Abstract
Polyethyleneimine (PEI) has been extensively investigated as an efficient carrier for nucleic acid delivery. Yet, it suffers from a high toxicity profile that hinders clinical translation. Fluorination has proven to be a valid approach to reduce the cytotoxicity of PEI and improve the in vitro siRNA delivery potency. Hydrophobicity and lipophobicity can be controllably introduced into the side chains of PEI. However, the effect of fluorination on siRNA delivery in vivo, particularly the biodistribution of siRNA polyplex nanoparticles with fluorinated PEIs, has not been extensively explored. Here, we introduce two series of fluorinated PEIs via amidation with ethyl trifluoroacetate and perfluorobutyryl chloride. Fluorination substantially improved the performance of PEI for siRNA delivery by reducing the cytotoxicity to MDA-MB-231 cells. Importantly, fluorinated PEI enabled the major accumulation of siRNA polyplex nanoparticles in the liver while non-fluorinated PEI delivered siRNA nanoparticles mainly to the lungs after intravenous administration to mice. It is envisioned that fluorination may be an important general strategy for lowering toxicity of cationic polymers, and that the fluorination-induced alteration of biodistribution may be applicable for improved delivery to different organs.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The use of short-interfering RNAs (siRNAs) is an established method to silence gene expression [1]. Effective delivery to targets, including tumors, requires balancing high potency with low carrier toxicity [2,3,4,5,6,7,8,9]. Cationic polymers, such as polyethyleneimine (PEI) and polylysine [2], are widely used as nucleic acid carriers; however, application of these materials to in vivo disease models is often limited by their cytotoxicity. Many strategies have been explored to translate formulations from in vitro to in vivo to overcome issues including toxicity, aggregation, degradation, and target site enrichment [10]. PEGylation and attachment of carbohydrates, alkyl chains, and cell targeting moieties are frequently applied strategies [11]. Modification of PEI with neutral or anionic moieties has been shown to reduce cytotoxic effects, sometimes without loss of the endosomal rupture abilities. In particular, hydrophobic modifications have been shown to improve siRNA delivery [12,13,14,15,16]. In addition, further functionalization of PEI with primary amines showed improved transfection efficiency and reduced cytotoxicity for nucleic acid delivery [17]. Given the smaller size of siRNA versus pDNA, one could reason that lower MW PEI (or BPEI) might improve delivery. For example, although oliogoethylenimine (OEI) 800 is inactive for siRNA delivery, hydrophobic modification of this construct with ten hexyl acrylate residues per OEI molecule yielded a promising carrier for siRNA delivery [12]. In an effort to improve delivery, not only intracellularly but also in vivo, full deacylation of PEI increased gene delivery to mouse lungs [18]. Hydrophobic modification of similar amidoamine carriers increased stability and efficacy [19]. Oligomers, such as guanidinium-rich amphipathic oligocarbonates, also serve to balance cationic and hydrophobic interactions with lower MW polymeric architectures to achieve effective delivery [20]. In vivo siRNA delivery to endothelial cells has also been achieved using alkyl-modified low molecular weight PEIs formulated with PEG lipids [21]. The effect of polymer composition and architecture on delivery was shown using triblock copolymers of poly(LPEI-b-(propylene glycol)-b-LPEI). Whereas LPEI50-b-PPG36-b-LPEI50 showed poor efficacy, decreasing the LPEI block length and increasing the hydrophobic block to LPEI14-b-PPG68-b-LPEI14 greatly improved delivery [22]. In these examples, it appears that incorporation of siRNA into ordered nanoparticles provides increased electrostatic interactions. Also, the addition of hydrophobic interactions further stabilizes the structures above the amine pKas.
There are many reports to illustrate that fluorination is an effective approach to improve the pharmacokinetic drug properties in terms of stability and effectiveness [23, 24]. In addition, fluorocarbon modification of delivery carriers benefits gene therapy with enhanced biocompatibility and serum stability [25,26,27,28,29,30,31,32,33,34,35,36,37]. Recent studies show that fluorination of PEIs or PAMAM dendrimers reduces the cytotoxicity and improves the potency of siRNA delivery to cells [38, 39]. However, the role of fluorine modification has not been fully recognized; particularly, effects on in vivo behavior of siRNA nanoparticles have not been extensively investigated.
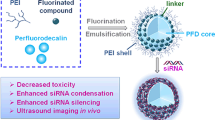
In this research, we used branched polyethyleneimine (PEI) as a model cationic polymer to explore the effect of fluorination on siRNA delivery both in vitro and in vivo. We chose two different kinds of fluorination strategies by amidation with two different lengths of fluorine chains, ethyl trifluoroacetate, and perfluorobutyryl chloride. By varying the modification degree, we obtained two series of polymers, CF3PEI (1-6) and F7PEI (1-6). Fluorinated PEI carriers mediated successful siRNA binding and luciferase reporter gene knockdown in the triple negative breast cancer (TNBC) cell line MDA-MB-231, while simultaneously decreasing cytotoxicity and aggregation versus unmodified control PEI. Interestingly, we found that PEI fluorination significantly shifted the biodistribution of siRNA from the lungs to the liver (Fig. 1), which provides insights for rational design of other fluorinated cationic polymers for liver targeting.
Guan and Chen et al. reported the fluorination of PEI using the reaction between the primary amine and epoxy or anhydride groups [26, 38]. Here, we proposed a facile approach to the fluorination of branched PEI (MW = 25,000 g/mol) with trifluoroacetate or perfluorobutyryl chloride under mild conditions (Scheme 1). By varying the mole ratio of PEI to fluorine compound from 1 to 6, we prepared two series fluorinated polymers with varying fluorination degree and the length of fluorine side chains (Supplementary Material Table S1). Fluorinated PEIs were characterized by gel permeation chromatography (GPC), 1H nuclear magnetic resonance spectroscopy (NMR), and 19F NMR (Supplementary Material Figure S1 and Supplementary Material Figure S2)
Successful siRNA delivery was demonstrated by the decrease in relative luciferase expression in MDA-MB-231 cells after treatment with polyplex NPs containing siRNA against luciferase (Fig. 2). The delivery efficacy increased with polymer/siRNA weight ratio for both polymer series implying that more fluorinated polymers may benefit the complexation with siRNA and facilitate endosomal release of siRNA inside MDA-MB-231 cells. At a weight ratio of 50:1 (Fig. 2c), siRNA delivery efficacy decreased with an increase of fluorination degree for CF3PEI polymers, while F7PEI polymers exhibited efficacious delivery even at high fluorination degree. Lead fluorinated polymers showed comparable siRNA delivery efficacy with the benchmark PEI under the same conditions. Importantly, there was a remarkable reduction in cytotoxicity for fluorinated PEI polyplexes comparing with unmodified PEI polyplexes, suggesting that fluorination maybe an approach to tackle the toxicity challenge of PEI-based carrier in nucleic acid delivery.
Luciferase expression and viability of MDA-MB-231 cells after treatment with siRNA polyplexes of a CF3PEI or b F7PEI at polymer/siRNA ratio (wt/wt) of 10, 20, and 50. c In vitro delivery of siRNA with fluorinated polymers at polymer/siRNA ratio (wt/wt) of 50. CF3PEI-2-10 denotes the sample of trifluoroacetate modified PEI (trifluoroacetate chloride/PEI = 2/1, mol/mol) and siRNA with a polymer/siRNA ratio (wt/wt) of 10/1. PEI-10 denotes the sample with PEI/siRNA ratio (wt/wt) of 10. Unmodified PEI was used as a control under the same conditions. Gray bars indicate the relative luciferase expression and black dots denote the cell viability compared with untreated cells
The binding affinity of the starting PEI and modified PEIs to siRNA was quantified using the Ribogreen binding assay at the optimized weight ratio of 50:1 (polymer:siRNA) (Fig. 3a). The result showed that the fluorination did not significantly change the binding affinity of siRNA to PEI under the test conditions. The size of siRNA polyplex nanoparticle with fluorinated PEIs varied with the fluorine modification ratio. For the F7PEI series, the diameter of most siRNA NPs was smaller than that for unmodified PEI-based NPs (Fig. 3b). Besides the decrease in particle size, the intrinsic hydrophobicity of fluorine groups in PEI should improve the stability of NPs, implying that these polymers may have better potential for siRNA delivery over unmodified PEI.
In vitro delivery results showed that fluorination benefited siRNA delivery by significantly reducing the cytotoxicity of higher molecular weight PEI, which is in agreement with a previous report on fluorinated low molecular weight PEI (Mn = 600) [38]. Here, we carried out animal experiments to further investigate how fluorination affects in vivo behavior of siRNA NPs (Fig. 4). It is now appreciated that the chemical structure of delivery carriers determines the physicochemical properties of corresponding siRNA NPs, which in turn affect serum protein-NP interactions and the fate of siRNA NPs in vivo [40, 41]. To examine this behavior, we employed fluorescent Cy5.5-labeled siRNA to track biodistribution of fluorinated and non-fluorinated siRNA polyplexes. We choose CF3PEI-5 polymer because it has lowest toxicity with moderate delivery efficacy (~ 60% luciferase knockdown). Mice were injected intravenously with 2.5 mg/kg Cy5.5-siRNA complexes with PEI and CF3PEI-5 at a polymer/siRNA ratio of 50 (wt/wt). Ex vivo organ imaging of mice injected with unmodified PEI Cy5.5-siRNA polyplexes showed that the majority siRNA polyplexes accumulated in the lungs, while there was considerable siRNA retention in the liver and kidneys. This is consistent with previous reports (Fig. 4a) [42, 43]. In contrast, Cy5.5 siRNA-fluorinated PEI polyplexes mainly accumulated in the liver and to lesser extent, the kidneys for CF3PEI-5-50 (Fig. 4b). There was no detectable signal in the lungs. This effect could be beneficial to reduce RES and endothelial cell uptake to promote liver delivery of PEI-based carriers. The fluorination of PEI altered the biodistribution of siRNA NPs from the lungs to the liver. In future work, we plan to examine the detailed effects of fluorination on in vivo siRNA delivery, additional chemical modifications, and the potential of fluorinated PEI for the treatment of liver diseases.
In this study, we developed a facile approach to synthesize fluorinated branched PEI and investigated the effect of fluorination on siRNA delivery both in vitro and in vivo. All fluorinated polymers showed above 90% binding affinity with siRNA and the resulting siRNA polyplexes were stable at a polymer/siRNA ratio (wt/wt) of 50. In vitro delivery studies showed that by fluorination, the cytotoxicity of PEI was significantly reduced, and the lead fluorinated polymers were still shown to be effective for siRNA delivery to MDA-MB-231 cells. The organ level biodistribution of siRNA was evaluated by ex vivo imaging following tail vein injection of Cy5.5 siRNA polyplexes in mice. Our results suggest that the fluorination can substantially alter the distribution of siRNA polyplexes, i.e., siRNA was mainly accumulated in the liver for siRNA-fluorinated PEI NPs in contrast to the major distribution of siRNA in the lungs for NPs with non-fluorinated PEI. Fluorination may provide a useful strategy to reduce the toxicity of cationic polymer-based siRNA carriers, while simultaneously increasing the liver targeting in vivo. Such chemical reactions could thereby reduce the adverse effect of lung-related toxicity of PEI.
Data availability
Materials, methods, and additional figures are included in the Supplementary Material and available on the website.
References
Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–38.
Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–93.
Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–77.
Kim HJ, Kim A, Miyata K, Kataoka K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv Drug Deliv Rev. 2016;104:61–77.
Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44(14):6518–48.
Zhou K, Nguyen LH, Miller JB, Yan Y, Kos P, Xiong H, et al. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc Natl Acad Sci U S A. 2016;113(3):520–5.
Hao J, Kos P, Zhou K, Miller JB, Xue L, Yan Y, et al. Rapid synthesis of a lipocationic polyester library via ring-opening polymerization of functional valerolactones for efficacious siRNA delivery. J Am Chem Soc. 2015;137(29):9206–9.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37.
Yan Y, Xiong H, Zhang X, Cheng Q, Siegwart DJ. Systemic mRNA delivery to the lungs by functional polyester-based carriers. Biomacromolecules. 2017;18(12):4307–15.
Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective ORgan Targeting (SORT) nanoparticles for tissue specific mRNA delivery and CRISPR/Cas gene editing. Nat Nanotechnol. 2020;15(4):313–20.
Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–27.
Philipp A, Zhao XB, Tarcha P, Wagner E, Zintchenko A. Hydrophobically modified oligoethylenimines as highly efficient transfection agents for siRNA delivery. Bioconjug Chem. 2009;20(11):2055–61.
Schroeder A, Dahlman JE, Sahay G, Love KT, Jiang S, Eltoukhy AA, et al. Alkane-modified short polyethyleneimine for siRNA delivery. J Control Release. 2012;160(2):172–6.
Yan Y, Zhou K, Xiong H, Miller JB, Motea EA, Boothman DA, et al. Aerosol delivery of stabilized polyester-siRNA nanoparticles to silence gene expression in orthotopic lung tumors. Biomaterials. 2017;118:84–93.
Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, et al. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc Natl Acad Sci U S A. 2011;108(32):12996–3001.
Kim M-C, Lin MM, Sohn Y, Kim J-J, Kang BS, Kim DK. Polyethyleneimine-associated polycaprolactoneSuperparamagnetic iron oxide nanoparticles as a gene delivery vector. J Biomed Mater Res B. 2017;105(1):145–54.
Bus T, Englert C, Reifarth M, Borchers P, Hartlieb M, Vollrath A, et al. 3rd generation poly(ethylene imine)s for gene delivery. J Mater Chem B. 2017;5(6):1258–74.
Thomas M, Lu JJ, Ge Q, Zhang CC, Chen JZ, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102(16):5679–84.
Schaffert D, Troiber C, Salcher EE, Frohlich T, Martin I, Badgujar N, et al. Solid-phase synthesis of sequence-defined T-, i-, and U-shape polymers for pDNA and siRNA delivery. Angew Chem Int Ed. 2011;50(38):8986–9.
Geihe EI, Cooley CB, Simon JR, Kiesewetter MK, Edward JA, Hickerson RP, et al. Designed guanidinium-rich amphipathic oligocarbonate molecular transporters complex, deliver and release siRNA in cells. Proc Natl Acad Sci U S A. 2012;109(33):13171–6.
Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9(8):648–55.
Brissault B, Leborgne C, Scherman D, Guis C, Kichler A. Synthesis of poly(propylene glycol)-block-polyethylenimine triblock copolymers for the delivery of nucleic acids. Macromol Biosci. 2011;11(5):652–61.
Boisdron-Celle M, Menei PH, Benoit JP. Preparation and characterization of 5-fluorouracil-loaded microparticles as biodegradable anticancer drug carriers. J Pharm Pharmacol. 1995;47(2):108–14.
Jud L, Košutić M, Schwarz V, Hartl M, Kreutz C, Bister K, et al. Expanding the scope of 2′-SCF3 modified RNA. Chem Eur J. 2015;21(29):10400–7.
Wang M, Liu H, Li L, Cheng Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat Commun. 2014;5(1):3053.
Lv J, Chang H, Wang Y, Wang MM, Xiao JR, Zhang Q, et al. Fluorination on polyethylenimine allows efficient 2D and 3D cell culture gene delivery. J Mater Chem B. 2015;3(4):642–50.
Wu T, Wang L, Ding S, You Y. Fluorinated PEG‐polypeptide polyplex micelles have good serum‐resistance and low cytotoxicity for gene delivery. Macromol Biosci. 2017;17(8):1700114.
Cai XJ, Jin RR, Wang JL, Yue D, Jiang Q, Wu Y, et al. Bioreducible fluorinated peptide dendrimers capable of circumventing various physiological barriers for highly efficient and safe gene delivery. ACS Appl Mater Interfaces. 2016;8(9):5821–32.
Fisicaro E, Compari C, Bacciottini F, Contardi L, Pongiluppi E, Barbero N, et al. Nonviral gene-delivery by highly fluorinated gemini bispyridinium surfactant-based DNA nanoparticles. J Colloid Interface Sci. 2017;487:182–91.
Wang LH, Wu DC, Xu HX, You YZ. High DNA-binding affinity and gene-transfection efficacy of bioreducible cationic nanomicelles with a fluorinated core. Angew Chem Int Ed. 2016;55(2):755–9.
Chen G, Wang K, Ullah A, Zhou Y, Ding L, Hu Q, et al. Self-assembled fluoropolycation nanomicelles for improved siRNA silencing. J Control Release. 2017;259:e35–e6.
Liu X, Wang Y, Chen C, Tintaru A, Cao Y, Liu J, et al. A fluorinated bola-amphiphilic dendrimer for on-demand delivery of siRNA, via specific response to reactive oxygen species. Adv Funct Mater. 2016;26(47).
Chen G, Wang K, Hu Q, Ding L, Yu F, Zhou Z, et al. Combining fluorination and bioreducibility for improved siRNA polyplex delivery. ACS Appl Mater Interfaces. 2017;9(5):4457–66.
Cai X, Zhu H, Zhang Y, Gu Z. Highly efficient and safe delivery of VEGF siRNA by bioreducible fluorinated peptide dendrimers for cancer therapy. ACS Appl Mater Interfaces. 2017;9(11):9402–15.
Wang H, Wang Y, Wang Y, Hu J, Li T, Liu H, et al. Self-assembled fluorodendrimers combine the features of lipid and polymeric vectors in gene delivery. Angew Chem Int Ed. 2015;54(40):11647–51.
Gong J-H, Wang Y, Xing L, Cui P-F, Qiao J-B, He Y-J, et al. Biocompatible fluorinated poly(beta-amino ester)s for safe and efficient gene therapy. Int J Pharm. 2018;535(1-2):180–93.
Xiao Y-P, Zhang J, Liu Y-H, Huang Z, Wang B, Zhang Y-M, et al. Cross-linked polymers with fluorinated bridges for efficient gene delivery. J Mater Chem B. 2017;5(43):8542–53.
Johnson ME, Shon J, Guan BM, Patterson JP, Oldenhuis NJ, Eldredge AC, et al. Fluorocarbon modified low-molecular-weight polyethylenimine for siRNA delivery. Bioconjug Chem. 2016;27(8):1784–8.
Wang MM, Cheng YY. Structure-activity relationships of fluorinated dendrimers in DNA and siRNA delivery. Acta Biomater. 2016;46:204–10.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
Yan Y, Liu L, Xiong H, Miller JB, Zhou K, Kos P, et al. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc Natl Acad Sci U S A. 2016;113(39):E5702–E10.
Gunther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2011;77(3):438–49.
Gao S, Dagnaes-Hansen F, Nielsen EJB, Wengel J, Besenbacher F, Howard KA, et al. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol Ther. 2009;17(7):1225–33.
Funding
D.J.S. received financial support from the Cancer Prevention and Research Institute of Texas (CPRIT) (R1212), the Welch Foundation (I-1855), the American Cancer Society (RSG-17-012-01), and the Mary Kay Foundation (049-15). Y.Y. received financial support from Zhejiang Provincial Natural Science Foundation of China (LY19B040004).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 144 kb)
Rights and permissions
About this article
Cite this article
Xue, L., Yan, Y., Kos, P. et al. PEI fluorination reduces toxicity and promotes liver-targeted siRNA delivery. Drug Deliv. and Transl. Res. 11, 255–260 (2021). https://doi.org/10.1007/s13346-020-00790-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00790-9