental period of July–September (rainfall >20 mm for 8 days, >10 mm for 11 days; see the “Appendix”), and it appears that Y. flavovittatus is more tolerant of rain effects than M. hiroglyphicus. This could be due to the difference in body size (length) between M. hiroglyphicus (2.5–3.5 mm) and Y. flavovittatus (3.5–4.0 mm), respectively (unpublished data).
The overall recapture rates of marked leafhoppers, M. hiroglyphicus and Y. flavovittatus, in our MRR experiments in the sugarcane field, were 10.1 and 13.4 %, respectively. This was low in comparison with some other leafhopper dispersal studies using the mark–release–recapture method [for example, 17.3–43.7 % in a report on sharpnosed leafhopper on blueberry plants (Whitney and Meyer 1988), and 5.4–32.4 % in a report on the vector leafhopper of Pierce’s disease in an alfalfa field which had only a few scattered plants (Blackmer et al. 2004)]; but was higher than the 1.8–3.7 % rate reported by Larsen and Whalon (1988) when studying the vector leafhopper of X-disease in peach and cherry orchards (some of which were commercial orchards regularly treated with pesticides). The lower rate in our study might be explained by the fact that vector movement (and thus dispersal) in a sugarcane field is comparatively difficult in comparison to that among orchards or vegetable crops, owing to the obstruction caused by the broad leaf types found in sugarcane plantations. Zhou et al. (2003) indicated that different crop types influenced the dispersal and spatial distribution of the aster leafhopper, Macrosteles quadrilineatus. Moreover, Taylor (1985) noted that the variance in dispersal activity present within the Cicadellidae might depend on species behavior itself.
The wind direction throughout the experimental period of our study was predominantly towards the south (S), southeast (SE), and southwest (SW) (see the “Appendix”). The total number of recaptured leafhopper individuals of both species was highest in the area south of the release point (Fig. 2), a result which appears to have been influenced by the prevailing wind direction. The release date did not influence the direction of leafhopper dispersal. The simple mean dispersal distances of M. hiroglyphicus and Y. flavovittatus in all releases combined were found to be 4.02 and 3.87 m/day, respectively (Table 3). The rate of dispersal would suggest that both species of leafhoppers moved an average of 4.0 m per day, and it seems that they are not capable of moving faster than this speed. However, in our experiment in the field, some individuals of vector leafhopper species could not move again once they landed on the sticky traps, while some might move over 50 m outside the trapped area. Therefore, the results for simple mean dispersal distance per day may be underestimated. Moreover, the simple mean dispersal distance (m) per day of M. hiroglyphicus in the first release experiment was found to be less than those for the second and third releases, while Y. flavovittatus showed similar mean dispersal distances in both releases (Table 3). We speculate that the high variability of wind direction and speed that occurred during the first release experiment of M. hiroglyphicus inhibited the flight movement of this species (“Appendix”). Wind appears to be the main factor influencing leafhopper dispersal direction and distance. Larsen and Whalon (1988) and Kobori et al. (2011) each reported that wind was one of the major factors influencing the dispersal of the leafhopper Paraphlepsius irroratus (Say) as a vector of X-disease and citrus greening disease (Asian citrus psyllid, Diaphorina citri Kuwayama), respectively. On the other hand, our results showed that the estimated natural mean dispersal distances (±SE) of M. hiroglyphicus and Y. flavovittatus were 162.1 (±47.2) m and 387.5 (±156.6) m, respectively, using the Yamamura method (Yamamura et al. 2003). Our estimation assumed that each trap had the same efficiency, but this cannot be asserted with confidence; and if the trap efficiency varied, then the estimated SE is an underestimation (less than the actual distance). In addition, our estimation assumed that the vectors’ movement was isotropic, which meant that variations in wind speed and wind direction in the actual field were not factored into the analysis (see “Appendix”).
Despite these caveats, our findings would be useful not only when considering prevention techniques for SCWL disease transmitted by M. hiroglyphicus and Y. flavovittatus, but as a first step in developing spread-estimation models for SCWL disease. Based on our results, we may conjecture that the disease risk decreases with increasing distance from infected fields, and that the SCWL infection risk is higher on the leeward (downwind) side of an infected field than on the windward (upwind) side. Therefore, wind speed and direction would be important variables to consider in a dispersal model in future investigations. The results from this study indicate that the shorter-bodied M. hiroglyphicus can disperse further than the longer-bodied Y. flavovittatus by wind. However, the Yamamura method suggests that Y. flavovittatus is dispersed further than M. hiroglyphicus, although this result could be due to the higher variability in the Y. flavovittatus data. One important factor that requires future research is how the body shapes, sizes, and masses of these species influence their flight and dispersal abilities. Further studies of the vectors’ transmission abilities, intrinsic rates of natural increase, and movement patterns are also needed in order to enhance SCWL disease management.
References
Blackmer JL, Hagler JR, Simmons GS, Canas LA (2004) Comparative dispersal of Homalodisca coagulate and Homalodisca liturata (Homoptera: Cicadellidae). Environ Entomol 33:88–99. doi:10.1603/0046-225X-33.1.88
Blackmer JL, Hagler JR, Simmons GS, Henneberry TJ (2006) Dispersal of Homalodisca vitripennis (Homoptera: Cicadellidae) from a point release site in citrus. Environ Entomol 35(6):1617–1625
Chen CT (1974) Sugarcane white leaf disease in Thailand and Taiwan. Sugarcane Pathologists’ Newsl 11(12):23
Darling ST (1925) Entomological research in malaria. South Med J 18:446–449
Hanboonsong Y (2008) The studies of ecology, behavior, efficiency of disease transmission of new insect vector of sugarcane white leaf disease and potential of its control (Sugarcane Project Report). Khon Kaen University, Khon Kaen (in Thai)
Hanboonsong Y, Choosai C, Panyim S, Damak S (2002) Transovarial transmission of sugarcane white leaf phytoplasma in the insect vector Matsumuratettix hiroglyphicus (Matsumura). Insect Mol Biol 11(1):97–103
Hanboonsong Y, Ritthison W, Choosai C, Sirithorn P (2006) Transmission of sugarcane white leaf phytoplasma by Yamatotettix flavovittatus, a new leafhopper vector. J Econ Entomol 99(5):1531–1537
Kobori Y, Nakata T, Ohto Y, Takasu F (2011) Dispersal of adult asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae), the vector of citrus greening disease, in artificial release experiments. Appl Entomol Zool 46:27–30. doi:10.1007/s13355-010-0004-z
Kumarasinghe NC, Jones P (2001) Identification of white leaf disease of sugarcane in Sri Lanka. Sugar Tech 3(1&2):55–58
Larsen KJ, Whalon ME (1988) Dispersal of Paraphlepsius irroratus (Say) (Homoptera: Cicadellidae) in peach and cherry orchards. Environ Entomol 17(5):842–851
Ling KC (1962) White leaf disease of sugarcane. Taiwan Sugar 9:1–5
Matsumoto T, Lee CS, Teng WS (1968) Studies on sugarcane white leaf disease of Taiwan with special reference to transmission by a leafhopper, Epitettix hiroglyphicus. Mats Pro Soc Sugarcane Tech 13:1090–1099
Nakashima K, Murata N (1993) Destructive plant disease caused by micoplasma-like organisms in Asia. Outlook Agric 22(1):53–58
Nakashima K, Wungkaew P, Sirithorn P (2001) Molecular detection and characterization of sugarcane white leaf phytoplasmas. In: Rao GP, Ford RE, Tosir M, Teakle TS (eds) Sugarcane pathology, vol II: virus and phytoplasma diseases. Science, Enfield, pp 157–175
Nakata T (2008) Effectiveness of micronized fluorescent powder for marking citrus psyllid, Diaphorina citri. Appl Entomol Zool 43:33–36. doi:10.1303/aez.2008.33
Phisitkul S, Kuntha C, Neera P, Wongkaew S, Chaihoi P, Chettharaj A (1989) Preliminary studies on insect vectors of sugarcane white leaf disease in Northeast Thailand. Khon Kaen Agric J 17:164–172
SAS Institute (2010) JMP 9 statistics and graphics guide. SAS Institute, Cary
Seber GAF (1982) The estimation of animal abundance and related parameters, 2nd edn. Charles Griffin, London
Taylor RAJ (1985) Migratory behaviour in the Auchenorrhyncha. In: Nault LR, Rodriguez JG (eds) The leafhoppers and planthoppers. Wiley, New York, pp 259–288
Thein MM, Jamjanya T, Hanboonsong Y (2011) Evaluation of color traps to monitor insect vectors of sugarcane white leaf phytoplasma. Bull Insectol 64(Suppl):117–118
Whitney SP, Meyer JR (1988) Movement between wild and cultivated blueberry by two species of sharpnosed leafhoppers (Homoptera: Cicadellidae) in North Carolina. J Entomol Sci 23:88–95
Yamamura K, Kishita M, Arakaki N, Kawamura F, Sadoyama Y (2003) Estimation of dispersal distance by mark-recapture experiments using traps: correction of bias caused by the artificial removal by traps. Popul Ecol 45:149–155. doi:10.1007/s10144-003-0152-x
Zhou L, Hoy CW, Miller SA, Nault LR (2003) Marking methods and field experiments to estimate aster leafhopper (Macrosteles quadrilineatus) dispersal rates. Environ Entomol 32(5):1177–1180
Acknowledgments
The authors are grateful for a Ph.D. scholarship funded by the Khon Kaen University—Khon Kaen University Scholarship for our Neighboring Country’s Personnel Program, and the sugarcane white leaf research fund from Khon Kaen University and Japan International Research Center for Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4
Rights and permissions
About this article
Cite this article
Thein, M.M., Jamjanya, T., Kobori, Y. et al. Dispersal of the leafhoppers Matsumuratettix hiroglyphicus and Yamatotettix flavovittatus (Homoptera: Cicadellidae), vectors of sugarcane white leaf disease. Appl Entomol Zool 47, 255–262 (2012). https://doi.org/10.1007/s13355-012-0117-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-012-0117-7