Abstract
Transdermal drug delivery is a viable and clinically proven route of administration. This route specifically requires overcoming the mechanical barrier provided by the Stratum Corneum of epidermis and vascular and nervous networks within the dermis. First-generation Transdermal patches and second-generation iontophoretic patches have been translated into commercial clinical products successfully. The current review reports different studies that aim to enhance the transdermal delivery of biopharmaceutical using microneedles and their effect on drug delivery. Microneedles (MN) are the micron-scale hybrid between transdermal patches and hypodermic syringes. Microneedles are tested and proven to show better delivery of the drugs, overcoming the drawbacks of hypodermic syringes. Multiple microneedles designs have been fabricated i.e. solid, coated, hollow, and polymer microneedles. Hollow microneedles are shorter in length but similar to hypodermic needles and have pore for infusion of liquid formulation of the drug. Solid microneedles a patch is applied after creating a hole in the skin; Drugs are coated on the surface of Coated microneedles; Polymer microneedles can be of different types like dissolving, non-dissolving or hydrogel-forming made up of polymers. Various advantages and limitations associated with the use of these techniques are discussed. Delivery of peptide and protein molecules with microneedles represents a significant opportunity for a better clinical outcome and hence value creation compared to standard injectable routes of administration. The advancement in various formulation and microfabrication techniques are currently being focused to aid the delivery of protein drugs via microneedles. The most recent advances and limitations in Microneedles -mediated protein and peptide delivery were discussed.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
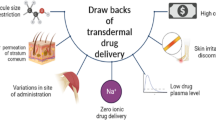
Within the discipline of drug delivery, in the course of advancement, many approaches have been developed following different routes, such as—oral, nasal and urogenital. Among the range of routes, the transdermal route of drug delivery is a major and exceedingly popular approach for drug delivery. Transdermal delivery refers to the administration of drugs through the skin. To understand the transdermal drug delivery system, it is crucial to understand the structure of skin. The transdermal drug delivery system has a fair share of advantages over other routes. It allows a constant infusion of the drug over prolonged durations; this also increases the bioavailability of the drug [1]. Transdermal delivery may also minimize the toxicity related side effects with increased compliance rate by the patients. It allows direct access of the drug to the systemic circulation [2]. The drug can be averted from the effects induced by the elements of gastro-intestinal passage (in contrast with oral administration of the drug) [3]. Along with these advantages, there are few disadvantages of transdermal drug delivery, such as limitation of the molecular weight of the drug to less than 500 Da [4]; very specific, relatively potent drugs are considered as appropriate treatment candidates due to the impermeable barrier created by the skin [5]; irritation or an allergic reaction in the skin [6].
Traditional hypodermic needles, commonly used with syringes, were first introduced in the 1800s.
The skin, being the largest and a prominent organ of the body, serves as the natural barrier against environmental stimuli. It is divided into three layers viz. Epidermis, Dermis and Hypodermis. The epidermis consists of a layer called the Stratum Corneum (SC), which is made up of dead skin cells. SC is organized into structural compartments, with defensive functions localized to extracellular matrix and corneocytes accordingly. The second layer, i.e. Dermis, consists of a broad vascular and nervous network and is also responsible for providing mechanical strength to the skin. The hypodermis is the deeper layer of the skin, which connects the bones or muscles to the dermis and is composed of reticular connective tissues. These layers put up a challenge and act as a barrier in transdermal drug delivery as it is rather strenuous to penetrate the stratum corneum without damaging the nerve endings present in the Dermis [7].
And have been a popular and most extensively used medical device since then. According to the World Health Organization, on an average, around 16 billion syringes are administered worldwide in a year [8]. The hypodermic needles can essentially be divided into three categories, based on needle length and needle gauze (diameter of needle), as described in Table 1. Despite the effective competence of these needles, there are many limitations and challenges associated with them. The most common issues that come with hypodermic needles are pain and anxiety [9]. The needle stimulates the pain receptors which are present in the dermis, primarily causing discomfort [10]. The transdermal drug delivery using these needles has been adversely limited due to an adequate number of drugs being unable to cross the underlying layers of the skin [11].
With the recent advancements, micron-scale syringes have popularly been fabricated and studied closely to overcome the limitations of traditional hypodermic needles. These micron-size syringes, known as microneedles (MN), are arranged in arrays attached to a patch (similar to a transdermal patch) that contains the drug. The needles penetrate the barrier, i.e. stratum corneum, and reach the epidermis, where the drug is released. Since the microneedles do not reach the dermis, it does not stimulate the pain receptors unlike hypodermic needles [10]. These microneedles were able to increase skin permeability and effectively increase the success rate of transdermal delivery.
Transdermal drug delivery using microneedles
The term microneedle was first coined in 1921. The microneedles were used to inject into the nucleus of an echinoderm egg, in order to perform a micro-dissection [12]. Microneedles being used for delivering a drug for the first time was reported in 1971 as ‘Drug Delivery Device’, when a patent was filed by M. S. Gerstel and V. A. Place [13] on 17th May, 1971 (Granted: 22nd June, 1976; Patent No. US3964482A). In a way, Gerstel and Vigil only introduced the concept of transdermal delivery of drugs using microneedles; the term ‘Microneedles’ was evidently introduced by S. Henry et al. [14] in 1998. The first appropriate application of microneedles for in vivo transdermal drug delivery (in rat model) was reported by Mikszta et al. [15] in 2002. In this experiment, topical gene transfer was carried out in the mouse model using microneedles. Hepatitis-B surface antigen encoding plasmid DNA was injected by scraping the mouse skin multiple times to induce an immune response, which resulted in enhanced expression (by 2880-folds) of the reporter gene.
By temporarily disrupting the stratum corneum, thus enhancing the skin permeability, the drug is delivered across the skin. The drug is placed on the epidermis or upper dermis, which is subsequently delivered to systemic circulation [10]. The drug delivery through the topical route follows either active diffusion or passive diffusion. These strategies can increase the efficiency of drug delivery. Passive diffusion (diffusion of a drug across a cell membrane in concentration gradient; high to low) depends on several factors such as permeation time, physio-chemical properties of the permeant, the density of sweat glands, thickness and integrity of stratum corneum, skin hydration. Drugs migrate through the intercellular lipids via a complex pathway in which hydrophilic molecules pass through the polar region of the intercellular lipids and lipophilic molecules travel through non-polar chains. Gels, ointments, and creams are used as channels for the passive diffusion of drugs. Active diffusion (drug is diffused against the concentration gradient; low to high) provides an ideal alternative route that reduces the barrier properties of the stratum corneum. It requires external energy to propel large and hydrophilic molecules through the stratum corneum. The permeation enhancers used in active diffusion enhance the drug solubility and/or reverse the lipid structure of skin and form partitions in the stratum corneum [16].
Types of microneedles
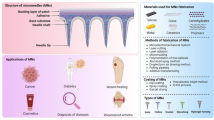
Microneedles are classified according to how they transmit drugs into the epidermis, as well as formulation parameters and materials used for their fabrication.
Solid microneedles
Solid microneedles work on the ‘poke and patch’ principle [17] whereby, skin is pre-treated by forming pores using pointed needles penetrated into the skin and forming micron-size channels (Fig. 1). This helps the drug to enter the epidermis where the drug is absorbed by capillaries and it reaches system circulation [10]. The drug formulation acts as an external reservoir and enables drug permeation. Solid microneedles are usually fabricated from polymer, silicon and metals like polyvinyl-pyrrolidone, titanium, stainless steel etc. [18].
Zhang et al. [19] fabricated solid silicon microneedles arrays of 150 μm in length for peptide delivery. The results show that the passive flux of acetyl hexapeptide-3 across the untreated porcine skin was 0.014 ± 0.002 μmoL/cm·h whereas the flux of post microneedles treatment increased to 0.44 ± 0.12 μmoL/cm·h. The rate of permeability of compounds were noted to be: acetyl hexapeptide-3 = 0.42 ± 0.14 μmoL/cm·h; hexapeptide = 0.84 ± 0.11 μmoL/cm·h; L-carnitine = 1.95 ± 0.21 μmoL/cm·h; oxytocin = 0.16 ± 0.05 μmoL/cm·h; tetrapeptide-3 = 0.90 ± 0.09 μmoL/cm·h. Hence, showing an inverse association between peptide permeability and their molecular weight.
Hoang et al. [20] showed the delivery of antiparkinsonian agents pramipexole dihydrochloride and amantadine hydrochloride across porcine ear skin with solid microneedles of 500 μm in length. The in vitro Diffusion studies show the passive transdermal flux of amantadine and pramipexole after 12 h. was 22.38 ± 4.73 μg/cm2/h and 134.83 ± 13.66 μg/cm2/h, respectively, while microneedle facilitated flux was 49.04 ± 19.77 μg/cm2/h and 134.04 ± 0.98 μg/cm2/h, respectively. The stainless steel microneedle roller was capable of producing microchannels in the stratum corneum, increasing the mean flux of Amantadine hydrochloride by 1.57-folds.
Martanto et al. [21] fabricated an array of solid microneedles for delivery of insulin in diabetic rats and designed an array of 105 microneedles with a length of 1000 μm. The insulin dose of 1.6–4.1 mU (0.06–0.15 μg) was delivered and blood glucose level tested after 4 h the treatment with microneedles was more effective, to a measure of 0.05–0.5 U, as compared to hypodermic needles.
In another experiment by Chiang et al. [22], to study the efficiency of microneedles on the round window membrane of the ear, the MNs were able to penetrate the membrane precisely despite its small size. Two sets of microneedles were fabricated, by 3-D printing technique, of sizes 100 μm and 150 μm, respectively. These MNs were then subjected to the round window ear membrane of a Guinea pig. Thickness of the membrane was determined by endoscopy: 60 ± 14.6 μm and the dimensions of MN penetration were found to be: (a) for 100 μm MN, depth = 7.7 ± 2.6 μm and width = 103.4 ± 17.1 μm; (b) for 150 μm MN, depth = 15.9 ± 7.9 μm and width = 153.9 ± 20.9 μm. In accordance with these results, they were able to conclude the efficiency of MNs for accurate perforation of the round window membrane without causing any damage to it.
The delivery of herbal medication Vitex agnus-castus and Tamarindus indica for cellulite using microneedles were studied by Reham I. Amer et al. [23]. Polymers such as Polyvinylpyrrolidone K-30 (PVP K-30), Chitosan, and Sodium alginate were used to create microneedles with a length of 600 μm and a base width of 300 μm. Four different formulations of the drug were used with percentage drug content to be 95.01 ± 1.90, 97.90 ± 1.25, 96.20 ± 1.20 and 98.95 ± 2.10 and the % of drug released after 90 min were 87.95 ± 1.4, 96.90 ± 2.6, 90.4 ± 3.4 and 98.01 ± 1.6, respectively. The in vitro drug release studies show that in 90 min more than 90% of encapsulated drugs in microneedles were released. Concluding that microneedles loaded with anti-cellulite drugs may help to improve the appearance of the skin by reducing inflammatory parameters and improving antioxidant power.
Coated microneedles
Coated microneedles follow the mechanism of the ‘coat and poke’ principle. Microneedles are usually coated with a water-soluble drug formulation which dissolves after the insertion of microneedles (Fig. 2) in the skin [24]. Coated microneedles, especially in pharmaceuticals, vaccines, DNA and biomolecules were studied widely. The coated microneedles can be fabricated using stainless steel, titanium and polymers. The drug-loaded on microneedles is determined by the thickness of the coating layer and the needle size [18], resulting in low drug delivery efficiency. Multiple coating methods, such as dip-coating, gas-jet drying, spray coating, electrohydrodynamic atomization (EHDA)-based technique, and ink-jet printing, have been developed to resolve the drawback of drug wastage.
Baek et al. [25] fabricated the microneedles of poly(L-lactide) acid (PLLA) coated with 290.6 ± 45.9 μg of lidocaine, which is mostly used for local anaesthesia. The amount of lidocaine delivered after 1, 2 and 5 min were 200.8 ± 43.9 μg, 224.2 ± 39.3 μg, and 244.1 ± 19.6 μg, respectively, which was compared with EMLA® cream. The results show that the delivered amount of lidocaine into the skin was remarkably higher by 22.0, 13.6, and 14.0-fold than the lidocaine delivered by EMLA® cream at the same intervals. These results suggested that coated microneedles arrays are more efficient and enhance the in vitro permeation of lidocaine compared to commercially available anaesthetics.
Yunzhe et al. [26] performed the delivery of hydrophobic drug lidocaine with hydrophilic matrix Polyethylene glycol (PEG). The microneedles are fabricated from stainless steel with a length of 700-µm and 5 microneedles per row. The array of microneedles coated with PEG and lidocaine in ratio 50:50 molten dispersion was inserted into porcine skin for 3 min. The results show thermal stability of PEG and lidocaine at 300 °C and 130 °C, respectively. The lidocaine content left on the microneedle and on the surface of the skin after insertion was 10.2 ± 6.7% and 3.2 ± 1.2%, respectively, and 86.6 ± 7.4% lidocaine was delivered into the tissue. In comparison to the topical application of 0.15 g of EMLA cream, microneedles were coated with 93 g of lidocaine and administered roughly double the amount of lidocaine into porcine tissue. The delivery efficiency of PEG-lidocaine coated microneedles is higher than that of hydrophilic lidocaine hydrochloride-coated microneedles. For water-insoluble molecules, solid dispersion-based coatings can provide similar delivery efficiency and wear-times as for water-soluble molecules.
To test the hypothesis for the delivery of a drug, using coated microneedles into the eye, Jiang et al. [27] fabricated the microneedles of length 750 µm coated with pilocarpine and sodium fluorescein in dose 10% (wt/vol) and 0.5% (wt/vol), respectively. The in vivo analyses of microneedles coated with 0.28 μg sodium fluorescein after injection in the rabbit's cornea were tracked for 24 h, with limited background fluorescence in the aqueous humour before the microneedle was inserted. A sharp increase in the concentration of intraocular fluorescein was observed just 1 min after microneedle insertion. A traditional topical fluorescein eye drop was added for contrast, and fluorescein concentration was measured over time in the rabbit eye. However, following topical application of an equal dose of fluorescein, there were low levels of delivery of fluorescein to the aqueous humour. Topical administration of 5.0 μg of pilocarpine caused 1 mm of pupil constriction and topical administration of 500 μg of pilocarpine caused 4 mm of pupil constriction. The transmission of microneedles caused 2.5 mm of pupil constriction. Since microneedles only delivered 5.5 g of pilocarpine, this represents a 45-fold increase in pilocarpine bioavailability over topical administration.
Coated and uncoated microneedles were prepared by Hye-Rin Jeong et al. [28] using four different polymers i.e. polyethylene (PE), nylon, polypropylene (PP) and polylactic acid (PLA). Three different subgroups were fabricated with different aspect ratios of height to width were 2.2, 2.5 and 3.0, for uncoated microneedles and 1.3, 1.4 and 1.6 for coated microneedles. The in-vitro penetration test on porcine skin shows the puncture performance of PE and PP for uncoated (3.0) was 8% and 53%, respectively, while in the case of coated microneedles fabricated of PP and PE it was 95% and 82%, respectively. The puncture performance in vivo with PP microneedles increased from 59% in uncoated to 96% in coated microneedles. With this, they concluded that coated microneedles are safer and reduce the chances of contamination.
Coated microneedles have also been tested for anti-cancer therapy. Hao et al. [29], fabricated poly(l-lactide) microneedles, coated with PEGylated gold nanorod (for photothermal imaging) combined with MPEG-PDLLA-DTX micelles for its anti-tumor activity. A431 tumors were treated with low doses of docetaxel, and the results showed that the coated MN mediated therapy was able to cure the tumors in subjected rat models.
In a similar study by Uddin [30], the anti-cancerous activity of cisplatin was exploited through 3-D printed microneedles. The coated microneedles showed a depth of 154.1 ± 18 μm in mice skin and achieved drug release rates of 80–90% within a time interval of 60 min. The tumor inhibition effect was tested and confirmed through histopathology. The results were able to exhibit the potential for transdermal delivery of anti-tumor drugs.
Hollow microneedles
Hollow microneedles work on the basis of the ‘poke and flow’ principle and work in a similar fashion as hypodermic needles. Microneedles are inserted in the skin, then liquid formulation flows into the skin (Fig. 3) and the flow rate can be controlled via machines [24]. For continuous delivery, various techniques such as diffusion, vibration, and electrical assistance may be used. High-molecular-weight substances such as antigens, proteins, and oligonucleotides are often used [18], and these microneedles can deliver a significant dose of a drug at a steady flow rate. [10]. These microneedles can be used for direct targeted delivery and help in increasing the efficiency of drugs by reducing wastage. It can also be utilised for signal monitoring, blood and tissue sampling applications, but also have certain drawbacks such as blockage of the needle upon insertion into the skin. Hollow microneedles can be fabricated with silicone, metal, polymers, glass and ceramic materials [24].
A hollow Stainless microneedle to release insulin was developed by Vinay Kumar et al. [31] The hollow microneedles of height 300 µm, outer diameter at the tip was 110 µm and 150 µm at the base were fabricated with a peristaltic pump was used for delivery of insulin in rat skin. The insulin successfully diffused in the bloodstream and blood glucose level decreased significantly after 5 h to normal level (80–120 mg dl-1), showing that subcutaneous insulin delivery can be efficiently supplemented with hollow microneedles.
Patel et al. [32] fabricated a hollow microneedle from a borosilicate micropipette tube for the delivery of sulforhodamine B in the suprachoroidal space of rabbit and pig ex vivo. They were administered volumes of up to 15–35 μL; large volumes leading to leakage. The most effective delivery was provided by 800–1000 μm lengths of the needle with applied pressures of 250–300 kPa.
Norman et al. [33] used an intradermal adapter, Mantoux technique, and hollow microneedle to deliver a fluorescent dye to pig skin in vivo to compare three intradermal delivery devices. The results showed similar reliability: 97.6 ± 1.5%, 95.4 ± 4.9% and 94.9 ± 0.3% delivered for the intradermal adapter, Mantoux technique, and hollow microneedle, respectively. The accuracy shown by all three devices were: 92 ± 21%, 97 ± 16% and 99 ± 12% delivered to the dermis, respectively, showing hollow microneedles may take over other techniques of drug delivery in future.
Polymeric nanoparticles (NPs) were delivered intradermally in rats using hollow microneedles by Niu et al. [34]. Nanoparticles were fabricated by poly(d,l-lactide-co-glycolide) (PLGA) with encapsulated antigen ovalbumin (OVA) and TLR agonists imiquimod and monophosphoryl Lipid A. A comparison of soluble OVA-based vaccines delivered by intramuscular injection and OVA loaded nanoparticles delivered by a hollow microneedle array. When compared to intramuscular injection, the microneedle produced higher levels of IgG2a antibody and IFN-secreting lymphocytes.
Dissolving microneedles
Dissolving microneedles deliver drugs on the ‘poke and release’ principle and are made from biodegradable polymers, with the drug encapsulated inside the polymer. The rate at which the drug releases is controlled by the degradation of the polymer upon insertion of a needle into the skin (Fig. 4). These microneedles have the convenience of use and a higher drug loading capacity, furthermore, it leaves no biohazardous wastes in the skin after getting completely dissolved. Dissolving microneedles face some problems while developing dissolving microneedles with drugs and they take time to dissolve completely and sometimes insertion can be difficult [10, 17]. Polyvinylpyrrolidone (PVP), carboxymethylcellulose, polyvinyl alcohol (PVA), poly (lactic acid), chitosan, sugar, dextran, poly (glycolic acid), poly (lactide-co-glycolide) (PLGA), and other materials may be used to make dissolving microneedles. [18].
In a dissolving microneedle study designed by González-Vázquez et al. [35], gentamicin drug was transdermally delivered in Sprague–Dawley rats using microneedles fabricated by PVP, PVA, N-acetylcysteine and polyethylene glycol. The rats were divided into four groups (10 rats in each group) which received the gentamicin administration in the following manner- (a) intramuscular injections with an average dose of 7.5 mg/kg; (b) transdermally administered low dose (1 array containing 30 mg of gentamicin); (c) transdermally administered medium dose (2 arrays containing 30 mg gentamicin each); (d) transdermally administered high dose (4 arrays containing 30 mg gentamicin each). From each group, five rats were sampled for blood plasma at 1 h and 4 h intervals, the other five sampled at 2 h and 6 h intervals and all the rats were sampled at a 24 h interval. The first group (IM injections) showed a mean gentamicin concentration of 5.72 ± 0.35 μg/mL after 1 h which was reduced to 2.52 ± 0.49 μg/mL after 2 h; following the progressive decrease in gentamicin level, the rest of the sample were found to be lower than the limits of quantification (0.099 μg/mL). gentamicin concentrations in second group (low dose) were observed to be 1.13 ± 0.42 μg/mL, 1.58 ± 1.31 μg/mL, 1.80 ± 1.22 μg/mL, 2.21 ± 1.46 μg/mL and 0.93 ± 1.11 μg/mL at 1 h, 2 h, 4 h, 6 h and 24 h intervals, respectively (gradual increase till 6 h, then reduced at 24 h). gentamicin concentrations in third group (medium dose) were 1.83 ± 1.13 μg/mL, to 5.34 ± 4.23 μg/mL, 2.37 ± 1.81 μg/mL, 4.58 ± 4.11 μg/mL and 1.68 ± 0.94 μg/mL at 1 h, 2 h, 4 h, 6 h and 24 h intervals, respectively (concentration drops at 4 h, increases at 6 h and again drops at 24 h). gentamicin concentration in the fourth group (high dose) was observed to be 4.30 ± 1.47 μg/mL at 1 h interval, followed by consistently lowered concentration at rest of the intervals (less than 3 μg/mL). The results demonstrated that gentamicin administered transdermally had better permeation (at low or medium doses) than intramuscular administration.
Yao et al. [36] delivered levonorgestrel (LNG) in vitro and in vivo using dissolving microneedles formed by chitosan and β-GP gel with height 800 μm in pig skin. The dissolving ability of microneedles, after a time period of 2 h, was found to be 69.32 ± 4.23%. The microneedles, loaded with LNG, delivered approximately 75.62 ± 22.79% of the drug across the skin. The pharmacokinetic parameters in the experiment showed Tmax to be 0.5 h (for transdermal and oral administration) and Cmax to be approximately 189.27 ± 57.46 ng/mL (transdermal) and 224.71 ± 55.23 ng/mL (oral). This indicates dissolving microneedles may be a better alternative to different administration routes of drugs.
Lahiji et al. [37] examined the in vivo delivery efficacy by applying insulin loaded dissolving microneedles in pig cadaver skin with a height of 600 μm. The drug release after 2 h by dissolving microneedles patch was 56 ± 5% and by Microlancer using single dissolving microneedles the release was 92 ± 2%. The biological activity of insulin was 99 ± 1% and for insulin loaded dissolving microneedles was 95 ± 3.3%. The maximum plasma insulin concentration for subcutaneous (SC) injection was 156 μIU/ml whereas for Microlancer and patch group was 128 μIU/ml and 61 μIU/ml, respectively, showing that subcutaneous injection can be effectively replaced by microneedles.
In the study by Panda et al. [38], Polyvinylpyrrolidone (PVP), hyaluronic acid (HA), and poly lactic-co-glycolic acid (PLGA) were used to fabricate dissolving microneedles with entrapped lysozyme (14 kDa). Rat models were used for carrying out the drug release tests in ex vivo. In case of PVP and HA, the microneedles dissolution was observed in 5–10 min and 50% of the drug was released in 20 min. After 20 min, 3.74 ± 1.22 µg/min and 3.94 ± 1.05 µg/min of lysozyme was released from PVP and HA microneedles, respectively. Furthermore, the PLGA microneedles released 29.53 ± 0.78% of drug after 72 h, demonstrating a consistent and long-term drug release profile.
Lee et al. [39] tested the dissolving microneedle made up of biodegradable polymer carboxymethyl cellulose (CMC, 90 kDa) and containing 331.20 ± 6.30 µg lidocaine (Li-DMN) for use in local anaesthesia. The average length and base diameter of microneedles with lidocaine were 369.62 ± 11.64 μm and 688.60 ± 16.56 μm, respectively and without lidocaine were 376.34 ± 8.39 μm and 671.05 ± 11.40 μm, respectively. Lidocaine doses and blank dissolving microneedle patches were applied to the inner surface of an adult rat's ear to determine tissue responses. The ear thickness was increased by 10% in 10 min which was recovered within 60 min, no sign of inflammation, bleeding etc. were observed in both cases. The microneedles were dissolved after 1 min of application. The in vivo anaesthetic efficacy was tested by applying the microneedles on the rat’s hind paws. The paw withdrawal threshold was 24.6 ± 0.5 g after the application of topical lidocaine cream which increased to 69.5 ± 16.9 g, 77.6 ± 12.3 g and 42.9 ± 6.0 g after 10, 30 and 60 min of microneedle application, respectively, indicating that the effect of topical lidocaine cream was less than that of the lidocaine dissolving microneedle.
Transdermal delivery of huperzine A (Hup-A) for treatment of Alzheimer’s disease (AD) using dissolving microneedles is studied by Yan et al. [40], The dissolving microneedles had a length of 500 μm, a base diameter of 250 μm and a tip diameter of 20 μm. The Hup-A microneedles were inserted into the skin of the Sprague–Dawley rat's abdomen for carrying out the in-vitro studies. For pharmacokinetic studies rats were divided into three groups, one received oral 0.5 mg Hup-A A and the other two groups received a different dosage of 0.5 mg and 1 mg Hup-A through dissolving microneedles. The maximum concentration (Cmax) at 3 h and 6 h by oral administration (0.5 mg), dissolving microneedles (0.5 mg) and dissolving microneedles(1 mg) were 8.48 ± 0.91 ng/mL, 5.53 ± 0.53 ng/mL and 11.70 ± 0.96 ng/mL, respectively. The half-life of oral administration (0.5 mg) was 3.44 ± 0.40 h whereas dissolving microneedles (0.5 mg) and dissolving microneedles (1 mg) were 15.21 ± 2.09 h and 14.32 ± 0.75 h, respectively. This study shows that dissolving microneedles are advantageous as they increase bioavailability and sustain the release of drug with minimum invasiveness into the skin.
Peipei et al. [41] demonstrated a study in which they targeted an immune checkpoint where the programmed cell death receptors (PD-1) interact over the anti-tumor T-cells and the related ligand (PD-L). Anti-PD-1 and anti-PD-L1 antibodies were delivered in combination with 1-methyl-d,l-tryptophan as the checkpoint inhibitor for melanoma. For this purpose, core–shell microneedles were fabricated from polydimethylsiloxane using centrifugation molding technology which consisted of micron-size cavities where the formulation was loaded. This indicated an efficient transdermal delivery that showed favorable anti-tumor effects in the rat models that were involved in the experiment.
Hydrogel-forming microneedles
Hydrogel-forming Microneedles are fabricated using super-swelling polymers. The polymers are the hydrophilic structure having a 3D-polymeric network that enables them to incorporate large quantities of water. The polymers swell after interacting with interstitial fluid, once the insertion into the skin takes place (Fig. 5). This leads to channels formed between the capillary and drug patches. Until needling, these microneedles are only used to disrupt the skin barrier. After swelling, they act as a membrane control rate. They are fabricated in a variety of shapes and sizes. The unique features of such microneedles include ease of sterilization and intact removal from the skin and for the manufacture of swellable microneedles for drug delivery, cross-linking polymers are used [10].
Migdadi et al. [42] have performed a study on hydrogel-forming microneedles for transdermal administration of metformin to decrease oral-related gastrointestinal side effects. The in vivo penetration was carried out with 16 Sprague–Dawley rats divided into two groups of 8 rats each. First group received a dose of 100 mg/kg metformin formulation orally. Second group was subjected to transdermal administration with two microneedles patches containing 50 mg metformin formulation each, using hydrogel-forming microneedles (prepared by crosslinking of a copolymer of methyl-vinyl-ether & maleic anhydride (20% w/w), with ‘poly-ethylene glycol’ (7.5% w/w); gel formed using this mixture with 3% w/w Na2CO3 in deionized water). Blood samples were obtained in the following pattern from the tail veins of rats from both the groups. (a) Four rats sampled after 1 h and 3 h of administration; (b) other four rats sampled after 2 h and 4 h of administration; (c) all rats sampled after 24 h. After 1 h, the measured concentration of metformin was 4.97 ± 2.57 μg/ml & 0.62 ± 0.51 μg/ml, for orally and transdermally administered groups, respectively. Blood samples from orally administered groups had a drug concentration of 1.42 ± 1.37 μg/ml after 4 h, which continued to reduce until the 24 h interval. The samples from the transdermal administration group showed a drug concentration of 3.21 ± 0.69 μg/ml, which was observed to increase to 3.77 ± 2.09 μg/ml. The concentration of the drug decreased with time when orally subjected, whereas the transdermal treatment showed comparatively steady and consistent levels of the drug. These results showed that the drug with designed microneedles had improved permeation and bioavailability.
Demir et al. [43] prepared hydrogel-forming microneedles using pectin (PE) and crosslinking poly (methyl-vinyl-ether-co-maleic acid) (PMVE/MA). 3D printing-based swellable microneedles array with a height range of 702.5 ± 11.9 μm to 726 ± 23.3 μm and aspect ratio 3.12 ± 0.20 to 3.29 ± 0.21. The hydrogel film was swelled after an hour, the surface quality of film was enhanced by cross-linking. After 7 days, the lowest % swell ratio of cross-linking PMVE/MA:PE (12.5:4% w/w) films was 485 ± 70%, with an equilibrium water content of 85.04 ± 1.55%. Concluding, a novel cross-linked polymer system was developed with active drug delivery and bio-analytical applications.
Eltayib et al. [44] prepared hydrogel-forming microneedle arrays from hydrolyzed poly (methyl-vinyl ether-co-maleic anhydride) that were then cross-linked with poly (ethylene glycol). Six rats were split into two classes for in vivo lithium carbonate control. The serum concentration in group 1 at 15 mg/kg was 0.1 ± 0.08 mmol/l, while in group 2 at 30 mg/kg it was 0.24 ± 0.06 mmol/l. The lithium levels extracted by applied microneedles were then calculated for group 1 and group 2 rats after an hour; the concentrations were 4.0 ± 2.5 μmol/l and 4.9 ± 2.2 μmol/l, respectively, indicating a mean increase of 22.5% in rats receiving higher doses. Hydrogel-forming microneedles can hence be considered as a potential tool with minimum invasiveness in the patients for drug monitoring.
In another study by Vicente-Pérez et al. [45] hydrogel-forming microneedles have been used for insertion feedback. The hydrogel-forming microneedles arrays were combined with pressure indicating sensor film (PISF). PISF demonstrates a change in colour depending upon the applied pressure, indicating whether or not the insertion was successful. 20 volunteers were given two microneedle patches each (one with PSIF and one without it) for self-application. The colour change in the PISF was compared with the range of penetration depth (measured by optical coherence tomography). The penetration depths were observed to be 245.2 ± 65.8 μm and 228.1 ± 53.7 μm for microneedles patches with and without PISF, respectively. This approach could be applied for getting visual feedback to the user, for successful insertion of the microneedles patch.
Microneedles for peptides delivery
A peptide is an amino acid chain that is linked together by peptide bonds. On an average, a peptide length ranges between 2 and 50 amino acids; the smaller size of the amino acid chain differentiates it from a protein. The peptides are important to contemplate because they have been studied to provide many health benefits. The smaller size of the peptides makes them easier for the body to absorb, as compared to the proteins. Potentially, peptides tend to show various properties such as anti-ageing, anti-microbial, anti-inflammatory or muscle building potential; peptides that have a positive effect on the body or health are termed as bioactive peptides.
With this context, the recent advancements have been focussing on developing peptides as therapeutic agents and the list of therapeutic peptides is expeditiously growing ever since [46, 47]. Despite this progress, the peptide delivery or administration to the body has been adversely limited. In lieu of the fact that oral administration is the preferred mode of peptide delivery, a vast proportion of these therapeutic peptides have been delivered subcutaneously, or intravenously for few peptides such as Bivalirudin (anticoagulant for patients undergoing coronary angioplasty) [48]. The orally administered peptides show low bioavailability and inadequate absorption in the gastrointestinal tract. The peptides are subjected to the gastric and intestinal enzymes (rich in proteases) when administered orally. This serves as a notable cause for the degradation of peptides that results in the loss of therapeutic effects [49]. In another study, Suyong et al. [50] demonstrated the potential of microneedles to deliver insulin in powder form Powder carrying microneedles were fabricated from stainless steel, having micron-sized cavities, using a laser cutting technique. The powder carrying MNs were able to deliver the required dose of insulin over an extended period of time, with minimal loss of the drug. This study presented an innovation that could be used for delivering the drugs that are formulated in dry form and therefore extending the applicability of the MNs in clinical practice. Evidently, the MNs have also been tested for the treatment of cancer. Consequently, transdermal delivery was recognized to be more efficient for the purpose. The microneedles have popularly been employed for the delivery of smaller proteins or peptides to carry out the systemic therapeutic effects with minimum skin invasiveness. Solid, hollow and dissolving type microneedles have been earnestly adopted in clinical research practice; relevant studies described in Table 2.
Recent Advances in MN-based biosensors
With many advances in the field of MNs and rising demand of minimal invasive techniques, use of MN-based biosensors have also been reported widely for diagnostics and drug delivery.
Ranamukhaarachchi et al. [51] performed a study to use hollow microneedles as biosensor for therapeutic drug monitoring (TDM). The interstitial fluid of volume 0.6 nl was used for measuring vancomycin concentration with a limit of detection < 100 nM. This concentration was detected by a proposed microneedle-optofluidic biosensor showing microneedles can be used as alternatives to hypodermic needles. In a more convoluted study, Mishra et al. [52] designed MN-based electrochemical biosensors for the detection of organophosphate chemicals, neurotoxins often used in chemical weapons. Hollow MNs were fabricated using 3D printing technique using acrylate polymer. The MNs were coupled with carbon paste electrode transducer which were further coated with a layer of organophosphorus hydrolase. These MNs were tested on rat skin models which were exposed to the methyl paraoxon (MPOx) neurotoxins and upon application the electrode transducer was able to detect the hydrolysis reaction of the organophosphorus hydrolase. The MN-based biosensors were able to detect the neurotoxin with high accuracy, in a range of 20–180 µM and hence they were able to further demonstrate the potential biosensor technique for on-body assessments..
Another study by Jayaneththi et al. [53] a controllable zero order and pulsatile drug release profiles was observed by constructing a drug dispensing and dosage sensing using hollow microneedles. The mean flow rate for fast pulsatile release was taken at two intervals showing 105 μL/min and 103 μL/min release. The drug reservoir containing 0.5 ml drug was emptied in the 330 s. These results show that the battery-less technology can deliver controlled medication release on demand.
Conclusion
With the continual research on the topic, varied types of microneedles have been introduced, with their unique set of advantages and disadvantages. Also, various techniques have been reported for the fabrication of microneedles arrays and patches. Some of the popularly exploited fabrication techniques may include micro-moulding, lithography, etching (wet or dry), 3D printing [54, 55]. In addition to these methods, some unique techniques have also been coined—electro drawing [56], thermal drawing [57], magnetorheological drawing lithography [58] and droplet-born air blowing [53]. Microneedles are generally fabricated by materials like metal, polymer, plastic or other inorganic materials [17], and the fabrication technique depends on the material of choice. These fabrication techniques and materials are being exploited and studied for developing an effective strategy for drug delivery, in terms of stability, safety and potency [59].
The ongoing studies show that microneedles are a promising alternative for transdermal administration of peptide compounds. Nevertheless, the results may vary upon administration, in terms of release rates, bioavailability, etc., due to the differences in the molecular weight of the peptides [19, 60]. Studies suggest that the lower molecular weight of the peptides accounts for inducing higher release rates, and vice versa. However, the current literature also demonstrates efficient in-vitro delivery of compounds with relatively higher molecular weights via. microneedles [61]. The use of hydrogel-forming microneedles for the delivery of therapeutic or cosmeceutical peptides still has a wide scope that can be exploited.
Comprehensively, it can be safely concluded that microneedles hold an undeniable potential for the transdermal delivery of drugs, or other therapeutic compounds.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Pfeiffer RF. Transdermal drug delivery in Parkinson’s disease. Aging Health. 2007;3:471–82.
Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13:175–87.
Henzl MR, Loomba PK. Transdermal delivery of sex steroids for hormone replacement therapy and contraption. A review of principles and practice. J Reprod Med. 2003;48:525–40.
Bos JD. Meinardi MMHM The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9.
Gaikwad AK. Transdermal drug delivery system: formulation aspects and evaluation. Compr J Pharm Sci. 2013;1:1–10.
Jampliek J. Transdermal application of drugs and techniques affecting skin barrier. J Bioequiv Bioavailab. 2013;5:233–5.
Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2011;64:11–29.
Allegranzi B. The burden of unsafe injections worldwide: highlights on recent improvements and areas requiring urgent attention. World Health Organization. https://www.who.int/medical_devices/Sun_pm_SAF_2_ALLEGRANZI.pdf. Accessed 1 Aug 2021
Sharma N, Parashar B, Sharma S, Mahajan U. Blooming pharma industry with transdermal drug delivery system. Indo Global J Pharm. 2012;2:262–78.
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, Dua K. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58.
Ita K. Transdermal delivery of drugs with microneedles—potential and challenges. Pharmaceutics. 2015;7:90–105.
Chambers R. Microdissection studies III, Some problems in the maturation and fertilization of the echinoderm egg. Biol Bull. 1921;41:318–50.
Gerstel MS, Place VA. Drug delivery device 1976. Google Patents.
Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–5.
Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–9.
Kochhar JS, Tan JJY, Kwang YC, Kang L. Microneedles for transdermal drug delivery. Cham: Springer; 2019.
He X, Sun J, Zhuang J, Xu H, Liu Y, Wu D. Microneedle system for transdermal drug and vaccine delivery: devices, safety, and prospects. Dose-Response. 2019;17.
Sharma S, Hatware K, Bhadane P, Sindhikar S, Mishra D. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater Sci Eng. 2019;103.
Zhang S, Qiu Y, Gao Y. Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharm Sin B. 2014;4:100–4.
Hoang MT, Ita KB, Bair DA. Solid microneedles for transdermal delivery of amantadine hydrochloride and pramipexole dihydrochloride. Pharmaceutics. 2015;7:379–96.
Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21:947–52.
Chiang H, Yu M, Aksit A, Wang W, Stern-Shavit S, Kysar JW, Lalwani AK. 3D-printed microneedles create precise perforations in human round window membrane in situ. Otol Neurotol. 2019;41:277–84.
Amer RI, El-Osaily GH, Bakr RO, El-Dine RS, Fayez AM. Characterization and pharmacological evaluation of anti-cellulite herbal product(s) encapsulated in 3D-fabricated polymeric microneedles. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-63271-6.
Ali R, Mehta P, Arshad MS, Kucuk I, Chang MW, Ahmad Z. Transdermal microneedles—a materials perspective. AAPS PharmSciTech. 2020;21:12.
Baek SH, Shin JH, Kim YC. Drug-coated microneedles for rapid and painless local anesthesia. Biomed Microdevices. 2017. https://doi.org/10.1007/s10544-016-0144-1.
Ma Y, Gill HS. Coating solid dispersions on microneedles via a molten dip-coating method: development and in vitro evaluation for transdermal delivery of a water-insoluble drug. J Pharm Sci. 2014;103:3621–30.
Jiang J, Gill HS, Ghate D, McCarey BE, Patel SR, Edelhauser HF, Prausnitz MR. Coated microneedles for drug delivery to the eye. Investig Opthalmol Vis Sci. 2007;48:4038.
Jeong H, Jun H, Cha H, Lee JM, Park JH. Safe coated microneedles with reduced puncture occurrence after administration. Micromachines (Basel). 2020;11:710.
Hao Y, Dong M, Zhang T, Peng J, Jia Y, Cao Y, Qian Z. Novel approach of using near-infrared responsive PEGylated gold nanorod coated poly(l-lactide) microneedles to enhance the antitumor efficiency of docetaxel-loaded MPEG-PDLLA micelles for treating an A431 tumor. ACS Appl Mater Interfaces. 2017;9:15317–27.
Uddin MJ, Scoutaris N, Economidou SN, Giraud C, Chowdhry BZ, Donnelly RF, Douroumis D. 3D printed microneedles for anticancer therapy of skin tumours. Mater Sci Eng. 2019;107:110248.
Vinayakumar KB, Kulkarni PG, Nayak MM, Dinesh NS. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J Micromech Microeng. 2016;26:65013.
Patel SR, Lin ASP, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2010;28:166–76.
Norman JJ, Gupta J, Patel SR, Park S, Jarrahian C, Zehrung D, Prausnitz MR. Reliability and accuracy of intradermal injection by Mantoux technique, hypodermic needle adapter, and hollow microneedle in pigs. Drug Deliv Transl Res. 2013;4:126–30.
Niu L, Chu LY, Burton SA, Hansen KJ, Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J Control Release. 2019;294:268–78.
Vázquez PG, Larrañeta E, McCrudden MTC, Jarrahian C, Rein-Weston A, Solares MQ, Zehrung D, McCarthy H, Donnelly F, et al. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J Control Release. 2017;265:30–40.
Yao G, Quan G, Lin S, Peng T, Wang Q, et al. Novel dissolving microneedles for enhanced transdermal delivery of levonorgestrel: in vitro and in vivo characterization. Int J Pharm. 2017;534:378–86.
Lahiji SF, Dangol M, Jung H. A patchless dissolving microneedle delivery system enabling rapid and efficient transdermal drug delivery. Sci Rep. 2015;5:7914.
Panda A, Shettar A, Sharma PK, Repka MA, Muthy SN. Development of lysozyme loaded microneedles for dermal applications. Int J Pharm. 2021;593:120104.
Lee B-M, Lee C, et al. Dissolving microneedles for rapid and painless local anesthesia. Pharmaceutics. 2020;12:366.
Yan Q, Wang W, et al. Dissolving microneedles for transdermal delivery of huperzine A for the treatment of Alzheimer’s disease. Drug Deliv. 2020;27:1147–55.
Yang P, Lu C, Qin W, Chen M, Quan G, Liu H, Wang L, Bai X, Pan X, Wu C. Construction of a core-shell microneedle system to achieve targeted co-delivery of checkpoint inhibitors for melanoma immunotherapy. Acta Biomater. 2020;104:147–57.
Migdadi EM, Courtenay AJ, Tekko IA, McCrudden MTC, et al. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J Control Release. 2018;285:142–51.
Demir YK, Metin AU, Şatıroğlu B, Solmaz ME, Kayser V, Mäder K. Poly (methyl vinyl ether-co-maleic acid)—pectin based hydrogel-forming systems: gel, film, and microneedles. Eur J Pharm Biopharm. 2017;117:182–94.
Eltayib E, Brady AJ, Caffarel-Salvador E, Gonzalez-Vazquez P, Alkilani AZ, McCarthy HO, McElnay JC, Donnelly RF. Hydrogel-forming microneedle arrays: potential for use in minimally-invasive lithium monitoring. Eur J Pharm Biopharm. 2016;102:123–31.
Vicente-Pérez EM, Quinn HL, McAlister E, O’Neill S, Hanna L-A, Barry JG, Donnelly RF. The use of a pressure-indicating sensor film to provide feedback upon hydrogel-forming microneedle array self-application in vivo. Pharm Res. 2016;33:3072–80.
Herwadkar A, Banga AK. Peptide and protein transdermal drug delivery. Drug Discov Today Technol. 2012;9:e147–54.
Walsh G. Biopharmaceuticals. West Sussex: Wiley; 2003.
Mitchell M. The medicines company reports full year and fourth quarter 2011 financial results. Boston: Vertex Pharmaceuticals Incorporated; 2012.
Delie F, Blanco-Príeto M. Polymeric particulates to improve oral bioavailability of peptide drugs. Molecules. 2005;10:65–80.
Kim S, Yang H, Eum J, Ma Y, Lahiji SF, Jung H. Implantable powder-carrying microneedles for transdermal delivery of high-dose insulin with enhanced activity. Biomaterials. 2020;232:119733.
Ranamukhaarachchi S, Padeste C, Dübner M, et al. Integrated hollow microneedle-optofluidic biosensor for therapeutic drug monitoring in sub-nanoliter volumes. Sci Rep. 2016;6:29075.
Mishra RK, Vinu Mohan AM, Soto F, Chrostowski R, Wang J. A microneedle biosensor for minimally-invasive transdermal detection of nerve agents. The Analyst. 2017;142:918–24.
Jayaneththi VR, Aw K, Sharma M, Wen J, Svirskis D, McDaid AJ. Controlled transdermal drug delivery using a wireless magnetic microneedle patch: preclinical device development. Sens Actuat B. 2019;297:126708.
Singh TRR, McMillan H, Mooney K, et al. Fabrication of microneedles. In: Dragicevic N, Maibach HI, editors., et al., Percutaneous penetration enhancers physical methods in penetration enhancement. Berlin: Springer; 2017. p. 305–23.
Ali R, Mehta P, Arshad M, et al. Transdermal microneedles—a materials perspective. AAPS PharmSciTech. 2019;21(1):12.
Ruggiero F, Vecchione R, Bhowmick S, et al. Electro-drawn polymer microneedle arrays with controlled shape and dimension. Sens Actuat B Chem. 2018;255:1553–60.
Choi CK, Lee KJ, Youn YN, et al. Spatially discrete thermal drawing of biodegradable microneedles for vascular drug delivery. Eur J Pharm Biopharm. 2013;83:224–33.
Chen Z, Ren L, Li J, Yao L, Chen Y, Liu B, Jiang L. Rapid fabrication of microneedles using magnetorheological drawing lithography. Acta Biomater. 2018;65:283–91.
Kim JD, Kim M, Yang H, Lee K, Jung H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J Control Release. 2013;170:430–6.
Dillon C, Hughes H, O’Reilly NJ, Allender CJ, Barrow DA, McLoughlin P. Dissolving microneedle based transdermal delivery of therapeutic peptide analogues. Int J Pharm. 2019;565:9–19.
Liu S, Jin M, Quan Y, Kamiyama F, Kusamori K, et al. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur J Pharm Biopharm. 2014;86:267–76.
Cancaster B. Selecting syringes and needles. Vitality Medical, 2015. https://www.vitalitymedical.com/blog/selecting-syringes-and-needles.html. Accessed on 1 Aug 2021.
Mohammed YH, Yamada M, et al. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE. 2014;9:e101956.
Zhao X, Coulman SA, et al. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J Control Release. 2017;265:2–13.
Chen J, Qiu Y, et al. Dissolving microneedle-based intradermal delivery of interferon-α-2b. Drug Dev Ind Pharm. 2016;42:890–6.
Kumar A, Li X, Sandoval MA, et al. Permeation of antigen protein-conjugated nanoparticles and live bacteria through microneedle-treated mouse skin. Int J Nanomed. 2011;6:1253–64.
Torrisi BM, Zarnitsyn V, et al. Pocketed microneedles for rapid delivery of a liquid- state botulinum toxin A formulation into human skin. J Control Release. 2013;165:146–52.
Dillon C, Hughes H, et al. Formulation and characterization of dissolving microneedles for the transdermal delivery of therapeutic peptides. Int J Pharm. 2017;526:125–36.
Cormier M, Johnson B, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–11.
Golombek S, Pilz M, Steinle H, et al. Intradermal delivery of synthetic mRNA using hollow microneedles for efficient and rapid production of exogenous proteins in skin. Mol Therapy-Nucleic Acids. 2018;11:382–92.
Vora LK, Courtenay AJ, et al. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int J Biol Macromol. 2020;146:290–8.
Pires LR, Amado IR, Gaspar J. Dissolving microneedles for the delivery of peptides—towards tolerance-inducing vaccines. Int J Pharm. 2020;586:119590.
Acknowledgements
The authors are thankful for the support and encouragement provided by the Department of Biotechnology, Jaypee Institute of Information Technology, Noida (U.P.), India, for this manuscript.
Author information
Authors and Affiliations
Contributions
KA and TS carried out information retrieval, designing the figures, compilation of the draft; Shweta Dang conceptualized, supervised and finalized the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
This is a review-type article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Consent for publication
Informed consent given by all authors involved in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aich, K., Singh, T. & Dang, S. Advances in microneedle-based transdermal delivery for drugs and peptides. Drug Deliv. and Transl. Res. 12, 1556–1568 (2022). https://doi.org/10.1007/s13346-021-01056-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01056-8