Abstract
Understanding that cancer is one of the most important health problems, especially in advanced societies, is not difficult. The term of targeted cancer therapy has also been well known as an ideal treatment strategy in the recent years. Peptides with ability to specifically recognize the cancer cells with suitable penetration properties have been used as the targeting motif in this regard. In the present review article, we focus on an individual RGD-derived peptide with ability to recognize the integrin receptor on the cancer cell surface like its ancestor with an additional outstanding feature to penetrate to extravascular space of tumor and ability to penetrate to cancer cells unlike the original peptide. This peptide which has been named “internalizing RGD” or “iRGD” has been the focus of researches as a new targeting motif since it was discovered. To date, many types of molecules have been associated with this peptide for their targeted delivery to cancer cells. In this review article, we have discussed a summary of penetration mechanisms of iRGD and all introduced peptides and proteins attached to this attractive cell-penetrating peptide and have expressed the results of the studies.
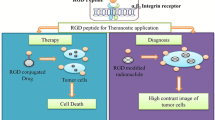
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Updated statistics show that about 19.3 million of new cancer cases and about 10 million deaths from cancer occurred in 2020 worldwide [1]. Targeted therapy of cancer on the other hand via recognition of cell surface molecules is an urgent approach to treat the cancer and diminish the toxic effects of various anticancer agents toward the normal tissues and non-cancerous cells [2].
Cancer targeted therapy approach was augmented after the introduction of monoclonal antibodies specifically attached to the receptors over-expressed on the cancer cell surface or cancer-specific antigens [3]. However, due to the obvious drawbacks of monoclonal antibodies such as their large molecular weight leading reduction of the cell and tissue penetration, difficult production and purification, essential post-translational modification and need to the usage of complex expression systems, attempts to find suitable alternatives are continuing [4]. Although the introduction of various forms of the single chain monoclonal antibodies overcome a lot of related problems, peptides are a better choice to achieve this goal because of their lower molecular weight which leads to more convenient production, lower immunogenicity and more permeability in comparison with the whole antibodies and even antibody fragments [5].
Cell-penetrating peptides, the small non-toxic peptides with 5- to 40- amino acid residues, are useful tools to facilitate drug delivery and increase the entry of various therapeutic molecules into the cell [6]. However, the biggest concern about these peptides is their poor selectivity, which limits their applications. So, it is interesting to investigate new peptides with higher selectivity and efficacy, generally known as tumor-specific penetrating peptides, for the targeted delivery of toxic agents to cancer cells as well as suitable stability in biological fluids [7].
Integrin receptor is highly expressed on the surface of some special tumor cells and the endothelial cells of feeding vessels with lower level of expression on the blood vessel cells of normal tissues. RGD, a motif with a high affinity to express integrin receptors (especially αvβ3 sub-type) on the vascular endothelial cell surface of several types of tumors, is an interesting ligand for targeting of the cytotoxic agents to cancer cells [8].
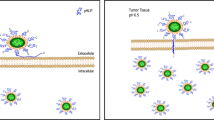
In recent years, a new type of RGD motif has been designed with increased penetration ability to the cells by means of a neurophilin receptor known as NRP-1, highly expressed on the tumor cell surface. This type of RGD motif enters the tumor cells in three stages. First, iRGD attaches to the integrin receptor; after that, this peptide is subjected to proteolytic cleavage and subsequent change to CRGDK/R with ability to attach to NRP-1 and internalizing to the cells. So, iRGD can improve the penetration of therapeutic agents to cancer cells as an ideal tool for increasing the selectivity and permeability of cytotoxic agents [9, 10]. In the recent years, iRGD has been used for the targeted delivery of various therapeutic agents, especially nanoparticles and small chemotherapeutic drugs such as doxorubicin [11], cisplatin [12] and 5-FU [13]. However, for therapeutic macromolecules, including peptides and proteins, there is an urgent need to find suitable delivery vehicle because most of them showed their biological activities after entering to the cells [14]. Figure 1 schematically presents the difference ability of RGD and iRGD in cancer cell penetration. In summary, this review focuses on the use of the iRGD motif for the delivery of peptides and proteins in the scope of cancer therapy. The summarized experimental data gathered in different stages up to in vivo studies are presented. Although the number of publications regarding this topic is limited, the high potential of the discussed peptide in the context of targeted delivery of peptide and protein drugs to cancer cells is clear. This review article gives an overview on the recent use of this motif and thus facilitates its future use.
RGD and its role in cancer
RGD is a tri-peptide motif containing arginine, glycine and aspartic acid with high affinity to integrin receptors. For the first time in 1984, this peptide motif was introduced as a highly conserved sequence in fibronectin protein recognized by integrin receptor with minimum amino acid residues [15]. RGD motif is present in many extracellular matrix proteins. Since the RGD motif was recognized, several peptidomimetics and modified peptides have been introduced as a ligand for integrin receptors especially αvβ3 sub-unit over-expressed on the surface of tumor vascular endothelial cells, while slightly expressed on the surface of vascular endothelial cells in normal tissues [16]. Subsequently, this peptide is an attractive subject to act as a targeting agent. There are various types of RGD peptidomimetics or RGD motif with the other amino acid residues in order to enhance their selectivity, integrin receptor affinity and stability. One of the most popular peptides is iRGD [17].
Internalizing RGD (iRGD) and its mechanism of action
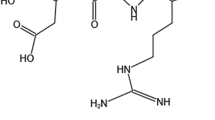
In 2009, for the first time, a different type of RGD was introduced by Sugahara et al. with the ability to transfer the chemotherapeutic agents to the deep parts of tumor cells. They named this peptide as internalizing RGD or iRGD. This penetrating peptide is a nonapeptide with the sequence CRGCK/RGPDC, widely used to increase the penetration of therapeutic agents to cancer cells via the attachment to the integrin receptor followed by cleavage by the specific cell surface proteases to create a free C-terminal named Cend-R with the sequence CRGDK/R. This end shows high affinity to neurophilin receptor, over-expressed on the surface of various types of cancer cells and enters the cells via receptor mediated endocytosis. Finally, the receptor–ligand complex enters the cell via receptor mediated endocytosis [18]. Figure 2 schematically expresses the receptor binding of iRGD peptide into the αvβ3 integrin receptor, its enzymatic cleavage and finally its attachment to the NRP-1 receptors on the cancer cell surface.
In one study, the inhibitory effects of iRGD on the cell migration were established. Sugahara, who introduced this tissue penetrating peptide for the first time, and his co-worker showed that this synthesized peptide causes inhibition in prostate cancer cell migration in mice bearing the GFP-PC-3 cells with over-expression of integrin receptors [19]. Actually, when the iRGD peptide was injected intravenously in 4 μmol/kg final dose, the inhibition of metastasis was dramatically established. These results was repeated during in vitro migration test. However, the action of this molecule as a cancer tissue-specific peptide is more obvious for delivering of various types of toxic agents [19]. In conclusion, internalizing RGD can act as a drug delivery vector for the targeted transportation of various therapeutic or diagnostic molecules to cancer cells. In addition, the attachment of iRGD to these agents is without a need for linker or chemical spacer between anticancer agent and iRGD [19].
iRGD usage for targeted drug delivery
Since the introduction of this peptide as an extravascular and cancer cell-penetrating molecule, it has been used for the targeted delivery of various types of agents with potential tumor toxicity in order to kill cancer cells. These agents include recognized chemotherapeutic agents such as doxorubicin [20], paclitaxel [21, 22], irinotecan [23], alone or entrapped in different types of nanoparticles, tyrosine kinase inhibitors such as sorafenib [24], vandetanib [25], and so on. Monoclonal antibodies such as cetuximab [26] are the other type of molecules attached to the iRGD for enhancing their selective penetration.
Natural compounds extracted from plants are the other example in this regard which have been targeted with iRGD. Isoliquiritigenin is one of these examples, a phenolic natural compound extracted from the Liquorice and loaded to the nanoparticles targeted with iRGD in order to enhance its anti-tumor effects against breast cancer cells [27]. The second example is Zerumbone with sesquiterpene structure derived from the Zingiber zerumbet smith. Similarly, this natural molecule with proven apoptotic effects was administered in nanoparticles with iRGD for the targeted delivery to breast cancer cells [7].
Recently, this motif was used to deliver the DNA- or RNA-based biomolecules to cancer cells. A new published paper in this regard is an attempt to transfer the thymidine kinase gene of HSV-1 to pancreatic carcinoma cell line PANC-1 followed by treatment of these cells with Ganciclovir, a routine type of suicide gene therapy [28].
In addition to the therapeutic agents, iRGD has been introduced as an attractive tool to deliver the diagnostic agents to cancer tissues. The most common example in this regard is iron oxide frequently used as a magnetic particle for imaging [29]. The other example is the synthesis of two novel iRGD analogues attached to the Ac-Cys(IRDye®800CW) and DOTA-Cys(IRDye®800CW), so that both analogues had significant tumor internalization in MDA-MB-435 tumor-bearing mice [30].
Finally, peptides and proteins are the last molecules which were associated with iRGD as a cancer-specific cell-penetrating peptide.
iRGD usage for peptide and protein delivery
Peptides and proteins are a large and diverse category of molecules, some of which have shown toxic or apoptotic effects on cancer cells. These are biomolecules with different mechanisms of action in cell killing. Cationic anti-microbial peptides, anticancer peptides, apoptosis inducing proteins, cytokines with potential anticancer effects and generally every peptide and proteins with significant toxicity to cancer cells can be associated with iRGD in order to enhance their penetration to tumor cells and tissues as well as specific toxic effects against cancer cells. In the following part of the paper, we reviewed all peptides and proteins attached to the iRGD and discussed the obtained results of each study.
TP5-iRGD
In 2014, a new peptide was designed by Lao et al. and then chemically synthesized with the name of TP5-iRGD. In the mentioned fusion peptide, five amino acid residues of Thymopoietin with established immunoregulatory effects on a host and suppression of tumor in mice model were attached to the iRGD in order to enhance the permeability of TP5 to the solid tumors. The proliferation inhibition of this peptide was assayed against three cell lines in various sources including B16F10, MCF-7 and H460, and the results of this test confirmed the significant enhancement of anti-proliferative effects of TP5-iRGD in comparison with the native TP5 for all surveyed cell lines. On the other hand, for the mice with melanoma induced by B16F10 cells, after two weeks of treatment, the tumor size decreased to 64% for the group treated with the fusion peptide compared to 41% for mice treated with the alone TP5. Finally, evaluation of tumor necrosis and increase in the melanoma cells attachment (leading to the less cancer cell migration) established more effect of TP5-iRGD than TP5. At the end of the present study, authors concluded that iRGD conjugation of TP5 can be assumed as an attractive way for increasing the anticancer effects of TP5. However, despite the selective toxicity of this constructed fusion protein, there was no evidence to confirm its safety on normal cell line. The only related test in this regard was the survey of murine spleen lymphocyte proliferation properties when treated with TP5 or TP5-iRGD. Actually, the results indicated that both peptides lead to the proliferation promotion of murine spleen lymphocyte in a concentration-dependent manner. It could be concluded from this test that neither TP5 nor TP5-iRGD has no cytotoxic effects against normal cells [31].
The other study used the same fusion peptide (TP5-iRGD in cyclic form) was evaluated its cytotoxic effects against breast cancer cell lines MDA-MB-231 and MCF-7. The results of MTT test showed no toxic effects of this cyclic molecule on cancer and normal cell lines (Hs27) after 72 h of incubation with 1000 µg/ml (about 300 µM) of the chimeric peptide [7]. Similarly, in the previous study, the IC50 value for the fusion peptide against various cancer cell lines (MCF-7, B16F10, and H460) was calculated as about 1000 µM (31). Nevertheless, in the mentioned project, the co-administration of TP5-iRGD and Zerumbone (Zer), a natural compound in sesquiterpene category which has been extracted from the wild ginger rhizomes with the scientific name Zingiber zerumbet smith was performed. The results revealed that when this natural compound was used in its IC50 concentration with the fusion protein (1:10 ratio) on MCF-7 cells, the most killing effects on cancer cells occurred and its IC50 value decreased by half time. Furthermore, when MCF-7 cells were treated with the Zer and TP5-iRGD, it was observed that apoptosis induction after 72 h is related to more penetration of Zer to cancer cells via co-administration with TP5-iRGD in comparison with the situation in which the natural compound was used alone. However, in this study, there is no discussion for data recorded for MDA-MB-231 as an over-expressed integrin receptor at least in comparison with the MCF-7 with established lower expression of αvβ3 integrin receptor [7]. When the authors surveyed the interaction of Zer with integrin αvβ3 receptor in the presence of TP5-iRGD using molecular modeling, they reached to attractive data which confirms the success of concurrent usage of two these molecules. In this paper TP5-iRGD was introduced as a non-toxic and safe fusion peptide which can improve the antitumor activity of Zer [7]. However, we think that this co-administration must be repeated for other cancer cell lines especially those with high expression of integrin receptors in order to gain more reliable data.
IL24-iRGD
In the related study, the iRGD coding sequence was added to the 3’ end of IL24 gene sequence and the recombinant expression was performed in E. coli expression system via pET-19b expression vector [32]. The mentioned fusion protein was then surveyed for its specific anti-proliferative effects of A549 cells as well as the NHLF as related normal cells. Furthermore, the ability of the mentioned biomolecule on the production of inflammatory cytokines especially IL-6 and TNF-γ was determined to be higher than the negative control in human monocytes. On the other hand, apoptosis induction in the cells treated with the fusion protein was established in A549 cells. Finally, the in vivo study results showed that the mentioned recombinant protein leads to about 60% of tumor growth inhibition in comparison with the A549 xenograft mice treated with the native IL-24 (with only 26% of tumor growth inhibition in the same molar concentration). Finally, the measurement of caspase 3 expression in active form in various treatment groups of mice showed significant difference especially between the IL-24 and IL24-iRGD with more than twofold increase in the caspase 3 concentration [32]. The authors have concluded that iRGD is a peptide which can increase the anticancer effects of IL-24 with higher efficacy to penetrate to the cancer cells.
HPRP-A1-iRGD
In a study, a novel chimeric peptide was designed in order to increase the selective anticancer effects of a cationic anti-microbial peptide, HPRP-A1. In two consecutive projects first this peptide was co-administered with iRGD in order to enhance the selective anticancer effects of the mentioned peptide during in vitro and in vivo tests. A549 cells were used to test the selective anticancer effects of these peptides as well as HUVEC as the negative control cell line. The results of MTT assay revealed that in the presence of iRGD, the cytotoxicity of HPRP-A1 increased in cancer cells. Surprisingly, concurrent usage of HPRP-A1 and iRGD reduced the IC50 value of the anti-microbial peptide on A549 by one half fold. So, the authors reached to this conclusion that iRGD enhances the killing properties of HPRP-A1. Furthermore, apoptosis evaluation on A549 cells showed enhancement in the early apoptosis from 9 to about 18% when this peptide was used with the iRGD peptide in comparison with the cells only treated with HPRP-A1 after 30 min of cell exposure. These data were repeated for the cell cycle arrest too. While this work only contained the in vitro study, the authors believed that co-administration of these two peptides can be used for the treatment of at least lung cancer as clinical studies [9].
The second paper in this regard was published by the same team of researchers. They chemically produced HPRP-A1-iRGD fusion peptide using solid phase technology and again test its selective toxicity against A549, MDA-MB-231, MCF-7, H-460 and BGC cells as well as HUVECs as the normal cell line. A549 was introduced as the most sensitive cell line after the HPRP-A1-iRGD treatment with the most difference with the IC50 of alone anti-microbial peptide. The highest IC50 value for the fusion peptide was calculated against the normal cell line, and the final finding from this test is this fact that for cells with low expression of integrin receptor, there was no significant difference between the IC50 value of the surveyed bio-conjugate and alone HPRP-A1. On the other hand, the cellular uptake of alone HPRP-A1 and HPRP-A1-iRGD labeled by FITC resulted in more penetration of HPRP-A1-iRGD than HPRP-A1 in A549 and MDA-MB-231 cells, though with apposite finding for HUVEC cells. Fortunately, in the mentioned study, the specific tumor penetration of this chimeric peptide was investigated for the first time in cancer mice model and it was observed that nude-mice injected with A549 cells and then treated with HPRP-A1-iRGD showed more intensity of fluorescence by rhodamine B in tumor site compared to the mice treated with alone HPRP-A1 [33]. However, till now, there is no evidence about the targeted anticancer effects of this designed biomolecule during in vivo studies for final decision about it.
sTRAIL-iRGD
The other example of iRGD usage is its attachment to the sTRAIL which is the soluble form of a transmembrane protein with established role in apoptosis induction [34]. The anti-tumor activity of this 168-amino acid residues protein was investigated during the in vitro and in vivo studies [35]. However, because of two important concerns about it, the short half-life as well as non-selective toxicity to hepatocytes, attempts to produce recombinant fusion proteins have been assumed as a suitable solution [36]. In this regard, sTRAIL-iRGD was designed and recombinantly produced in E. coli expression system and its cytotoxic effects were analyzed by MTT assay against MKN45 and KATO III gastric cancer cell lines. The results indicated that for MKN45 cells, there is significant difference between the inhibitory effects of sTRAIL-iRGD and alone sTRAIL. However, for the KATO III cells this difference was not statistically significant which can be attributed to the lower level of NRP-1 receptor on the cell surface. The apoptosis induction of the fusion protein was more than the alone sTRAIL when MKN45 cells were treated. Furthermore, the internalization of the fusion protein to the mentioned cells which was evaluated after FITC labeling showed confirming results; sTRAIL-iRGD was more effectively taken up into MKN45 cells within 30 min, but not into KATO III cells. In addition, the in vivo test with mice bearing the tumor induced by MKN45 cells established its localization in to the tumor site. Finally, when these mice were analyzed for their tumor size, the results confirmed the inhibitory effects of this fusion protein of the tumor growth even after three days of injection in comparison with the injection of native sTRAIL leading to the increase in the tumor size [10]. So, this novel recombinant molecule was introduced as a new selective and safe anti-gastric cancer.
KLA-iRGD
In a related paper, iRGD was fused to a cationic amphipathic peptide named KLA with the ability to increase the mitochondrial membrane permeability and induction of apoptosis to increase its penetration to the eukaryotic cells as well as to enhance its selective toxicity [37]. In this study conducted by HuANG et al. the fourteen amino acids of KLA peptide were attached to the iRGD sequence in mediation of a flexible linker containing two repeats of GGGGS sequence. The mentioned fusion protein KLA-iRGD was produced by solid-phase peptide synthesis procedure, and its internalization as well as its anti-proliferative effects was surveyed against KATO III and MKN45 gastric cancer cell lines. The cell toxicity assay revealed more toxicity of KLA-iRGD in comparison with KLA in MKN45 cells with IC50 value of about 200 µg/ml unlike the other cell line with same anti-proliferative effects of two these peptides. Furthermore, the fusion peptide induced apoptosis about 70% on the MKN45 cells versus the 4% of apoptosis induction by the KLA peptide on the same cell line. Finally, the in vivo test for evaluating the internalizing and anti-tumor effects confirmed that this fusion peptide when injected to the mice bearing MKN45 cells in 30 mg/kg final dose, even after three days, the inhibitory effects on tumor growth was seen and its penetration to the deep parts of the tumor was established [38]. However, although the pharmacokinetics and pharmacodynamics studies are essential tests to be performed, it seems that this chimeric peptide showed anti-proliferative and anticancer effects in the high doses which is attributed to the milder cytotoxicity of KLA peptide attached to the iRGD.
BIF1-iRGD and DFF40-iRGD
Recently, we designed two novel fusion proteins according to the related previous studies and produced them by recombinant DNA technology in E. coli expression system. After the optimization of the expression in order to obtain the most soluble and active protein, evaluation of their anti-proliferative effects was performed against MDA-MB-231 and MCF-7 cells with over-expressed and median levels of integrin receptor on their surface, respectively. The results from the treatment of MDA-MB-231 cells with BIF1-iRGD showed significant differences between the two proteins (BIF-1 and BIF1-iRGD), especially after 24 h compared to 48 and 72 h incubation times. Furthermore, the apoptotic effects of the fusion protein in IC50 concentration (650 ng/ml) were shown against MDA-MB-231 (article in press). These results were repeated for the other recombinant fusion protein (DFF40-iRGD) in the lower concentrations. Actually, this recombinant protein led to the induction of apoptosis as about 70% in concentration of about 5 nM [39]. The investigation about the anti-tumor effects of these fusion proteins on the mice bearing 4T1-induced tumor is ongoing.
Anti-EGFR-iRGD
The last different example of iRGD motif usage as the targeting moiety is its fusion to the anti-EGFR antibody to increase the targeting efficacy of this antibody. The antibody against EGFR which is expressed on the surface of various cancer cells in large amounts was attached to the iRGD as a bispecific molecule to transfer gadolinium‑diethylene triamine pentaacetate (DTPA-Gd) as an imaging molecule [8]. The ability of this biomolecule to transfer to the human gastric cell line (BCG-823) was established. On the other hand, in vivo studies on xenograft mice showed the penetration ability of this bispecific molecule to the tumor created by these gastric cancer cells in mice with safe profile. Actually, the effects of anti-EGFR-iRGD-DTPA-Gd in cell survival were not significantly different by cells treated with the same concentrations of anti-EGFR-DTPA-Gd. In addition, when the penetration ability of each construct was determined by fluorescence intensity, the speed of penetration increased for anti-EGFR-iRGD-DTPA-Gd in comparison with the anti-EGFR-DTPA-Gd, 30 min versus 60 min in maximum intensity. These results were repeated for the final amounts of penetration [8].
In another related study performed by the same researchers, the designed bispecific antibody was used to increase the penetration of paclitaxel to gastric cancer cells. However, because the toxic moiety in the mentioned study was a non-protein molecule, it is not discussed here. Finally, in an attempt to increase the infiltration and toxic effects of cytotoxic T cells in the tumor site, this bispecific molecule co-administered with T cells. The results showed that this molecule has no cytotoxic and apoptotic effects on lymphocyte even in the final concentration as 240 μg/ml. Over 21 days, this recombinant protein had no effects on the proliferation rate of the cells. Finally, the expression of protein markers on the cell surface of lymphocytes was without any significant differences in comparison with the cells without the treatment. However, anti-EGFR-iRGD induced more proliferation inhibition in BGC-823 cell line (as positive EGFR) compared to the MKN-45 and MGC-803 cell lines with low expression of EGF receptors. Furthermore, T cells co-administered with the fusion protein showed more cytolytic activity against gastric cancer cells, especially in the ratios of 1:40 (cytotoxic T cell/fusion protein). Actually, when the BGC-823 cells were treated with a lone T cells, they showed about 54.5% of apoptosis which increased to 65.5% in combination therapy. In this example, iRGD was used to enhance the targeting efficacy of an antibody against EGFR in order to augment the cytotoxicity of T cells [40].
These examples are the most studied fusion proteins or chimeric peptides, and Table 1 represents a summary of each biomolecule in addition to a few studies which used iRGD for targeting of some other peptides or proteins.
Conclusion
Although the active targeting leads to the more complications in construction of drug conjugates, there are several undeniable advantages for this strategy such as increasing in drug efficacy, frugality in used doses and particularly diminishing in side effects of toxic agents used for the treatment of various cancers. However, iRGD peptide is a good example which acts as an active targeting moiety. As mentioned, this peptide can penetrate to the extravascular space of the tumor as well as the ability to internalize to the cancer cells. Although the addition of a targeting moiety such as iRGD to the nanoparticles containing cytotoxic agents leads to the increasing in their production complexity, the distinction between normal and cancer cells is pursued with greater vigor. In this review article, considering that the authors' research field is the production of anticancer chimeric proteins, only peptides and proteins fused to iRGD, which are often recombinantly produced, have been discussed which are with no need to use nanoparticles. Several important results were obtained from reviewing these articles. The most important finding is this fact that all these bio-conjugates had the ability to penetrate to cell lines with the over-expression of integrin receptors and in significant differences with the cell lines with no expression or lower expression of the mentioned receptor. The other important conclusion from all studies was the differences in the ability of native peptide and protein with one fused to the iRGD peptide. These two well-proven results indicate that the present peptide has an undeniable role as a specific cancer cell-penetrating peptide. On the other hand, in vivo tests, which were performed in several examples, could establish the acceptable penetration of peptides or proteins fused to the iRGD peptide as well as good inhibitory effects on the tumor growth. So, there is no doubt that this peptide has been passed its examination for its ability to penetrate to tumors and cancer cells. However, as shown for each bio-conjugate, the IC50 value for the treatment of cell lines or dose which was injected to the tumor-bearing mice was different case by case. These observed variations completely relate to the toxic agents attached to the iRGD peptide rather than the targeting moiety. Actually, the peptide or protein used in each study showed different biological activities with various mechanisms of action. iRGD has similar ability to internalize the toxic motif regardless of its type to be a large protein or a short peptide. Another conclusion that can be drawn from this review is that almost all the investigated studies have been advanced to the level of animal test and evaluation of the anticancer efficacy of designed molecules in preclinical studies. However, a complete pattern of pharmacokinetics and pharmacodynamics studies, safety and toxicity tests as well as clinical phase must be performed in order to judge accurately about the effectiveness and safety profile of each designed molecule; however, it seems that the lack of these data is due to the short time since the discovery of this peptide and the new design of each of these biomolecules. It can be said that the first chimeric protein in this category was designed and evaluated in 2014. As the final conclusion, iRGD can be assumed as an ideal cancer-specific cell-penetrating peptide to act as a targeting moiety in order to decrease the side toxic effects on the normal tissues although it is a peptide and its stability may be less than chemical ligands which can be used as a targeting moiety. We hope that researchers will achieve good results in clinical trials, if they continue their studies on each of these molecules as a big step in introducing a new therapeutic approach to cancer.
Availability of data and materials
This is a review article, and all data have been presented in the text of manuscript or in table.
Abbreviations
- NRP1:
-
Neurophilin-1
- CPP:
-
Cell-Penetrating Peptide
- GFP:
-
Green Florescent Protein
- IL24:
-
Interleukin 24
- EGFR:
-
Epidermal Growth Factor Receptor
- TNF:
-
Tumor Necrosis Factor
- HPRP-A1:
-
Helicobacter pylori Ribosomal Protein-A1
- IC50:
-
Inhibitory Concentration 50%
- FITC:
-
Fluorescein Isothiocyanate
- sTRAIL:
-
Soluble Tumor Necrosis Factor (TNF)-Related Apoptosis-Inducing Ligand
- AMP:
-
Anti-Microbial Peptide
- BIF-1:
-
BAX Interacting Factor-1
- DFF-40:
-
DNA Fragmentation Factor-40
- CDD:
-
Cell Death Domain
- Tα1:
-
Thymosin α1
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Can J Clin. 2021. https://doi.org/10.3322/caac.21660
Shafiee F, Aucoin MG, Jahanian-Najafabadi A. Targeted diphtheria toxin-based therapy: a review article. Frontiers Microbiol. 2019. https://doi.org/10.3389/fmicb.2019.02340.
Das M, Mohanty C, Sahoo SK. Ligand-based targeted therapy for cancer tissue. Exp Opin Drug Del. 2009. https://doi.org/10.1517/17425240902780166.
Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Can Immun Arch. 2012. https://doi.org/10.3390/antib9030034.
Shafiee F, Minaiyan G, Moazen F, Jahanian-Najafabadi A. Recombinant production and intein-mediated purification of an antimicrobial peptide, BR2. Int J Pep Res Ther. 2017. https://doi.org/10.1007/s10989-017-9583-7.
Shafiee F, Rabbani M, Jahanian-Najafabadi A. Production and evaluation of cytotoxic effects of DT386-BR2 fusion protein as a novel anti-cancer agent. J Microbiol Meth. 2016. https://doi.org/10.1016/j.mimet.2016.09.004.
EM Eid E, S Alanazi A, Koosha S, A Alrasheedy A, Azam F, M Taban I et al. Zerumbone induces apoptosis in breast cancer cells by targeting αvβ3 integrin upon co-administration with TP5-iRGD peptide. Molecules. 2019. https://doi.org/10.3390/molecules24142554
Xin X, Sha H, Shen J, Zhang B, Zhu B, Liu B. Coupling Gd-DTPA with a bispecific, recombinant protein anti-EGFR-iRGD complex improves tumor targeting in MRI. Oncol Rep. 2016. https://doi.org/10.3892/or.2016.4712.
Hu C, Chen X, Huang Y, Chen Y. Co-administration of iRGD with peptide HPRP-A1 to improve anticancer activity and membrane penetrability. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-20715-4.
Huang Y, Li X, Sha H, Zhang L, Bian X, Han X et al. sTRAIL-iRGD is a promising therapeutic agent for gastric cancer treatment. Sci Rep. 2017. https://doi.org/10.1038/s41598-017-00688-6.
Yu K-F, Zhang W-Q, Luo L-M, Song P, Li D, Du R et al. The antitumor activity of a doxorubicin loaded, iRGD-modified sterically-stabilized liposome on B16–F10 melanoma cells: in vitro and in vivo evaluation. Int J Nanomed. 2013. https://doi.org/10.2147/IJN.S46962.
Cho H-J, Park S-J, Lee Y-S, Kim S. Theranostic iRGD peptide containing cisplatin prodrug: Dual-cargo tumor penetration for improved imaging and therapy. J Ctrl Rel. 2019. https://doi.org/10.1016/j.jconrel.2019.02.043.
Zhang L, Xing Y, Gao Q, Sun X, Zhang D, Cao G. Combination of NRP1-mediated iRGD with 5-fluorouracil suppresses proliferation, migration and invasion of gastric cancer cells. Biomed & Pharmacother. 2017. https://doi.org/10.1016/j.biopha.2017.06.103.
Allahyari H, Heidari S, Ghamgosha M, Saffarian P, Amani J. Immunotoxin: A new tool for cancer therapy. Tumor Biol. 2017. https://doi.org/10.1177/1010428317692226.
Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–3. https://doi.org/10.1038/309030a0.
Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987. https://doi.org/10.1126/science.2821619.
Schittenhelm J, Klein A, Tatagiba MS, Meyermann R, Fend F, Goodman SL et al. Comparing the expression of integrins αvβ3, αvβ5, αvβ6, αvβ8, fibronectin and fibrinogen in human brain metastases and their corresponding primary tumors. Int J Clin Exp Pathol. 2013;6(12):2719.
Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009. https://doi.org/10.1016/j.ccr.2009.10.013.
Sugahara KN, Braun GB, de Mendoza TH, Kotamraju VR, French RP, Lowy AM et al. Tumor-penetrating iRGD peptide inhibits metastasis. Mol Can Ther. 2015;14(1):120–8. https://doi.org/10.1158/1535-7163.MCT-14-0366.
Liu M, Ma W, Zhao D, Li J, Li Q, Liu Y et al. Enhanced Penetrability of a Tetrahedral Framework Nucleic Acid by Modification with iRGD for DOX-Targeted Delivery to Triple-Negative Breast Cancer. ACS Appl Mat Int. 2021. https://doi.org/10.1021/acsami.1c07297.
Hu H, Wang B, Lai C, Xu X, Zhen Z, Zhou H et al. iRGD-paclitaxel conjugate nanoparticles for targeted paclitaxel delivery. Drug Dev Res. 2019. https://doi.org/10.1002/ddr.21589.
Li L, Yang M, Li R, Hu J, Yu L, Qian X. iRGD Co-Administration with Paclitaxel-Loaded PLGA Nanoparticles Enhance Targeting and Antitumor Effect in Colorectal Cancer Treatment. Anti-Can Ag Med Chem. 2021. https://doi.org/10.2174/1871520620666200721134919.
Ruoslahti E. Access granted: iRGD helps silicasome-encased drugs breach the tumor barrier. J Clin Inv. 2017. https://doi.org/10.1172/JCI93955.
Liu X, Zhu X, Qi X, Meng X, Xu K. Co-administration of iRGD with Sorafenib-loaded iron-based metal-organic framework as a targeted ferroptosis agent for liver cancer therapy. Int J Nanomed. 2021. https://doi.org/10.2147/IJN.S292528.
Wang J, Wang H, Li J, Liu Z, Xie H, Wei X et al. iRGD-decorated polymeric nanoparticles for the efficient delivery of vandetanib to hepatocellular carcinoma: preparation and in vitro and in vivo evaluation. ACS Appl Mat Int. 2016. https://doi.org/10.1021/acsami.6b03166.
Zhang Y, Yang J, Ding M, Li L, Lu Z, Zhang Q et al. Tumor-penetration and antitumor efficacy of cetuximab are enhanced by co-administered iRGD in a murine model of human NSCLC. Oncol Lett. 2016. https://doi.org/10.3892/ol.2016.5081.
Gao F, Zhang J, Fu C, Xie X, Peng F, You J et al. iRGD-modified lipid–polymer hybrid nanoparticles loaded with isoliquiritigenin to enhance anti-breast cancer effect and tumor-targeting ability. Int J Nanomed. 2017. https://doi.org/10.2147/IJN.S134148
Egorova A, Shtykalova S, Selutin A, Shved N, Maretina M, Selkov S et al. Development of iRGD-modified peptide carriers for suicide gene therapy of uterine leiomyoma. Pharmaceutics. 2021. https://doi.org/10.3390/pharmaceutics13020202.
Hamilton AM, Aidoudi-Ahmed S, Sharma S, Kotamraju VR, Foster PJ, Sugahara KN et al. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J Mol Med. 2015. https://doi.org/10.1007/s00109-015-1279-x.
Ye Y, Zhu L, Ma Y, Niu G, Chen X. Synthesis and evaluation of new iRGD peptide analogs for tumor optical imaging. Bioorg Med Chem Lett. 2011. https://doi.org/10.1016/j.bmcl.2010.12.112.
Lao X, Li B, Liu M, Chen J, Gao X, Zheng H. Increased antitumor activity of tumor-specific peptide modified thymopentin. Biochimie. 2014. https://doi.org/10.1016/j.biochi.2014.09.013.
Yang J, Wei Y, Yin H, Fang L, Chai D, Li H et al. Modification of IL-24 by tumor penetrating peptide iRGD enhanced its antitumor efficacy against non-small cell lung cancer. Int Immunopharmacol. 2019. https://doi.org/10.1016/j.intimp.2019.02.027.
Hu C, Huang Y, Chen Y. Targeted modification of the cationic anticancer peptide HPRP-A1 with iRGD to improve specificity, penetration, and tumor-tissue accumulation. Mol Pharma. 2018. https://doi.org/10.1021/acs.molpharmaceut.8b00854.
Refaat A, Abd-Rabou A, Reda A. TRAIL combinations: The new ‘trail’for cancer therapy. Oncol Lett. 2014. https://doi.org/10.3892/ol.2014.1922.
Liu R, Ma X, Wang H, Xi Y, Qian M, Yang W et al. The novel fusion protein sTRAIL-TMTP1 exhibits a targeted inhibition of primary tumors and metastases. J Mol Med. 2014. https://doi.org/10.1007/s00109-013-1093-2.
Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nature Med. 2001. https://doi.org/10.1038/86397.
Hyun S, Lee S, Kim S, Jang S, Yu J, Lee Y. Apoptosis inducing, conformationally constrained, dimeric peptide analogs of KLA with submicromolar cell penetrating abilities. Biomacromol. 2014. https://doi.org/10.1021/bm501026e.
Huang Y, Li X, Sha H, Zhang L, Bian X, Han X et al. Tumor-penetrating peptide fused to a pro-apoptotic peptide facilitates effective gastric cancer therapy. Oncol Rep. 2017. https://doi.org/10.3892/or.2017.5440.
Amrollahi-Nia R, Akbari V, Shafiee F. DFF40-iRGD, a novel chimeric protein with efficient cytotoxic and apoptotic effects against triple-negative breast cancer cells. Biotechnol Lett. 2021. https://doi.org/10.1007/s10529-021-03178-y.
Zhu A, Sha H, Su S, Chen F, Wei J, Meng F et al. Bispecific tumor-penetrating protein anti-EGFR-iRGD efficiently enhances the infiltration of lymphocytes in gastric cancer. Am J Can Res. 2018;8(1):91.
Ren W, Sha H, Yan J, Wu P, Yang J, Li R et al. Enhancement of radiotherapeutic efficacy for esophageal cancer by paclitaxel-loaded red blood cell membrane nanoparticles modified by the recombinant protein anti-EGFR-iRGD. J Biomat Appl. 2018. https://doi.org/10.1177/0885328218809019.
Chen H, Sha H, Zhang L, Qian H, Chen F, Ding N et al. Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein. Int J Nanomed. 2018. https://doi.org/10.2147/IJN.S165109.
Zhang Z, Qian H, Huang J, Sha H, Zhang H, Yu L et al. Anti-EGFR-iRGD recombinant protein modified biomimetic nanoparticles loaded with gambogic acid to enhance targeting and antitumor ability in colorectal cancer treatment. Int J Nanomed. 2018. https://doi.org/10.2147/IJN.S170148.
Sha H, Li R, Bian X, Liu Q, Xie C, Xin X et al. A tumor-penetrating recombinant protein anti-EGFR-iRGD enhance efficacy of paclitaxel in 3D multicellular spheroids and gastric cancer in vivo. Eur J Pharma Sci. 2015. https://doi.org/10.1016/j.ejps.2015.05.020.
Sha H, Zou Z, Xin K, Bian X, Cai X, Lu W et al. Tumor-penetrating peptide fused EGFR single-domain antibody enhances cancer drug penetration into 3D multicellular spheroids and facilitates effective gastric cancer therapy. J Ctrl Rel. 2015. https://doi.org/10.1016/j.jconrel.2014.12.039.
Yang J, Yin H, Wei Y, Fang L, Chai D, Zhang Q et al. Tumor-penetrating peptide enhances antitumor effects of IL-24 against prostate cancer. Translation Oncol. 2019. https://doi.org/10.1016/j.tranon.2018.12.002.
Fadeev R, Chekanov A, Solovieva M, Bezborodova O, Nemtsova E, Dolgikh N et al. Improved anticancer effect of recombinant protein izTRAIL Combined with Sorafenib and Peptide iRGD. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20030525.
Qifan W, Fen N, Ying X, Xinwei F, Jun D, Ge Z. iRGD-targeted delivery of a pro-apoptotic peptide activated by cathepsin B inhibits tumor growth and metastasis in mice. Tumor Biol. 2016. https://doi.org/10.1007/s13277-016-4961-x.
Hu C, Chen X, Huang Y, Chen Y. Co-administration of kla-TAT peptide and iRGD to enhance the permeability on A549 3D multiple sphere cells and accumulation on xenograft mice. Chem Biol Drug Des. 2018. https://doi.org/10.1038/s41598-018-20715-4.
Chu Y, Chen N, Yu H, Mu H, He B, Hua H et al. Topical ocular delivery to laser-induced choroidal neovascularization by dual internalizing RGD and TAT peptide-modified nanoparticles. Int J Nanomed. 2017. https://doi.org/10.2147/IJN.S126865.
Xin L, Yuan Y-W, Liu C, Zhou L-Q, Liu L, Zhou Q et al. Preparation of internalizing RGD-modified recombinant methioninase exosome active targeting vector and antitumor effect evaluation. Dig Dis Sci. 2021. https://doi.org/10.1007/s10620-020-06262-x.
Kolesanova E, Melnikova M, Bolshakova T, Rybalkina EY, Sivov I. Bacteriophage MS2 as a tool for targeted delivery in solid tumor chemotherapy. Acta Naturae. 2019. https://doi.org/10.32607/20758251-2019-11-2-98-101
Wang F, Li B, Fu P, Li Q, Zheng H, Lao X. Immunomodulatory and enhanced antitumor activity of a modified thymosin α1 in melanoma and lung cancer. Int J Pharma. 2018. https://doi.org/10.1016/j.ijpharm.2018.06.041.
Lao X, Li B, Liu M, Shen C, Yu T, Gao X et al. A modified thymosin alpha 1 inhibits the growth of breast cancer both in vitro and in vivo: suppressment of cell proliferation, inducible cell apoptosis and enhancement of targeted anticancer effects. Apoptosis. 2015. https://doi.org/10.1007/s10495-015-1151-z.
Lao X, Liu M, Chen J, Zheng H. A tumor-penetrating peptide modification enhances the antitumor activity of thymosin alpha 1. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0072242.
Peng Z-H, Kopeček J. Synthesis and activity of tumor-homing peptide iRGD and histone deacetylase inhibitor valproic acid conjugate. Bioorg Med Chem Lett. 2014. https://doi.org/10.1016/j.bmcl.2014.03.006.
Dai J, Han S, Ju F, Han M, Xu L, Zhang R et al. Preparation and evaluation of tumour microenvironment response multistage nanoparticles for epirubicin delivery and deep tumour penetration. Art Cell Nanomed Biotechnol. 2018. https://doi.org/10.1080/21691401.2018.1470528.
Bao X, Zeng J, Huang H, Ma C, Wang L, Wang F et al. Cancer-targeted PEDF-DNA therapy for metastatic colorectal cancer. Int J Pharma. 2020. https://doi.org/10.1016/j.ijpharm.2019.118999.
Kawano T, Murata M, Kang J-H, Piao JS, Narahara S, Hyodo F et al. Ultrasensitive MRI detection of spontaneous pancreatic tumors with nanocage-based targeted contrast agent. Biomaterials. 2018. https://doi.org/10.1016/j.biomaterials.2017.10.029.
De G, Ko JK, Tan T, Zhu H, Li H, Ma J. Amphipathic tail-anchoring peptide is a promising therapeutic agent for prostate cancer treatment. Oncotarget. 2014. https://doi.org/10.18632/oncotarget.2301.
Gu G, Gao X, Hu Q, Kang T, Liu Z, Jiang M et al. The influence of the penetrating peptide iRGD on the effect of paclitaxel-loaded MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials. 2013. https://doi.org/10.1016/j.biomaterials.2013.03.036.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014. https://doi.org/10.1016/j.biomaterials.2013.11.083.
Karandish F, Froberg J, Borowicz P, Wilkinson JC, Choi Y, Mallik S. Peptide-targeted, stimuli-responsive polymersomes for delivering a cancer stemness inhibitor to cancer stem cell microtumors. Coll Surf B: Biointer. 2018. https://doi.org/10.1016/j.colsurfb.2017.12.036.
Gregory JV, Kadiyala P, Doherty R, Cadena M, Habeel S, Ruoslahti E et al. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat Com. 2020. https://doi.org/10.1038/s41467-020-19225-7.
Yang X, Hu C, Tong F, Liu R, Zhou Y, Qin L et al. Tumor microenvironment-responsive dual drug dimer-loaded pegylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv Fun Mat. 2019. https://doi.org/10.1002/adfm.201901896.
Chen R, Braun GB, Luo X, Sugahara KN, Teesalu T, Ruoslahti E. Application of a proapoptotic peptide to intratumorally spreading cancer therapy. Can Res. 2013. https://doi.org/10.1158/0008-5472.CAN-12-1979.
Song Y, Xu M, Li Y, Li Y, Gu W, Halimu G et al. An iRGD peptide fused superantigen mutant induced tumor-targeting and T lymphocyte infiltrating in cancer immunotherapy. Int J Pharma. 2020. https://doi.org/10.1016/j.ijpharm.2020.119498.
Ghobadi M, Shafiee M. Recombinant Production of BIF1-iRGD Fusion Protein with Cytotoxic and Apoptotic Effects against Breast Cancer Cell Lines. Biomed Res Ther. 2022; in press.
Acknowledgements
NA.
Funding
This research has been done under the spiritual support of the Isfahan University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
ZD has reviewed the paper, prepared the table and revised the manuscript. FSH has prepared the text of manuscript and designed the figures.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This paper is a review article without any in vitro or in vivo test.
Consent for publication
All authors agree to publish this review article to this journal.
Competing interests
All authors explain that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davoodi, Z., Shafiee, F. Internalizing RGD, a great motif for targeted peptide and protein delivery: a review article. Drug Deliv. and Transl. Res. 12, 2261–2274 (2022). https://doi.org/10.1007/s13346-022-01116-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01116-7