Abstract
Over the past two decades, polymersomes have been widely investigated for the delivery of diagnostic and therapeutic agents in cancer therapy. Polymersomes are stable polymeric vesicles, which are prepared using amphiphilic block polymers of different molecular weights. The use of high molecular weight amphiphilic copolymers allows for possible manipulation of membrane characteristics, which in turn enhances the efficiency of drug delivery. Polymersomes are more stable in comparison with liposomes and show less toxicity in vivo. Furthermore, their ability to encapsulate both hydrophilic and hydrophobic drugs, significant biocompatibility, robustness, high colloidal stability, and simple methods for ligands conjugation make polymersomes a promising candidate for therapeutic drug delivery in cancer therapy. This review is focused on current development in the application of polymersomes for cancer therapy and diagnosis.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally cancer is one of the prominent causes of death, thus representing a major public health-related problem. Many medical interventions, such as chemotherapy, radiation therapy, and surgical removal for the conventional management of cancer, lack specificity and also damage the nearby healthy cells of the body [1]. The use of nanotechnology has currently become a popular technique for cancer treatment and management. Nanotechnology-based approaches to target tumor cells mainly focus on enhancing the bioavailability of anti-cancer drugs and reducing toxicity. Polymersome-based therapeutic drug delivery strategies have shown remarkable potential in the therapy of cancer due to their physical and chemical robustness, high drug loading capability, high colloidal stability, significant biocompatibility, and their ability to encapsulate both hydrophilic and hydrophobic molecules [2]. Polymersomes are spherical nanostructures made up of amphiphilic block copolymers, in which a hydrophobic bilayer encloses an aqueous core [3, 4]. Surface modification of polymersomes with target-specific ligands, which binds especially to the receptors over-expressed on tumor cells, can improve the cellular uptake of anti-cancer molecules [5, 6]. In comparison with healthy cells, polymersomes are progressively internalized by cancer cells and are less toxic, even at high levels [7]. After systemic administration, polymersomes assemble passively at the tumor site via enhanced permeation and retention (EPR) effect and release the drug at the target site [8]. The integration of stimuli-sensitive chemistry can also control the drug release from polymersomes [9]. Because of these benefits, polymersomes have been widely explored in numerous biomedical applications, for example, in drug delivery [10], gene delivery [11], and diagnostics [12].
Design and preparation of polymersomes
The scientific development of a number of controlled radical polymerization (CRP) methods, such as reversible addition-fragmentation chain transfer polymerization (RAFT), atom transfer radical polymerization (ATRP), and nitroxide-mediated polymerization (NMP), has resulted in the synthesis of well-defined amphiphilic polymers [13]. Through CRP, it is possible to synthesize functional polymers with specific molecular weight, precise architectures, and low dispersity, such as block, triblock, and graft copolymers [14]. Many studies have reported the self-assembly of amphiphilic polymers in the form of spheres, cylinders, and polymersomes [15]. It is mainly the packing parameter (p = v / al) that predicts the most likely structure of the self-assembled aggregate in solution by taking into account the volume of the hydrophobic block (v), the contact area of the head group (a), and the length of the hydrophobic block (l). If p < 1/3, spheres are formed; between 1/3 < p < 1/2, cylinders are formed; and when 1/2 < p < 1, polymersomes are formed [16].
When the concentration of block copolymers exceeds the critical aggregate concentration in a solution, block copolymer undergoes self-assembly to form high molecular weight aggregates [17]. Amphiphilic block copolymers show extremely slow chain exchange dynamics and exhibit a low critical aggregate concentration [17,18,19]. As a result, polymersomes are retained in the bloodstream for a longer duration. The thickness of the membrane in polymersomes can be tailored by adjusting the molecular weight of the amphiphilic polymers, which can affect their physicochemical properties such as mechanical stability and toughness [20]. Non-biodegradable polymers such as poly (styrene), poly(dimethylsiloxane), poly(ethyl ethylene), poly(butadiene), and biodegradable polymers like poly(lactide) and poly(ε-caprolactone) primarily constitute the hydrophobic part of the block copolymer. The hydrophilic part is mainly formed by polymers like poly(acrylic acid), poly(ethylene glycol) (PEG), and poly (L-glutamic acid) [21]. PEG is commonly used as a hydrophilic constituent of di- and triblock copolymers to prevent the plasma protein adsorption and thus maintains the stability of the carrier in the plasma [22, 23]. Its neutral and hydrophilic nature makes polymersomes imperceptible to dendritic and phagocytic cells [24]. Figure 1 illustrates the typical structure of polymersomes. The core section of the polymersomes contains hydrophilic molecules, and the hydrophobic bilayer can be designed to load hydrophobic molecules [25, 26].
The preparation of polymersomes generally involves the addition of an amphiphilic polymer in the selective organic solvent to form an organic phase. The polymer containing organic phase is then added to the aqueous phase. Mixing enables the formation of a fine dispersion of the polymer in the aqueous phase and the amphiphiles self-assemble to form polymersomes [15]. Several techniques can be exploited to prepare polymersomes such as nanoprecipitation (solvent-exchange method), film rehydration, electroformation, and emulsification methods (oil-in-water emulsion method and double emulsion method) [27, 28]. The technique used to self-assemble the polymersomes can influence the size of polymersomes. Methods such as electroformation, double-emulsion result in the formation of polymersomes in micrometer size range, while polymersomes in the size range of nanometer are prepared using the film rehydration and nanoprecipitation method [29,30,31,32]. The polymersomes structure can be additionally modified by external factors such as sonication, freeze/thaw cycles, and extrusion through polycarbonate filters [32,33,34,35]. Though it is possible to load the molecules during the formulation processes, it is more often favored to load molecules post-fabrication to circumvent harmful effects on the carrier. Some of the post-fabrication techniques to encapsulate drugs in polymersomes are extrusion, electroporation, and ultrasonication [36]. Table 1 gives an overview of the methods used for the preparation of polymersomes.
Several methodologies have been adopted to conjugate ligands on the surface of nanoparticles such as click chemistry, oxime chemistry, thiol-ene chemistry, and inverse-electron demand Diels-Alder reactions [41]. Among them, click reactions are widely employed to conjugate ligands onto the surface of polymersomes [42, 43]. The thickness and fluidity of the membrane are affected by the nature of the block copolymer (tri or di-block) and molecular weight of the building blocks [28, 44, 45]. Triblock copolymers, having the same molecular weight as di-block copolymers, form thinner membranes and block copolymer with high molecular weight, form a thicker membrane [26]. The fast absorption of drug carriers by the mononuclear phagocyte system is one of the important challenges in targeted drug delivery [46]. Several factors, such as the degree of PEGylation, size and surface charge of nanoparticles, can influence the uptake of polymeric nanoparticles by the mononuclear phagocyte system [47, 48]. In addition, the presence of a tumor also significantly influences the circulation time of polymersomes [49]. Therefore, it is important that long-circulating nanocarriers should be designed judiciously for the adequate accumulation of nanocarriers via passive targeting at the tumor site. The presence of high charges on the surface of carriers may also decrease their circulation time by enhancing hepatic uptake [28]
Drug encapsulation in polymersomes
Drug encapsulation is an important factor that needs to be considered in the preparation of drug delivery systems. For the encapsulation of hydrophobic and hydrophilic molecules in the polymersomes, both active and passive loading approaches have been employed. In the passive loading approach, the hydrophilic molecules are typically added to the aqueous phase during the preparation of polymersomes. Several active liposomal loading techniques (such as salt and pH gradient) can also be employed if the water solubility of drugs is low or to achieve high encapsulation efficiency [50, 51]. The hydrophobic molecules are generally added to the requisite organic solvent along with the amphiphilic polymers and incorporated into the polymersome membrane during vesicle formation [27, 37].
Advantages and limitations of polymersomes in comparison with those of liposomes
Polymersomes and liposomes have their respective advantages and limitations. Liposomes have been extensively studied for the delivery of a variety of therapeutic agents and are considered one of the best drug delivery vehicle systems. A significant drawback of liposomes is their poor biochemical and mechanical stability in complex fluids, such as blood, which might result in the premature and uncontrolled release of therapeutic payload [52]. Polymersomes are more stable in comparison to their lipid counters, such as liposomes due to their membrane thickness, lateral diffusivity, and entanglement [53]. However, such properties, in particular, depend on many different aspects such as the size of polymeric vesicles, method of preparation, nature of amphiphiles, and storage conditions [16]. The bilayer membrane in polymersomes is typically much thicker (~ 50 nm) in comparison with that in liposomes (3–5 nm) [16, 53, 54]. Therefore, higher amounts of hydrophobic drug molecules can be accommodated into the thicker hydrophobic bilayer of polymersomes compared to liposomes. Moreover, thicker membranes may result in a slower release of hydrophobic drug molecules because of the large diffusion distances [55]. However, polymersomes have relatively low loading capacity for hydrophilic molecules, limiting their applications as drug carriers, particularly for anti-cancer therapy [56]. The robustness and stablility of bilayer membrane of polymersomes can also be a disadvantage. However, due to their chemical versatility, it is also possible to program the release of the cargo using several external and internal stimuli. The polymersomes show better colloidal stability against mechanical shear and osmotic pressure in the bloodstream than liposomes because of the high molecular weight of block copolymers [57]. Polymersomes exhibit relatively low permeability toward macromolecules, ions, and small molecules, and this drawback also hinder their use in biomedical applications [58]. Liposomes are considered more biocompatible in comparison to polymersomes as the polymersomes are prepared using synthetic building blocks. Therefore, polymersomes degrade slowly and also show higher toxicity compared to liposomes [59].
The pharmacokinetics and biodistribution profile of polymersomal and liposomal formulations have been reviewed in several papers. Alibolandi M. et al. investigated the distribution and therapeutic efficacy of doxorubicin-loaded polymersomes (Poly-DOX) with a liposomal formulation in murine colon adenocarcinoma model [60]. Findings of the study revealed that Poly-DOX favorably accumulated in the tumor and was present in significantly lower concentrations in the liver 48 h post-treatment than the liposomal formulation. The tolerated dose in mice receiving Poly-DOX was remarkably lower than those receiving the liposomal formulation. Poly-DOX demonstrated better therapeutic efficacy in terms of tumor growth rate and can potentially limit off-site effects. In another study, a comparison between the pharmacokinetic properties of DOX-loaded polymersomes (PolyDoxoSomes) and a commercially available liposomal DOX (Lipo-DOX) have been reported [61]. It was observed that the PolyDoxoSomes exhibited shorter plasma half-life (22 vs. 35 h) and lower area under the curve (AUC) (568 vs. 229 μg h/ml), which could be valuable for reducing the side effects and dose-related toxicities. The therapeutic efficacy of the polymersomal formulation was equivalent to that of the Lipo-DOX. In a similar study, Zou et al. prepared DOX-loaded cNGQGEQc peptide decorated redox-sensitive polymersomes [62]. Pharmacokinetic and biodistribution studies revealed that the prepared formulation had a longer circulation time and significantly higher tumor accumulation in mice when compared with treatment with Lipo-DOX. The prepared formulation showed a 6-fold higher maximum-tolerated dose in mice than Lipo-Dox. Youssef S. F et al. prepared flutamide-loaded polymersomes and investigated their in vivo pharmacokinetics compared with the liposomal formulation after oral administration [63]. They observed that flutamide-loaded polymersomes exhibited 3.9- and 4.7-times higher plasma concentration than liposomes and drug suspension. GE11 peptide-modified polymersomes loaded with DOX were prepared by Zou Y et al. for the treatment of SKOV3 human ovarian tumors [64]. In vivo biodistribution studies showed a more significant tumor accumulation of the polymersomal formulation compared to Lipo-DOX. The prepared formulation effectively suppressed the progression of SKOV3 tumors (IC50 = 8.7 DOX equiv./mL) and showed low toxicity compared to Lipo-DOX in in vitro studies.
Mechanisms of drug release from polymersomes
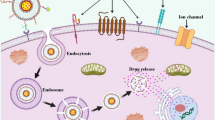
Drug release from polymersomes generally occurs through passive diffusion, which is mainly driven by concentration gradients. In recent years several stimuli-responsive polymersomes have also been developed to achieve controlled release of their payload on reaching to the target site in response to external or internal stimuli [65]. Their ability to trigger drug release at the target site can significantly enhance therapeutic efficiency and may diminish its adverse effects. Stimuli-responsive polymersomes can be designed to release the therapeutic agent in response to either chemical (mainly induced by pH and redox molecular chain composition) and physical triggers (temperature, light, magnetic, electrical, and ultrasound) at the target site [25, 66]. These intelligent nanostructures can effectively receive, transmit, and react to stimuli. Figure 2 displays stimuli-responsive, intracellular drug release by polymersomes in the target diseased cell. The stimuli characterized as abnormal temperature, pH, oxidative stress, and abnormal glucose levels, typical to the unhealthy cells, prompt the intracellular dissolution of the polymersome backbone to release the encapsulated drug molecules. Over the years, several types of stimuli-responsive polymersomes [25] like pH- [67], enzyme- [68], photo- [69], glucose- [70], voltage- [71], and magnetic field-responsive polymersomes [72] have been developed.
Categories of polymersomes
Based on stimuli response, polymersomes can be categorized in various classes, which are discussed below:
pH-responsive polymersomes
pH-responsive polymersomes have recently gained significant attention due to the existence of physiological pH gradients within the body. Acidic extracellular pH is the main feature of the tumors and inflammatory tissues (pH ∼ 6.5 − 7.2) [73]. Several pH-responsive polymersomes have been developed by the incorporation of pH-sensitive or ionizable bonds in the amphiphilic polymer to trigger the polymersome degradation and drug release [65]. Some of the earliest pH-responsive polymersomes developed, comprised of hydrolysis-susceptible polyester such as poly(lactic acid) (PLA) or poly(ε-caprolactone) (PCL) [74]. The hydrolysis of the ester bonds linking the polymer blocks leads to polymersome degradation and drug release. Ahmed et al. prepared ph-responsive polymersomes based on PEG-PLA and PEG-PCL for the delivery of anticancer drugs to the tumor tissues. The pH-gradient method was used to load DOX into the polymersomes and loading of paclitaxel was done after vesicle formation. The authors found that the prepared polymersomes accumulated in tumor tissue in mice and released the encapsulated payloads in the acidic tumor environment [51]. In recent years, linkers comprising various acid-sensitive groups like hydrazine, imine, acetal, orthoester, and vinyl ester have also been employed to prepare polymersomes, which can induce the cleavage of such systems in a pH-dependent manner and release of the encapsulated cargo [66, 75,76,77].
Hydrazone linkage is one of the most commonly used pH-sensitive bonds that have been employed in drug delivery systems on account of its faster rate of hydrolysis in an acidic pH environment [78]. Brinkhuis et al. formulated polymersomes composed of a mixture of polybutadiene–PEG amphiphilic block copolymers coupled via an acid-sensitive hydrazone linkage. The prepared polymersomes retained their colloidal stability at physiological pH and disassembled under slightly acidic conditions [77]. pH-triggered degradation of polymersomes can also be achieved by incorporating ionizable groups into the polymer blocks. pH-sensitive polymers with ionizable groups typically have weakly acidic (carboxylic acids/sulfonic acids) or weak basic groups (amine) that undergo protonation or deprotonation in response to change in environmental pH. When the environmental pH is lower than the pKa for specific group side chains of copolymers, the basic groups are protonated, and the integrity of the vesicle is affected by electrostatic repulsion [79]. Structural transformation can subsequently cause polymersome formation/deformation, resulting in a higher therapeutic index due to the rapid release of therapeutics at the target site. Armes and co-workers formulated zwitterionic poly(2-(methacryloyloxy)ethylphosphorylcholine)-b-poly(2-(diisopropylamino) ethyl methacrylate) (PMPC-b-PDPA) block copolymer to prepare stable vesicles [80]. The vesicles were stable at physiological pH due to the acidic pKa of the tertiary amine group in PDPA. Under acidic conditions, degradation of vesicles occurred due to the protonation of the amine groups in PDPA, which is hydrophobic at physiological pH and hydrophilic under acidic solutions. In another study, Massignani M. et al. formulated polymersomes comprising PMPC-PDPA diblock copolymers. The prepared polymersomes exhibited both cell affinity and pH-sensitivity and effectively delivered the cargo within the cell cytosol [81].
Cationic polymers, including poly(lysine), poly(histidine), and poly(β-amino ester), and anionic polymers, like poly(methacrylic acid) and poly(acrylic acid), are pH-responsive [25, 82,83,84,85]. Cationic polymers with pH-sensitivity can modify the drug taste and gastric release of drugs by reacting to low pH of the stomach. Anionic polymers sensitive to the high intestinal pH are utilized to prevent the degradation of the drug in the stomach, drug delivery in the colon, and to increase the bioavailability of weakly basic drugs [78]. Cationic polymers comprising amino groups show greater solubility at acidic pH in aqueous solutions as compared to neutral pH [86]. This results in the pH dissolution of the polymer and the release of drugs into the target cell.
Temperature-responsive polymersomes
Temperature-responsive polymersomes consist of temperature-sensitive polymers that exhibit a phase transition or conformational changes in response to temperature stimuli. The phase transitions induce changes in hydrophilicity/hydrophobicity balance and solubility of polymers that lead to the formation/disintegration of vesicles [87]. Some thermo-responsive polymers applied in drug delivery systems exhibit a low critical solution temperature (LCST), which become insoluble upon heating. Many thermo-responsive polymers have an upper critical solution temperature (UCST), which become soluble upon heating [88]. This property has been exploited in cancer therapy, as the local temperature is slightly higher in solid tumors than the temperature in adjacent normal tissues. The local cooling of the tissues by the application of external ice packs or by penetrated cryoprobes can also be used to distort the polymersome vesicles to release the therapeutic drug [89].
Poly-N-isopropyl acrylamide (PNIPAm) is a thermo-responsive polymer that is often used to prepare temperature-sensitive polymersomes. PNIPAm, which has an LCST of 32 °C, can self-assemble at normal body temperature (37 °C) to form stable polymersomes and disassemble to release the loaded therapeutic agent at temperatures below its LCST [66]. Qin et al. formulated thermoresponsive polymersomes based on diblock copolymer PEO-b-PNIPAM [90]. These polymers could self-assemble into polymersomes in water above body temperature, encapsulating both hydrophobic and hydrophilic molecules. With a decrease in temperature (below 32 °C), the PNIPAM becomes hydrophilic, causing vesicles to disassemble and release the encapsulated cargo.
The tri-block copolymers of PEG-PAA-PNIPAm have been used to prepare polymersome with dual triggers response (temperature and reduction responsive) [91]. Increasing the temperature over the LCST of polymer contributed to the development of polymersomes in the aqueous environment. Crosslinking the vesicles with cystamine produced robust polymersomes at human body temperature. However, due to the presence of cystamine in the structure, these polymers were sensitive to a reductive environment. Kharlampieva and co-workers prepared DOX-loaded temperature-sensitive polymersomes of poly (3-methyl-N-vinylcaprolactam)-block-poly (N-vinylpyrrolidone) (PMVC-PVPON) [92]. By reducing the length of the PVPO block, the LCST of the copolymer PMVC-PVPON can be varied from 19.2 to 15.2 °C. The prepared polymersomes showed a remarkably high loading capacity of 49%. The formulation exhibited good structural stability in serum.
Redox-responsive polymersomes
The development of redox-responsive polymersomes permits the loading of both hydrophobic and hydrophilic therapeutic agents, and the simultaneous release of multiple therapeutic loads can enhance the therapeutic efficacy of the delivery system [93]. Redox potentials differ significantly between the tumor cells and normal tissue, the extracellular and intracellular environment, and between different cell organelles [94]. Polymersome, which reacts to change in redox potential, often contain disulfide bonds [95]. Disulfide bonds are susceptible to reduction and get reduced to two thiols in reductive environments. The concentration of glutathione (GSH) is typically low in plasma and normal tissues but much higher in the nuclei, cytosol, and tumor tissue, which produces a high intracellular redox gradient [65]. Disulfide bonds are incorporated into the polymeric backbone and subsequent cleavage of these bonds triggers the degradation of the polymersomes resulting in the release of the therapeutic payload [95]. The intracellular reducing environment and oxidizing nature of the extracellular environment may be utilized to activate the drug release from polymersomes.
Ren et al. prepared DOX-loaded redox-responsive polymeric vesicles self-assembled from triblock copolymers with disulfide bond linked PEG and poly(ε-benzyloxycarbonyl-l-lysine) (PzLL) for glutathione (GSH)-mediated drug release [96]. The cleavage of the disulfide bonds by the GSH can activate the disassembly of the vesicles to release the cargo within the cells. DOX-loaded vesicles demonstrated higher nuclear accumulation after incubation with HeLa cervical cancer cells after exposure to GSH and were helpful in reversing drug resistance. In a similar study, Kumar A. et al. prepared DOX-loaded polymersomes based on redox-sensitive amphiphilic triblock copolymer pPEGMA-PCL-ss-PCL-pPEGMA [97]. The intracellular GSH triggered the degradation of the polymersomes after the cleavage of the disulfide bonds in the polymeric backbone, leading to intracellular drug release. Furthermore, the prepared redox-responsive polymersomes showed enhanced anti-tumor efficacy without showing any significant cardiotoxicity.
Several recent studies have reported the preparation of oxidation-sensitive polymersomes that can deliver the therapeutic agents to the target site. Oxidation by reactive oxygen species like hydrogen peroxide (H2O2) or oxidation stress at the tumor site leads to the destruction of polymeric assemblies. Li et al. prepared glucose oxidase-loaded polymersomes made up of diblock copolymers of PEG and copolymer of camptothecin (CPT) and piperidine-modified methacrylate [98]. At the tumor site, the acidic environment of the tumor triggered the generation of H2O2. High levels of H2O2 induces self-destruction of the polymersomes to release CPT, which suppressed the growth of tumor through the synergistic action.
Other stimuli-responsive polymersomes
Polymersomes loaded with magnetic nanoparticles have also been investigated for the controlled delivery of therapeutic and imaging agents when exposed to an external magnetic field. By the application of local hyperthermia, the encapsulated hydrophilic magnetic core can be released at the target site [72, 99]. Photo-responsive polymersomes have also received great attention because of their ability to release the cargo at specific sites by exposure to light. Photo-sensitive moieties can be incorporated into the amphiphilic block polymers to make them sensitive to the wavelengths of light [100]. A photo-cleavable linker (o-nitrobenzyl) that forms a photo-labile bond between the hydrophilic and hydrophobic sections of polymers has also been reported. UV radiation exposure at 365 nm induces bond cleavage and eventually shrinks the material, which allows the release of an encapsulated drug. The frequency of UV light can also be used to monitor the release of material as well [101]. A major drawback of adopting this strategy is that UV irradiation is required for efficient release of encapsulated cargo at 365 nm wavelength, which can destroy healthy tissues as well.
The specific overexpression of certain enzymes in the tumor microenvironment can be utilized to deliver drugs at a particular site. In recent years several enzyme-sensitive polymersomes have been prepared in which the polymer blocks are linked through enzyme cleavable groups that enable the release of encapsulated payloads from nanocarriers at the target cell. Lee et al. formulated polymersomes based on mPEG and PDLLA (mPEG: methoxy poly(-ethylene glycol), PDLLA: poly(d,l-lactide) block copolymer [102]. A Gly-Phe-Leu-Gly-Phe (GFLGF) linker was then incorporated in between the two blocks. The block copolymer was cleaved at the tumor site by Cathepsin B (found in abundance in tumor tissues) causing disassembly and the release of chemotherapy agents from the prepared systems.
Iatridi et al. investigated pH and temperature-responsive, star-graft quarterpolymer-based polymersomes as carriers for the controlled co-delivery of paclitaxel (hydrophobic) and DOX (hydrophilic) [103]. The release profile of both the chemotherapeutic agents was controlled in a different way depending upon the environmental conditions (temperature and pH). The polymersomes displayed higher cytotoxicity in the A549 cancer cell lines in comparison with the single-loaded polymersomes. In another study, Chen et al. prepared ultrasound and pH-responsive polymersomes loaded with DOX. Upon irradiation with ultrasound and by decreasing the pH of the solution, the vesicles became smaller and released the encapsulated anti-cancer drugs in a controlled manner [104].
Polymersomes in various cancer therapy
Recent advances to encapsulate and deliver various therapeutic agents using polymersomes for the treatment of different types of cancer are discussed below. The characteristics and anti-cancer activity of the polymersomes based formulations are summarized in Table 2.
Polymersomes for brain cancer therapy
In brain tumor treatment, drug delivery via the blood-brain barrier (BBB) that primarily prevents the entry of large numbers of molecules into the brain has become an important concern among the scientific community [33, 137]. Innovation in nanomedicine has contributed to polymersome development as an appropriate carrier for drug delivery in brain cancer treatment. BBB is highly expressed with a plethora of receptors, such as low-density lipoprotein receptor (LDLR), transferrin receptor, and insulin receptor, which can be utilized for receptor-mediated transcytosis (RMT) of nanomedicine through BBB [138, 139].
Surface functionalization of polymersomes with antibodies such as lactoferrin (Lf) and transferrin (Tf) has been reported to improve the delivery of therapeutic agents to the brain. Pang et al. developed biodegradable polymersomes loaded with DOX (Tf-PO-DOX) using the nanoprecipitation method to improve the DOX delivery into brain tumor cells [108]. The researchers eventually conjugated transferrin to the polymersome surface. In contrast to the free DOX, the developed formulation considerably improved the intracellular delivery and chemotherapeutic effect of DOX in glioma-bearing rats. The pharmacokinetics studies revealed that Tf-PO-DOX significantly reduced the plasma clearance of DOX (from 1038 to 101 mL/h/kg) and showed 19-fold higher AUC compared with free DOX. In addition, tissue accumulation of polymersomes was higher in the liver but lower in the heart, suggesting that the polymersome DOX may have the same potency as Doxil® (liposomal doxorubicin). Pang and co-workers formulated tetrandrine and DOX-loaded biodegradable polymersomes conjugated with lactoferrin (Lf-PO-DOX/Tet) [140]. The outcomes of the study revealed the enhanced cellular uptake of LF-PO-DOX/Tet and cytotoxicity toward C6 cells. The prepared formulation effectively inhibited the glioma growth and enhanced the survival time of glioma-bearing rats. In another study, Pang et al. compared the relative superiority of polymersomes conjugated with Tf and Lf (Tf-PS and Lf-PS) in brain targeting [141]. The findings of the in vitro cellular uptake studies in mice revealed enhanced brain uptake of Tf-PS in comparison with Lf-PS. The pharmacokinetic study displayed a similar plasma concentration-time profile for both Tf-PS and Lf-PS. Tf-PS showed a 1.84-fold higher AUC compared with Lf-PS. Furthermore, the distribution and elimination rate constants for Lf-PS were 2.11- and 3.12- fold higher compared with that for Tf-PS.
Apolipoprotein E peptide (ApoE) has an active penetrating function across the BBB due to its high affinity to multiple LDLRs and can be used to target intracranial glioma [142]. In 2018, Jiang Y. et al. prepared apolipoprotein E peptide modified chimeric polymersomes (ApoE-CP) carrying saporin (SAP) [143]. The in vitro BBB model studies indicated that ApoE-CP exhibited 2.2-fold higher penetration compared with Angiopep-2–modified chimeric polymersomes. Administration of the prepared formulation via the systemic route in the glioblastoma bearing mouse model resulted in total inhibition of orthotopic U-87 MG glioblastoma cells (IC50 = 14.2 nM), and no adverse effects were observed. The histological analyses revealed that ApoE-CP-SAP did not induce damage to major organs. Qin et al. prepared ApoE peptide targeted chimaeric polymersomes loaded with rigosertib (ApoE-CP-RGS) to overcome the BBB barrier [10]. The polymersomal formulation exhibited a 3.8-fold higher cellular uptake in the U-87 MG cells in comparison to the non-targeted formulation. The ApoE-CP showed steady loading of RGS into its lumen and reduction-responsive release behavior of the drug. ApoE-CP-RGS showed significant cytotoxicity towards U-87 MG glioblastoma cells with an IC50 of 2.8 μg/mL. The formulation exhibited considerable inhibitory effects on glioblastoma cells and prolonged survival time in nude mice bearing U-87 MG glioblastoma. Furthermore, little damage to major organs like the heart, spleen, liver, lung, and kidney was observed in the mice treated with ApoE-CP-RGS.
Angiopep-2 peptide can specifically bind to low-density lipoprotein receptor–related protein 1 (LRP1) and have been employed to deliver drugs through the BBB. DOX-loaded biodegradable polymersomes conjugated with Angiopep-2 (Ang-PS-DOX) were formulated by Lu et al. to target LRP1 over-expressed both in glioma cells and BBB [105]. The encapsulation efficiency and drug loading of polymersomes were found to be 95% and 7.94%. Ang-PS-DOX showed considerably higher cytotoxicity and cellular uptake in C6 cells in contrast to PS-DOX. The in vivo brain distribution and pharmacokinetic studies in glioma-bearing rat model confirmed the wider distribution and more abundant accumulation of Ang-PS-DOX in glioma cells as compared to PS-DOX. Ang-PS-DOX exhibited significantly higher mean residence time (MRT) (4.30 vs. 1.49 h) and AUC (4.96 vs. 0.52 h/ml) compared with free DOX. The prepared formulation significantly increased the survival time of the glioma-bearing rats in comparison with treatment with free DOX and PS-DOX. Figueiredo et al. prepared polymersomes conjugated with Angiopep-2 and evaluated their potential of active targeting and delivery of DOX to brain cancer cells [106]. It was observed that DOX-loaded Ps-Angiopep-2 demonstrated an enhanced cytotoxic effect on U87 MG glioblastoma in comparison with non-targeted polymersomes.
Chen et al. formulated des-octanoyl ghrelin and folate conjugated polymersomal DOX (GFP-D) to aid in transportation through the BBB to target tumors [107]. Pharmacokinetic studies in mice revealed that GFP-D solutions showed significantly higher AUC (724.90 vs. 15.4 mg/mL*h), longer terminal half-life (47.66 vs. 1.6 h), and lower blood clearance (2.06 vs. 97.2 mL*h−1) in comparison with free DOX. However, no significant difference in AUC and blood clearance was observed between GFP-D, des-octanoyl ghrelin–conjugated polymersomal DOX, unmodified polymersomal DOX, and folate–conjugated polymersomal DOX. GFP-D enhanced the accumulation of DOX by up to 3.7-fold in tumors compared with the group treated with unmodified polymersomal DOX. Furthermore, GFP-D demonstrated significant anti-glioma efficacy and greatly improved the overall survival of tumor-bearing mice.
Polymersomes for breast cancer therapy
Breast cancer is one of the most common cancers in women, affecting one in eight women worldwide [144]. Several authors have investigated the potential of polymersome-based drug delivery systems for the treatment of breast cancer. Targeted delivery of chemotherapeutics at the tumor location provides a possible strategy by raising the therapeutic index as compared with non-targeted drugs. Folic acid conjugation has commonly been exploited to improve the tumor disposition of polymersomes in breast cancer cells and in preventing nonspecific cell damage. Alibolandi et al. developed folic acid conjugated PEG-PLGA nano-polymersomes loaded with DOX and quantum dot (FA-QD-DOX) for the chemotherapy and imaging of breast cancer [109]. Folic acid conjugated nano-polymersomes achieved active targeting of cancer cells through folate receptor directed delivery. In vivo imaging experiments on 4T1 breast adenocarcinoma-bearing, BALB/c mice revealed that FA-QD-DOX nano-polymersomes accumulated at the site of the tumor for 6 h post intravenous injection. In comparison with the non-targeted and free DOX, the developed FA-QD-DOX nano-polymersomes demonstrated enhanced therapeutic effectiveness. Furthermore, the animals treated with FA-QD-DOX nano-polymersomes exhibited no evidence of long-term detrimental physiological and histopathological effects. Trastuzumab and folic acid conjugated redox-sensitive biodegradable polymersomes comprising triblock copolymer were fabricated by Lale et al. using the nanoprecipitation method [112]. The polymersomes exhibited a significantly high DOX loading content of 32%. The conjugation of trastuzumab and folic acid significantly enhanced the cellular uptake and apoptosis in MCF-7 breast cancer cell lines (IC50 = 46.22 μg/mL). The in vivo experiments in mice showed enhanced chemotherapeutic efficiency of the prepared nanosystem in breast cancer treatment with negligible cardiotoxicity in comparison with free DOX.
A stem cell marker, Epithelial cell adhesion molecule (EpCAM) has emerged as a potential target in cancer treatment due to its over-expression in adenocarcinoma cell lines. Alibolandi et al. formulated (EpCAM)-targeted PEG–PLGA nano-polymersomes encapsulated with DOX using a pH gradient method [145]. The authors found that the prepared nano-polymersomes could effectively deliver DOX to EpCAM-positive tumor cells (MCF-7) as compared to non-targeted nano-polymersomes.
The hyaluronic acid (HA) based polymersomes have shown advantages in terms of solubility and the targeting ability of cancer cells. In order to deliver mertansin (MM1) toxin to the Ehrlich-ascites tumor (EAT) bearing Swiss albino mice model, Zhang et al. prepared HA shelled and disulfide crosslinked polymersomes loaded with DOX [113]. The prepared polymersomal formulation showed a high drug loading content of 16.7%, low drug leakage under physiological environments, and fast GSH-triggered drug release. Results suggested that the prepared formulation could effectively target breast cancer cells and had considerable inhibitory effects on the growth of the tumor in mice. In another study, DOX-loaded HA-based polymersomes (HA-PCL-DOX) were developed by Shahriari et al. to target the HA-binding glycoprotein CD44 on breast cancer cells [8]. The prepared polymersomes exhibited a loading content and encapsulation efficiency of 3.6% and 54.9%. The prepared formulation also showed significant bio-distribution and in vivo anti-cancer activity in the mouse 4 T1 breast tumor model. In addition, HA-PCL-DOX showed no signs of toxicity in major organs. The uptake of HA-PCL-DOX was significantly lower in the mice liver, which could be attributed to lesser immunogenicity of the HA shell.
Tamoxifen-loaded PEG-PCL polymersomes were prepared by Bessone et al. to investigate the effect of iRGD peptide functionalization on cellular uptake and breast cancer cell viability [111]. Functionalization of the polymersomes with iRGD peptide significantly enhanced the uptake of the polymersomes in both MCF7 and T47D breast cancer cells and reduced the proliferation of cancer cells with self-renewing capacity. To increase the bioavailability of DOX, Kumar et al. formulated redox-responsive polymersomes with disulfide linkage [95]. The prepared formulation exhibited significantly higher cell uptake in breast cancer cell lines. In vivo tests in EAT-bearing mice revealed a superior regression of tumors compared to free DOX with reduced cardiotoxicity. In 2014, Xu et al. prepared polymersomes by self-assembly of amphiphilic PEGylated polyphosphazene (PEP) to encapsulate hydrophobic DOX base and hydrophilic DOX [114]. The authors found that DOX, both in its hydrophilic or hydrophobic forms, encapsulated into PEP polymersomes with superior encapsulation efficacy. In vivo studies on nude mice indicated that PEP polymersomes significantly increased the survival rates without compromising therapeutic efficiency.
Polymersomes for lung cancer therapy
Several authors have investigated the potential of polymersome-based drug delivery systems for the treatment of lung cancer. Aptamer conjugation has been utilized to enhance the accumulation of polymersomes at the tumor site. AS1411 aptamer surface conjugated polyethyleneglycolepoly(lactic-co-glycolicacid) nanopolymersome (Apt-GEM-NP) loaded with an anti-cancer drug, gemcitabine (GEM) were fabricated by Alibolandi et al. to target nucleolin-overexpressing A549 cancer cells [120]. The prepared formulation showed loading content and encapsulation efficiency of 8.61% and 95.32%. It was observed that Apt-GEM-NP showed enhanced cellular uptake and cytotoxicity in nucleolin-overexpressing non-small cell lung cancer cells (A549). The results of the study suggested that AS1411-GEM-NPs could be a potential therapeutic approach for non-small cell lung cancer treatment.
Zhong and co-workers prepared granzyme B–loaded reduction-sensitive polymersomes (GrB-CPRPs) and investigated their effectiveness in inhibiting the progression of orthotopic human lung tumors in vivo [115]. GrB-d CPRPs were found to be highly effective against A549 lung cancer cells (IC50 = 20.7 nM). GrB-d CPRPs completely inhibited the growth of tumors in orthotopic A549-Luc lung tumor–bearing mice and significantly improved the survival rates. Yang et al. reported the preparation of reduction-responsive chimeric polymersomes (RCPs) functionalized with lung cancer-specific CSNIDARAC (CC9) peptide for the targeted delivery of pemetrexed disodium (PEM), an anti-cancer drug to tumor cells [116]. Results validated a superior potency of PEM-loaded CC9-RCPs to H460 cells compared with free PEM and non-targeting PEM-RCPs. The prepared PEM-CC9-RCPs showed higher accumulation in H460 human lung cancer cells in comparison with clinical formulation Alimta®. PEM-CC9-RCPs effectively reduced the growth of H460 xenografts and extended the survival time in mice in comparison with clinical formulations, Alimta®. In addition, PEM-CC9-RCPs showed comparable levels of aspartate aminotransferase, alanine aminotransferase, serum creatinine, and blood urea nitrogen with PBS control indicating the absence of kidney and liver toxicity. Another study reported the development of polymersomes containing cyclic RGD peptide and disulfide-crosslinked DOX (cRGD-PS-DOX) for targeted delivery of chemotherapeutic agents to human non-small cell lung cancer cells (NSCLC) [117]. Findings of the study revealed that cRGD-PS-DOX exhibited 15.2% of drug loading, excellent stability, and outstanding targeting capacity to αvβ3 integrin over-expressed in A549 human lung cancer cells. The in vivo pharmacokinetics and biodistribution of cRGD-PS-DOX showed comparable elimination half-lives but 2-fold greater tumor accumulation in comparison to Lipo-DOX and PS-DOX. cRGD-PS-DOX effectively inhibited the growth of A549 lung tumors in both orthotopic and subcutaneous models as compared with treatment with Lipo-DOX and PS-DOX.
Yang et al. investigated the loading and targeting efficiency of disulfide-crosslinked chimeric polymersomes loaded with MTX sodium in sigma receptor overexpressing H460-NSCLC xenografts [118]. The developed formulation showed high MTX sodium loading, good in vivo stability and high accumulation at the tumor site. The formulation showed high potency against H460 cells with a low IC50 of 2.4 μg mL−1, which was 10-fold lower than that of non-targeted control. In addition, the prepared polymersomes significantly suppressed the tumor growth and enhanced the rate of survival in H460 tumor xenografted mice in contrast to Trexall™. In another study, Yang and co-workers formulated selective cell-penetrating peptide (SCPP-PS) functionalized polymersomes for targeted delivery of MTX to human lung cancer cells in vivo [119]. The authors observed that MTX loaded SCPP-PS (MTX-SCPP-PS) significantly increased drug accumulation in the A549 tumor and demonstrated 2.8-fold lower IC50 (5.8 μg/mL) compared with MTX-PS and free MTX. Most remarkably, the prepared formulation significantly suppressed the progression of tumor and enhanced survival rates in mice having A549 lung tumor xenografts as compared to treatment with MTX-PS and free MTX.
Another study reported the development of a novel composite polyphosphazene vesicle system comprising amphiphilic and weakly cationic polymers loaded with microRNA 200c (miR-200c) [121]. The prepared system showed a significant anti-tumor effect in nude mice bearing tumor xenografts and PTX-resistant human lung cancer cells (A549/T). Zou et al. prepared cyclic peptide cNGQGEQc-functionalized polymersomes for DOX delivery to lung cancer cells [62]. The prepared formulation showed superior stability under physiological conditions and quickly disassembled in response to GSH with minimal drug leakage. The prepared formulation showed a significantly higher maximum-tolerated dose of over 100 mg/kg in mice. Pharmacokinetic and biodistribution studies showed that the prepared formulation had a longer circulation time and significantly improved tumor accumulation when compared to treatment with Lipo-DOX. The prepared DOX-loaded polymersomes displayed high anti-tumor activity in A549 lung cancer cells over-expressing the α3 and β1 integrin with quick intracellular drug release, and effective tumor growth suppression was observed in orthotropic A549 lung tumor-bearing mice.
Polymersomes for pancreatic cancer therapy
Matrix metalloproteinase (MMP) enzymes are a family of endopeptidases that plays important roles in pathological and physiological processes. The matrix metalloproteinase-7 (MMP-7) isozyme is overexpressed at the tumor site in pancreatic cancer [146]. Anajafi et al. prepared nuclear localizing peptide (NLS) conjugated redox-responsive vesicles in order to deliver DOX and curcumin to pancreatic tumors [123]. In the pancreatic cancer microenvironment, MMP-7 isozyme hydrolyzed the peptide and exposed the NLS on the polymersome surface, which facilitated the release of encapsulated cytotoxic drugs in the pancreatic tumors. The obtained results demonstrated that the developed polymersomes were more toxic towards the pancreatic cancer cells compared to the normal cells. In another study, Anajafi et al. prepared polymeric redox-responsive polymersomes loaded with anti-cancer drugs, DOX, and GEM by using pH gradient method [124]. To provide nuclear-localizing property, the redox-responsive polymersomes were conjugated with acridine orange (AO). The authors found that the redox-sensitive polymersomes released most of its embedded contents and significantly decreased the viability and volume of the pancreatic cancer cell spheroids.
Iatrou et al. developed temperature and pH-responsive polymersomes made up of amphiphilic polymers of type ABA and ABC where A, B, and C represented poly(l-lysine hydrochloride) [PLL], poly(γ-benzyl-(d7) l-glutamate) [PBLG(-d7)] and poly(ethylene oxide) [PEO], respectively [126]. The polymersomes were also loaded with a hydrophobic drug, PTX or hydrophilic drug, DOX. It was observed that vesicles made up of amphiphilic polymers of the ABC architecture were smaller. In vivo toxicity studies in mice confirmed the non-toxic nature of the prepared polymersome formulation. The in vitro drug release data revealed the faster release of DOX and PTX from the polymersomes at lower and higher pH, respectively. In vitro studies in BXPC3 human pancreatic cancer cell lines revealed that DOX-loaded polymersome made up of amphiphilic polymers of ABA type showed comparable activity to non-PEGylated liposomal DoxMyocet, whereas the ABC type displayed lower activity.
Karandish et al. prepared stimuli-responsive polymersomes for anti-cancer drug delivery to the nuclei of BxPC3 cells in a nucleus-mimicking environment [122]. The authors synthesized an alkyne derivative of dexamethasone, which was conjugated with N3–PEG–polylactic acid polymer via click chemistry. The combination of the developed conjugate with a stimuli-sensitive polymer resulted in the development of stable polymersomes. Reportedly, the prepared redox-sensitive polymersomes released most of its encapsulated contents in about 80 min under a high concentration of reducing agents. Polymersomes were also loaded with the cancer inhibitor, BBI608. Results revealed that BBI608 loaded polymersomes notably decreased the viability of BxPC3 pancreatic cancer cells. Kulkarni et al. formulated erlotinib and GEM-loaded hypoxia-responsive polymersomes [125]. Analysis of hypoxia-responsive release of anti-cancer drugs from polymersomes on three-dimensional (3D) spheroid cultures of BxPC-3 cells revealed that the prepared polymersomes could significantly reduce the viability of hypoxic spheroidal cultures of BxPC-3 pancreatic cancer cells.
Polymersomes for prostate cancer therapy
The surface of prostate cancer cells is overexpressed with prostate-specific membrane antigen (PSMA) receptors. In recent years, the PSMA receptors have been extensively explored as a targeting receptor for the targeted delivery of drugs to prostate cancer cells. Karandish et al. prepared folic acid conjugated reduction-triggered polymersomes containing anti-cancer drugs, mocetinostat, and docetaxel (DTX) for proficient PSMA receptor-mediated targeting followed by internalization in pancreatic cancer cell spheroids [127]. These results indicated that the conjugation of folic acid enhanced the tumor-targeting efficiency and subsequent internalization of the polymersomes into cultured cells. Notably, the polymersomes encapsulating both DTX and mocetinostat significantly reduced the cell viability of 3D spheroidal cultures of LNCaP prostate cancer cells as compared with control and free drugs. In another study, Li et al. developed pH-sensitive chimeric polymersome formulation decorated with 2-[3-[5-amino-1-carboxypentyl]-ureido]-pentanedioic acid (Acupa) for efficient delivery of granzyme B, a therapeutic protein to prostate cancer cells [128]. The authors found that the prepared system resulted in efficient apoptosis of LNCaP cells (IC50 = 1.6 nM). The flow cytometry and CLSM studies demonstrated an apparent decrease of mitochondrial membrane potential in LNCaP human prostate adenocarcinoma cells on treatment with the prepared polymersomal formulation. The prepared formulation exhibited a prolonged circulation time and elimination half-life of 3.3 h.
Demirgoz et al. designed polymersomes functionalized with PR_b peptide for the treatment of prostate cancer [129]. PR_b peptide effectively targets a5b1 receptors and mimics the binding site in fibronectin. Results suggested the effective internalization of developed polymersomes in prostate cancer cells. Later on, the PR_b-functionalized polymersomes were encapsulated with a therapeutic protein, tumor necrosis factor-α (TNFα). Reportedly, the delivery of TNFα via PR_b-decorated polymersomes significantly enhanced the cytotoxic potential of TNFα as compared with non-functionalized polymersomes in vitro.
Polymersomes for liver cancer therapy
Liver cancer is the most lethal malignancies across the world and has a less than 20% 5-year survival rate [147]. The chemotherapeutic agents administered via a systemic route are usually unsuccessful for liver cancer because of low tumor uptake and rapid clearance. Targeted delivery of chemotherapeutics to cancer cells in the liver provides a possible strategy by raising the therapeutic index as compared to non-targeted drugs. In 2017, Fang and co-workers formulated DOX-loaded polymersomes functionalized with GE11 peptide in order to effectively target the epidermal growth factor receptor (EGFR)-overexpressing SMMC-7721 xenografts in mice [130]. MTT assays, confocal microscopy, and flow cytometry studies confirmed that GE11-PS-DOX could efficiently deliver DOX into SMMC7721 cells as compared with non-targeting PS-DOX and Lipo-DOX. In vivo experiments demonstrated that GE11-PS-DOX showed a prolonged circulation time and high accumulation in tumor cells. Moreover, the prepared formulation demonstrated much better treatment and improved the survival rate of mice bearing SMMC7721 orthotopic liver tumor as compared to treatment with PS-DOX and Lipo-DOX. Wei et al. prepared transferrin anchored polycarbonate-based polymersomal DOX for effective liver cancer therapy [131]. Most remarkably, the prepared formulation demonstrated high accumulation in the transferrin receptor-expressing human liver tumor SMMC-7721 model in contrast to treatment with non-targeted PS-DOX and Lipo-DOX. The prepared formulation significantly improved the survival rates in mice and showed low systemic toxicity.
Reduction-responsive hepatoma-targeted chimeric biodegradable polymersomes were fabricated by Wang et al. for intracellular protein delivery [132]. Later on, the polymersomes were loaded with various proteins like cytochrome C (CC), bovine serum albumin (BSA), and ovalbumin (OVA). Confocal microscopy confirmed that the FITC-CC-laden galactose-decorated reduction-sensitive chimeric polymersomes effectively delivered the FITC-CC into HepG2 liver cancer cells, as compared with cells treated with free CC and FITC-CC–loaded non-targeting polymersomes. The results indicated that CC-loaded galactose-decorated chimeric polymersomes showed efficient targeting capacity and anti-tumor activity in liver cancer cells (IC50 = 2.7 nM). Also, GrB-loaded galactose-decorated chimeric polymersomes resulted in significant apoptosis of liver cancer cells.
Polymersomes for colorectal cancer therapy
Alibolandi and co-workers prepared PEG-PLGA polymersomes functionalized with tetraiodothyroacetic acid (tetrac) for efficient camptothecin delivery to the colon adenocarcinoma [133]. Tetrac attaches specifically to the integrin αvβ3 receptor with high affinity. Camptothecin was loaded in the polymersomes with a loading content and encapsulation efficiency of 4.2% and 84%. In vitro drug release studies indicated that tetrac-conjugated PEG-PLGA polymersomes released camptothecin in a sustained release pattern. The polymersomal formulation demonstrated significant cytotoxicity against integrin over-expressed C26 and HT29 colorectal cancer cells. Moreover, in vivo studies in mice bearing C26 tumor confirmed the enhanced tumor inhibition activity of developed polymersomes as compared to the non-targeted formulation and free camptothecin. In another study, Goñi-de-Cerio et al. fabricated poly(trimethylene carbonate)–block–poly(l-glutamic acid) based polymersomes for targeting EGFR [134]. The plitidepsin-loaded polymersome was later evaluated for its specificity and efficacy in LS174T and HT29 colorectal cancer cell lines. Cytotoxicity assay and cellular uptake studies in colorectal cancer cell lines confirmed that prepared formulation was more sensitive to cell lines in contrast to non-targeted drug-loaded polymersome formulation.
Polymersomes for bone marrow cancer therapy
Multiple myeloma is a malignant cancer of the plasma cells of the bone marrow. Polymersomes offers the opportunity to selectively deliver therapeutic agents to the bone marrow for the effective treatment of multiple myeloma. Zhong and co-workers prepared CD44-targeted reduction-sensitive chimeric polymersomes loaded with granzyme B for targeted therapy of multiple myeloma [135]. The polymersomes showed a drug loading content of 1 wt%. The prepared formulation showed high targeting efficiency and was found to be highly effective against LP1 cells with a low IC50 of 8.1 nM. The in vivo biodistribution studies confirmed the enhanced accumulation of the formulation in both subcutaneous and orthotopic LP1 multiple myeloma tumor models. Furthermore, the formulation showed reduction-responsive protein release and potent inhibition of subcutaneous and orthotopic LP1 multiple myeloma tumor models in comparison with non-targeted counterparts. In a similar study, Gu et al. prepared CD44-specific A6 short peptide-functionalized polymersomes loaded with epirubicin for the treatment of multiple myeloma [136]. The polymersomes showed a drug loading content of ≈ 11 wt%. The formulation displayed enhanced anti-cancer activity against LP-1 cells with an IC50 of 3.9 μg mL−1. The in vivo pharmacokinetic studies revealed that the polymersomal formulation had a prolonged circulation time with an elimination half-life of 5.1 h. Moreover, the formulation showed enhanced targetability and anti-cancer activity against orthotopic human multiple myeloma xenografts in vivo compared with non-targeted polymersomes.
Polymersomes for cancer diagnosis
Polymersomes have also been investigated as a theranostic platform that is engineered to deliver imaging and therapeutic agents simultaneously. Several imaging agents such as magnetic particles [99, 148], quantum dots (QDs) [109, 149, 150], radionuclides [110], and fluorescent dyes [151] have successfully been integrated with polymersomes. Chiang et al. formulated SPION-loaded pH-responsive polymersomes for chemotherapy and magnetic resonance imaging (MRI) [148]. The polymersomes were then loaded with DOX, and folic acid was conjugated onto the surfaces of the polymersomes by layer-by-layer adsorption technique. The formulation was effectively internalized into the HeLa cells and released its payload under the combined influence of pH and high-frequency magnetic fields. The formulation exhibited significant cytotoxicity and showed good MRI sensitivity. Recently, Zavvar et al. investigated the potential of PEG-PCL polymersomes loaded with gadolinium-based QDs and DOX for MR/fluorescence imaging and anti-tumor activity [150]. The outer surfaces of the polymersomes were then conjugated with AS1411 DNA aptamer for targeted delivery. MR and fluorescence imaging confirmed the accumulation of theranostic platform at the site of the tumor. The prepared formulation significantly inhibited tumor growth in 4T1 tumor-bearing mice compared with the non-targeted formulation. In another study, Cao et al. investigated the potential of iodine-rich polymersomes for single-photon emission computed tomography/computed tomography (SPECT/CT) imaging and radioisotope therapy of 4T1 murine breast cancer in mice. [110]. 125I-radiolabeled polymersomes showed a prolonged circulation time and distributed mainly in organs of the reticuloendothelial system and in tumor cells. These iodine-rich polymersomes enabled SPECT/CT imaging and effectively inhibited tumor growth cells in mice with significant survival benefits.
Conclusion and future perspectives
The use of polymersomes in drug delivery has attained significant importance in recent years due to their ability to load a wide range of drugs. The surface modification of polymersomes with ligands and peptides, which can attach to the specific receptors located on the cancer cells can improve the targeting efficacy and cellular uptake of the therapeutic agents towards cancer cells. In this regard, numerous studies have been conducted in recent years for improving the cellular uptake and intracellular delivery of polymersomes against cancer cell membrane markers. Identification of specific targets and related ligands is important to reduce off-toxicity. The design and development of novel copolymers will undoubtedly pave the way for the fabrication of polymersomes with ideal features for incorporating a variety of therapeutic and imaging agents. Until now, a very limited number of polymersome-based systems have actually entered into clinical trials. Most of the techniques used for the preparation of polymersomes have low feasibility and reproducibility for upscaling, which limits their translation into clinical application. Most stimuli-responsive studies have been carried out under relatively static conditions in vitro and do not guarantee their efficacy in the human body. The in vivo pharmacokinetics and biodistribution parameters are vital aspects for evaluating the biosafety of nanocarriers. To date, there have been limited investigations on the pharmacokinetics and biodistribution of polymersomes. Furthermore, the safety of polymersomes should be a primary consideration for practical applications. Long-term toxicity and immunogenicity of polymersomes must be evaluated to enhance this strategy further. The effect of biodegradation on polymersome stability, drug release, and plasma half-life has not been extensively explored. More studies comparing the drug loading efficiency, therapeutic efficacy, pharmacokinetics, and biodistribution of polymersomes against strong liposome controls are needed, whose clinical safety profile is well recognized. The development of highly stable polymersomes with multi stimuli-linkers, which could efficiently deliver the therapeutic load into tumor cells, might be a promising novel strategy.
References
Baudino TA. Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol. Bentham Science Publishers. 2015;12:3–20.
Mohammadi M, Ramezani M, Abnous K, Alibolandi M. Biocompatible polymersomes-based cancer theranostics: towards multifunctional nanomedicine. Int J Pharm. Elsevier. 2017;519:287–303.
Yin H, Kang S-W, Bae YH. Polymersome formation from AB2 type 3-miktoarm star copolymers. Macromolecules. ACS Publications. 2009;42:7456–64.
Hu Y, Qiu L. Polymersomes: preparation and characterization. Pharm Nanotechnol. Springer. 2019:247–65.
Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers (Basel). Multidisciplinary Digital Publishing Institute. 2019;11:640.
Alibolandi M, Shahriari M, Ramezani M. Smart polymersomes as intelligent nanomedicines in cancer treatment. Polym Nanoparticles as a Promis Tool Anti-cancer Ther. Elsevier. 2019:343–71.
Pegoraro C, Cecchin D, Gracia LS, Warren N, Madsen J, Armes SP, et al. Enhanced drug delivery to melanoma cells using PMPC-PDPA polymersomes. Cancer Lett. 2013;334:328–37.
Shahriari M, Taghdisi SM, Abnous K, Ramezani M, Alibolandi M. Synthesis of hyaluronic acid-based polymersomes for doxorubicin delivery to metastatic breast cancer. Int J Pharm Elsevier. 2019;118835.
Wang X, Yao C, Zhang G, Liu S. Regulating vesicle bilayer permeability and selectivity via stimuli-triggered polymersome-to-PICsome transition. Nat Commun Nature Publishing Group. 2020;11:1–13.
Qin H, Jiang Y, Zhang J, Deng C, Zhong Z. Oncoprotein inhibitor rigosertib loaded in ApoE-targeted smart polymersomes reveals high safety and potency against human glioblastoma in mice. Mol Pharm. 2019;16:3711–9.
Ge X, Zhang Q, Cai Y, Duan S, Chen S, Lv N, et al. PEG–PCL–DEX polymersome–protamine vector as an efficient gene delivery system via PEG-guided self-assembly. Nanomedicine. Future Medicine. 2014;9:1193–207.
Kim HO, Lim JW, Choi J, Lee H, Son HY, Kim J, et al. Anchored protease-activatable polymersomes for molecular diagnostics of metastatic cancer cells. J Mater Chem B. 2017;5:9571–8.
Lansalot M, Rieger J, D’Agosto F. Polymerization-induced self-assembly: the contribution of controlled radical polymerization to the formation of self-stabilized polymer particles of various morphologies. Macromol self-assembly. Wiley Online Library. 2016:33–82.
Matyjaszewski K, Spanswick J. Controlled/living radical polymerization. Mater Today. Elsevier. 2005;8:26–33.
Balasubramanian V, Herranz-Blanco B, Almeida PV, Hirvonen J, Santos HA. Multifaceted polymersome platforms: spanning from self-assembly to drug delivery and protocells. Prog Polym Sci. Elsevier. 2016;60:51–85.
Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev Royal Society of Chemistry. 2018;47:8572–610.
Meng F, Zhong Z, Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules. ACS Publications. 2009;10:197–209.
Barros SM, Whitaker SK, Sukthankar P, Avila LA, Gudlur S, Warner M, et al. A review of solute encapsulating nanoparticles used as delivery systems with emphasis on branched amphipathic peptide capsules. Arch Biochem Biophys Elsevier. 2016;596:22–42.
Yin H, Kang HC, Huh KM, Bae YH. Biocompatible, pH-sensitive AB 2 miktoarm polymer-based polymersomes: preparation, characterization, and acidic pH-activated nanostructural transformation. J Mater Chem Royal Society of Chemistry. 2012;22:19168–78.
Men Y, Peng F, Tu Y, van Hest JCM, Wilson DA. Methods for production of uniform small-sized polymersome with rigid membrane. Polym Chem. Royal Society of Chemistry. 2016;7:3977–82.
Krishnamoorthy B, Karanam V, Chellan VR, Siram K, Natarajan TS, Gregory M. Polymersomes as an effective drug delivery system for glioma–a review. J Drug Target. Taylor & Francis. 2014;22:469–77.
Kricheldorf HR. Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew Chem Int Ed. Wiley Online Library. 2006;45:5752–84.
Vlakh E, Ananyan A, Zashikhina N, Hubina A, Pogodaev A, Volokitina M, et al. Preparation, characterization, and biological evaluation of poly (glutamic acid)-b-polyphenylalanine polymersomes. Polymers (Basel). Multidisciplinary Digital Publishing Institute. 2016;8:212.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. Elsevier. 2016;99:28–51.
Thambi T, Lee DS. Stimuli-responsive polymersomes for cancer therapy. Stimuli Responsive Polym Nanocarriers Drug Deliv Appl. Elsevier. 2019:413–38.
Zhang X, Zhang P. Polymersomes in nanomedicine-A review. Curr Nanosci. Bentham Science Publishers. 2017;13:124–9.
Matoori S, Leroux J-C. Twenty-five years of polymersomes: lost in translation? Mater Horizons. Royal Society of Chemistry. 2020.
Lee JS, Feijen J. Polymersomes for drug delivery: design, formation and characterization. J Control Release Elsevier. 2012;161:473–83.
Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng Annual Reviews. 2006;8:323–41.
Brinkhuis RP, Rutjes FPJT, van Hest JCM. Polymeric vesicles in biomedical applications. Polym Chem Royal Society of Chemistry. 2011;2:1449–62.
Bleul R, Thiermann R, Maskos M. Techniques to control polymersome size. Macromolecules. ACS Publications. 2015;48:7396–409.
Leong J, Teo JY, Aakalu VK, Yang YY, Kong H. Engineering polymersomes for diagnostics and therapy. Adv Healthc Mater. Wiley Online Library. 2018;7:1701276.
Lee JC, Bermudez H, Discher BM, Sheehan MA, Won Y, Bates FS, et al. Preparation, stability, and in vitro performance of vesicles made with diblock copolymers. Biotechnol Bioeng. Wiley Online Library. 2001;73:135–45.
Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. Elsevier. 2003;90:323–34.
Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release. 2006;116:150–8.
Guan L, Rizzello L, Battaglia G. Polymersomes and their applications in cancer delivery and therapy. Nanomedicine. Future Medicine. 2015;10:2757–80.
Anajafi T, Mallik S. Polymersome-based drug-delivery strategies for cancer therapeutics. Ther Deliv. Future Science. 2015;6:521–34.
Matoori S, Bao Y, Schmidt A, Fischer EJ, Ochoa-Sanchez R, Tremblay M, et al. An investigation of PS-b-PEO polymersomes for the oral treatment and diagnosis of hyperammonemia. Small. Wiley Online Library. 2019;15:1902347.
Lorenceau E, Utada AS, Link DR, Cristobal G, Joanicot M, Weitz DA. Generation of polymerosomes from double-emulsions. Langmuir. ACS Publications. 2005;21:9183–6.
Allen S, Osorio O, Liu Y-G, Scott E. Facile assembly and loading of theranostic polymersomes via multi-impingement flash nanoprecipitation. J Control Release. Elsevier. 2017;262:91–103.
Rijpkema SJ, Langens SGHA, van der Kolk MR, Gavriel K, Toebes BJ, Wilson DA. Modular approach to the functionalization of polymersomes. Biomacromolecules. ACS Publications. 2020.
Meghani NM, Amin HH, Lee B-J. Mechanistic applications of click chemistry for pharmaceutical drug discovery and drug delivery. Drug Discov Today. Elsevier. 2017;22:1604–19.
Rasheed T, Bilal M, Abu-Thabit NY, Iqbal HMN. The smart chemistry of stimuli-responsive polymeric carriers for target drug delivery applications. Stimuli Responsive Polym Nanocarriers Drug Deliv Appl. 2018;1. Elsevier:61–99.
Itel F, Chami M, Najer A, Lörcher S, Wu D, Dinu IA, et al. Molecular organization and dynamics in polymersome membranes: a lateral diffusion study. Macromolecules. ACS Publications. 2014;47:7588–96.
Lee K, David AE, Zhang J, Shin MC, Yang VC. Enhanced accumulation of theranostic nanoparticles in brain tumor by external magnetic field mediated in situ clustering of magnetic nanoparticles. J Ind Eng Chem. 2017;54:389–97.
Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. Elsevier. 2015;10:487–510.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. ACS Publications. 2008;5:505–15.
Lu W-L, Qi X-R, Zhang Q, Li R-Y, Wang G-L, Zhang R-J, et al. A pegylated liposomal platform: pharmacokinetics, pharmacodynamics, and toxicity in mice using doxorubicin as a model drug. J Pharmacol Sci. The Japanese Pharmacological Society. 2004;95:381–9.
de Kruijff RM, Raavé R, Kip A, Molkenboer-Kuenen J, Roobol SJ, Essers J, et al. Elucidating the influence of tumor presence on the polymersome circulation time in mice. Pharmaceutics. Multidisciplinary Digital Publishing Institute. 2019;11:241.
Choucair A, Lim Soo P, Eisenberg A. Active loading and tunable release of doxorubicin from block copolymer vesicles. Langmuir. ACS Publications. 2005;21:9308–13.
Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, et al. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm. ACS Publications. 2006;3:340–50.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. Dove Press. 2015;10:975.
Chandrawati R, Caruso F. Biomimetic liposome-and polymersome-based multicompartmentalized assemblies. Langmuir. ACS Publications. 2012;28:13798–807.
Schulz M, Binder WH. Mixed hybrid lipid/polymer vesicles as a novel membrane platform. Macromol Rapid Commun. Wiley Online Library. 2015;36:2031–41.
Lee JH, Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem Eng Sci. Elsevier. 2015;125:75–84.
Zhao Y, Li X, Zhao X, Yang Y, Li H, Zhou X, et al. Asymmetrical polymer vesicles for drug delivery and other applications. Front Pharmacol. Frontiers. 2017;8:374.
Cho HK, Cheong IW, Lee JM, Kim JH. Polymeric nanoparticles, micelles and polymersomes from amphiphilic block copolymer. Korean J Chem Eng. Springer. 2010;27:731–40.
Yao C, Wang X, Hu J, Liu S. Cooperative modulation of bilayer permeability and microstructures of polymersomes. Acta Polym Sin. 2019;6:553–66.
Mumtaz Virk M, Reimhult E. Phospholipase A2-induced degradation and release from lipid-containing polymersomes. Langmuir. ACS Publications. 2018;34:395–405.
Alibolandi M, Abnous K, Mohammadi M, Hadizadeh F, Sadeghi F, Taghavi S, et al. Extensive preclinical investigation of polymersomal formulation of doxorubicin versus doxil-mimic formulation. J Control Release. 2017;264:228–36.
Ayen WY, Kumar N. In vivo evaluation of doxorubicin-loaded (PEG) 3-PLA nanopolymersomes (PolyDoxSome) using DMBA-induced mammary carcinoma rat model and comparison with marketed LipoDox™. Pharm Res. Springer. 2012;29:2522–33.
Zou Y, Meng F, Deng C, Zhong Z. Robust, tumor-homing and redox-sensitive polymersomal doxorubicin: a superior alternative to doxil and caelyx? J Control Release. 2016;239:149–58.
Youssef SF, Elnaggar YSR, Abdallah OY. Elaboration of polymersomes versus conventional liposomes for improving oral bioavailability of the anticancer flutamide. Nanomedicine. Future Medicine. 2018;13:3025–36.
Zou Y, Xia Y, Meng F, Zhang J, Zhong Z. GE11-directed functional polymersomal doxorubicin as an advanced alternative to clinical liposomal formulation for ovarian cancer treatment. Mol Pharm. 2018;15:3664–71.
Hu X, Zhang Y, Xie Z, Jing X, Bellotti A, Gu Z. Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules. ACS Publications. 2017;18:649–73.
Che H, van Hest JCM. Stimuli-responsive polymersomes and nanoreactors. J Mater Chem B Royal Society of Chemistry. 2016;4:4632–47.
Puglisi A, Bayir E, Timur S, Yagci Y. pH-Responsive polymersome microparticles as smart cyclodextrin-releasing agents. Biomacromolecules. ACS Publications. 2019;20:4001–7.
Li Y, Liu G, Wang X, Hu J, Liu S. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew Chem Int Ed. Wiley Online Library. 2016;55:1760–4.
Sun Z, Liu G, Hu J, Liu S. Photo-and reduction-responsive polymersomes for programmed release of small and macromolecular payloads. Biomacromolecules. ACS Publications. 2018;19:2071–81.
Zhou J, Tang Q, Zhong J, Lei Z, Luo H, Tong Z, et al. Construction of glucose and H 2 O 2 dual stimuli-responsive polymeric vesicles and their application in controlled drug delivery. J Mater Sci Springer. 2018;53:14063–74.
Yan Q, Yuan J, Cai Z, Xin Y, Kang Y, Yin Y. Voltage-responsive vesicles based on orthogonal assembly of two homopolymers. J Am Chem Soc ACS Publications. 2010;132:9268–70.
Oliveira H, Pérez-Andrés E, Thevenot J, Sandre O, Berra E, Lecommandoux S. Magnetic field triggered drug release from polymersomes for cancer therapeutics. J Control Release. 2013;169:165–70.
Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. Springer. 2013;13:89.
Meng F, Hiemstra C, Engbers GHM, Feijen J. Biodegradable polymersomes. Macromolecules ACS Publications. 2003;36:3004–6.
Chen W, Meng F, Cheng R, Zhong Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: a comparative study with micelles. J Control Release Elsevier. 2010;142:40–6.
Qiao Z-Y, Cheng J, Ji R, Du F-S, Liang D-H, Ji S-P, et al. Biocompatible acid-labile polymersomes from PEO-b-PVA derived amphiphilic block copolymers. RSC Adv Royal Society of Chemistry. 2013;3:24345–53.
Brinkhuis RP, Visser TR, Rutjes FPJT, van Hest JCM. Shedding the hydrophilic mantle of polymersomes. Polym Chem Royal Society of Chemistry. 2011;2:550–2.
Yoshida T, Lai TC, Kwon GS, Sako K. pH-and ion-sensitive polymers for drug delivery. Expert Opin Drug Deliv Taylor & Francis. 2013;10:1497–513.
Lodge TP, Rasdal A, Li Z, Hillmyer MA. Simultaneous, segregated storage of two agents in a multicompartment micelle. J Am Chem Soc ACS Publications. 2005;127:17608–9.
Du J, Tang Y, Lewis AL, Armes SP. pH-sensitive vesicles based on a biocompatible zwitterionic diblock copolymer. J Am Chem Soc ACS Publications. 2005;127:17982–3.
Massignani M, Canton I, Sun T, Hearnden V, MacNeil S, Blanazs A, et al. Enhanced fluorescence imaging of live cells by effective cytosolic delivery of probes. PLoS One Public Library of Science. 2010;5.
Min KH, Kim J-H, Bae SM, Shin H, Kim MS, Park S, et al. Tumoral acidic pH-responsive MPEG-poly (β-amino ester) polymeric micelles for cancer targeting therapy. J Control Release Elsevier. 2010;144:259–66.
Kim DH, Seo YK, Thambi T, Moon GJ, Son JP, Li G, et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1α using a dual ionic pH-sensitive copolymer. Biomaterials Elsevier. 2015;61:115–25.
Caon T, Porto LC, Granada A, Tagliari MP, Silva MAS, Simões CMO, et al. Chitosan-decorated polystyrene-b-poly (acrylic acid) polymersomes as novel carriers for topical delivery of finasteride. Eur J Pharm Sci Elsevier. 2014;52:165–72.
Bellomo EG, Wyrsta MD, Pakstis L, Pochan DJ, Deming TJ. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat Mater. Nature Publishing Group. 2004;3:244.
Hashimoto Y, Tanaka M, Kishimoto H, Shiozawa H, Hasegawa K, Matsuyama K, et al. Preparation, characterization and taste-masking properties of polyvinylacetal diethylaminoacetate microspheres containing trimebutine. J Pharm Pharmacol. Wiley Online Library. 2002;54:1323–8.
Onaca O, Enea R, Hughes DW, Meier W. Stimuli-responsive polymersomes as nanocarriers for drug and gene delivery. Macromol Biosci. Wiley Online Library. 2009;9:129–39.
Gandhi A, Paul A, Sen SO, Sen KK. Studies on thermoresponsive polymers: phase behaviour, drug delivery and biomedical applications. Asian J Pharm Sci. Elsevier. 2015;10:99–107.
Pfleiderer SOR, Marx C, Camara O, Gajda M, Kaiser WA. Ultrasound-guided, percutaneous cryotherapy of small (≤ 15 mm) breast cancers. Investig Radiol. LWW. 2005;40:472–7.
Qin S, Geng Y, Discher DE, Yang S. Temperature-controlled assembly and release from polymer vesicles of poly (ethylene oxide)-block-poly (N-isopropylacrylamide). Adv Mater. Wiley Online Library. 2006;18:2905–9.
Cheng R, Meng F, Ma S, Xu H, Liu H, Jing X, et al. Reduction and temperature dual-responsive crosslinked polymersomes for targeted intracellular protein delivery. J Mater Chem. Royal Society of Chemistry. 2011;21:19013–20.
Kozlovskaya V, Liu F, Yang Y, Ingle K, Qian S, Halade GV, et al. Temperature-responsive polymersomes of poly (3-methyl-N-vinylcaprolactam)-block-poly (N-vinylpyrrolidone) to decrease doxorubicin-induced cardiotoxicity. Biomacromolecules. ACS Publications. 2019;20:3989–4000.
Tan J, Deng Z, Liu G, Hu J, Liu S. Anti-inflammatory polymersomes of redox-responsive polyprodrug amphiphiles with inflammation-triggered indomethacin release characteristics. Biomaterials. Elsevier. 2018;178:608–19.
de Oliveira MF, Amoêdo ND, Rumjanek FD. Energy and redox homeostasis in tumor cells. Int J Cell Biol. Hindawi. 2012;2012.
Kumar A, Lale SV, Mahajan S, Choudhary V, Koul V. ROP and ATRP fabricated dual targeted redox sensitive polymersomes based on pPEGMA-PCL-ss-PCL-pPEGMA triblock copolymers for breast cancer therapeutics. ACS Appl Mater Interfaces. 2015;7:9211–27.
Ren T, Wu W, Jia M, Dong H, Li Y, Ou Z. Reduction-cleavable polymeric vesicles with efficient glutathione-mediated drug release behavior for reversing drug resistance. ACS Appl Mater Interfaces ACS Publications. 2013;5:10721–30.
Sun H, Meng F, Cheng R, Deng C, Zhong Z. Reduction and pH dual-bioresponsive crosslinked polymersomes for efficient intracellular delivery of proteins and potent induction of cancer cell apoptosis. Acta Biomater. 2014;10:2159–68.
Li J, Li Y, Wang Y, Ke W, Chen W, Wang W, et al. Polymer prodrug-based nanoreactors activated by tumor acidity for orchestrated oxidation/chemotherapy. Nano Lett ACS Publications. 2017;17:6983–90.
Sanson C, Diou O, Thévenot J, Ibarboure E, Soum A, Brûlet A, et al. Doxorubicin loaded magnetic polymersomes: theranostic nanocarriers for MR imaging and magneto-chemotherapy. ACS Nano. 2011;5:1122–40.
García MC. Stimuli-responsive polymersomes for drug delivery applications. Stimuli Responsive Polym Nanocarriers Drug Deliv Appl. Elsevier. 2019:345–92.
Yamamoto S, Yamada T, Kubo G, Sakurai K, Yamaguchi K, Nakanishi J. Preparation of a series of photoresponsive polymersomes bearing photocleavable a 2-nitrobenzyl group at the hydrophobic/hydrophilic interfaces and their payload releasing behaviors. Polymers (Basel). Multidisciplinary Digital Publishing Institute. 2019;11:1254.
Lee JS, Groothuis T, Cusan C, Mink D, Feijen J. Lysosomally cleavable peptide-containing polymersomes modified with anti-EGFR antibody for systemic cancer chemotherapy. Biomaterials Elsevier. 2011;32:9144–53.
Iatridi Z, Angelopoulou A, Voulgari E, Avgoustakis K, Tsitsilianis C. Star-Graft quarterpolymer-based polymersomes as nanocarriers for co-delivery of hydrophilic/hydrophobic chemotherapeutic agents. ACS Omega. 2018;3:11896–908.
Chen W, Du J. Ultrasound and pH dually responsive polymer vesicles for anticancer drug delivery. Sci Rep. Nature Publishing Group. 2013;3:2162.
Lu F, Pang Z, Zhao J, Jin K, Li H, Pang Q, et al. Angiopep-2-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) polymersomes for dual-targeting drug delivery to glioma in rats. Int J Nanomedicine. 2017;12:2117–27.
Figueiredo P, Balasubramanian V, Shahbazi MA, Correia A, Wu D, Palivan CG, et al. Angiopep2-functionalized polymersomes for targeted doxorubicin delivery to glioblastoma cells. Int J Pharm. 2016;511:794–803.
Chen YC, Chiang CF, Chen LF, Liang PC, Hsieh WY, Lin WL. Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials. 2014;35:4066–81.
Pang Z, Gao H, Yu Y, Guo L, Chen J, Pan S, et al. Enhanced intracellular delivery and chemotherapy for glioma rats by transferrin-conjugated biodegradable polymersomes loaded with doxorubicin. Bioconjug Chem. 2011;22:1171–80.
Alibolandi M, Abnous K, Sadeghi F, Hosseinkhani H, Ramezani M, Hadizadeh F. Folate receptor-targeted multimodal polymersomes for delivery of quantum dots and doxorubicin to breast adenocarcinoma: in vitro and in vivo evaluation. Int J Pharm. 2016;500:162–78.
Cao J, Wei Y, Zhang Y, Wang G, Ji X, Zhong Z. Iodine-rich polymersomes enable versatile SPECT/CT imaging and potent radioisotope therapy for tumor in vivo. ACS Appl Mater Interfaces. 2019;11:18953–9.
Bessone MID, Simón-Gracia L, Scodeller P, de los Angeles Ramirez M, Huvelle MAL, Soler-Illia GJAA, et al. iRGD-guided tamoxifen polymersomes inhibit estrogen receptor transcriptional activity and decrease the number of breast cancer cells with self-renewing capacity. J Nanobiotechnol. BioMed Central. 2019;17:1–14.
Lale SV, Kumar A, Prasad S, Bharti AC, Koul V. Folic acid and trastuzumab functionalized redox responsive polymersomes for intracellular doxorubicin delivery in breast cancer. Biomacromolecules. 2015;16:1736–52.
Zhang Y, Wu K, Sun H, Zhang J, Yuan J, Zhong Z. Hyaluronic acid-shelled disulfide-cross-linked nanopolymersomes for ultrahigh-efficiency reactive encapsulation and CD44-targeted delivery of mertansine toxin. ACS Appl Mater Interfaces. 2018;10:1597–604.
Xu J, Zhao Q, Jin Y, Qiu L. High loading of hydrophilic/hydrophobic doxorubicin into polyphosphazene polymersome for breast cancer therapy. Nanomedicine Nanotechnology, Biol Med. 2014;10:349–58.
Yang W, Wei Y, Yang L, Zhang J, Zhong Z, Storm G, et al. Granzyme B-loaded, cell-selective penetrating and reduction-responsive polymersomes effectively inhibit progression of orthotopic human lung tumor in vivo. J Control Release. 2018;290:141–9.
Yang W, Yang L, Xia Y, Cheng L, Zhang J, Meng F, et al. Lung cancer specific and reduction-responsive chimaeric polymersomes for highly efficient loading of pemetrexed and targeted suppression of lung tumor in vivo. Acta Biomater. 2018;70:177–85.
Zou Y, Wei J, Xia Y, Meng F, Yuan J, Zhong Z. Targeted chemotherapy for subcutaneous and orthotopic non-small cell lung tumors with cyclic RGD-functionalized and disulfide-crosslinked polymersomal doxorubicin. Signal Transduct Target Ther. 2018;3:32.
Yang W, Zou Y, Meng F, Zhang J, Cheng R, Deng C, et al. Efficient and targeted suppression of human lung tumor xenografts in mice with methotrexate sodium encapsulated in all-function-in-one chimeric polymersomes. Adv Mater. 2016;28:8234–9.
Yang W, Xia Y, Fang Y, Meng F, Zhang J, Cheng R, et al. Selective cell penetrating peptide-functionalized polymersomes mediate efficient and targeted delivery of methotrexate disodium to human lung cancer in vivo. Adv Healthc Mater. 2018;7:1701135.
Alibolandi M, Ramezani M, Abnous K, Hadizadeh F. AS1411 Aptamer-decorated biodegradable polyethylene glycol-poly(lactic-co-glycolic acid) nanopolymersomes for the targeted delivery of gemcitabine to non-small cell lung cancer in vitro. J Pharm Sci. 2016;105:1741–50.
Peng Y, Zhu X, Qiu L. Electroneutral composite polymersomes self-assembled by amphiphilic polyphosphazenes for effective miR-200c in vivo delivery to inhibit drug resistant lung cancer. Biomaterials. 2016;106:1–12.
Karandish F, Mamnoon B, Feng L, Haldar MK, Xia L, Gange KN, et al. Nucleus-targeted, echogenic polymersomes for delivering a cancer stemness inhibitor to pancreatic cancer cells. Biomacromolecules. 2018;19:4122–32.
Anajafi T, Yu J, Sedigh A, Haldar MK, Muhonen WW, Oberlander S, et al. Nuclear localizing peptide-conjugated, redox-sensitive polymersomes for delivering curcumin and doxorubicin to pancreatic cancer microtumors. Mol Pharm. 2017;14:1916–28.
Anajafi T, Scott MD, You S, Yang X, Choi Y, Qian SY, et al. Acridine orange conjugated polymersomes for simultaneous nuclear delivery of gemcitabine and doxorubicin to pancreatic cancer cells. Bioconjug Chem. 2016;27:762–71.
Kulkarni P, Haldar MK, You S, Choi Y, Mallik S. Hypoxia-responsive polymersomes for drug delivery to hypoxic pancreatic cancer cells. Biomacromolecules. 2016;17:2507–13.
Iatrou H, Dimas K, Gkikas M, Tsimblouli C, Sofianopoulou S. Polymersomes from polypeptide containing triblock co-and terpolymers for drug delivery against pancreatic cancer: asymmetry of the external hydrophilic blocks. Macromol Biosci Wiley Online Library. 2014;14:1222–38.
Karandish F, Haldar MK, You S, Brooks AE, Brooks BD, Guo B, et al. Prostate-specific membrane antigen targeted polymersomes for delivering mocetinostat and docetaxel to prostate cancer cell spheroids. ACS Omega. 2016;1:952–62.
Li X, Yang W, Zou Y, Meng F, Deng C, Zhong Z. Efficacious delivery of protein drugs to prostate cancer cells by PSMA-targeted pH-responsive chimaeric polymersomes. J Control Release. 2015;220:704–14.
Demirgöz D, Pangburn TO, Davis KP, Lee S, Bates FS, Kokkoli E. PR-b-targeted delivery of tumor necrosis factor-α by polymersomes for the treatment of prostate cancer. Soft Matter. 2009;5:2011–9.
Fang Y, Yang W, Cheng L, Meng F, Zhang J, Zhong Z. EGFR-targeted multifunctional polymersomal doxorubicin induces selective and potent suppression of orthotopic human liver cancer in vivo. Acta Biomater. 2017;64:323–33.
Wei Y, Gu X, Cheng L, Meng F, Storm G, Zhong Z. Low-toxicity transferrin-guided polymersomal doxorubicin for potent chemotherapy of orthotopic hepatocellular carcinoma in vivo. Acta Biomater. 2019;92:196–204.
Wang X, Sun H, Meng F, Cheng R, Deng C, Zhong Z. Galactose-decorated reduction-sensitive degradable chimaeric polymersomes as a multifunctional nanocarrier to efficiently chaperone apoptotic proteins into hepatoma cells. Biomacromolecules. 2013;14:2873–82.
Alibolandi M, Rezvani R, Farzad SA, Taghdisi SM, Abnous K, Ramezani M. Tetrac-conjugated polymersomes for integrin-targeted delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int J Pharm. 2017;532:581–94.
Goñi-de-Cerio F, Thevenot J, Oliveira H, Pérez-Andrés E, Berra E, Masa M, et al. Cellular uptake and cytotoxic effect of epidermal growth factor receptor targeted and plitidepsin loaded co-polymeric polymersomes on colorectal cancer cell lines. J Biomed Nanotechnol. 2015;11:2034–49.
Zhong Y, Meng F, Zhang W, Li B, van Hest JCM, Zhong Z. CD44-targeted vesicles encapsulating granzyme B as artificial killer cells for potent inhibition of human multiple myeloma in mice. J Control Release Elsevier. 2020;320:421–30.
Gu W, An J, Meng H, Yu N, Zhong Y, Meng F, et al. CD44-Specific A6 short peptide boosts targetability and anticancer efficacy of polymersomal epirubicin to orthotopic human multiple myeloma. Adv Mater. Wiley Online Library. 2019;31:1904742.
Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. Royal Society of Chemistry. 2019.
Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood–brain barrier (BBB). J Drug Target. Taylor & Francis. 2011;19:125–32.
Abdul Razzak R, Florence GJ, Gunn-Moore FJ. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int J Mol Sci. Multidisciplinary Digital Publishing Institute. 2019;20:3108.
Pang Z, Feng L, Hua R, Chen J, Gao H, Pan S, et al. Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol Pharm. 2010;7:1995–2005.
Pang Z, Fan L, Hu K, Wu B, Jiang X. Effect of lactoferrin-and transferrin-conjugated polymersomes in brain targeting: in vitro and in vivo evaluations. Acta Pharmacol Sin Nature Publishing Group. 2010;31:237–43.
Chung NS, Wasan KM. Potential role of the low-density lipoprotein receptor family as mediators of cellular drug uptake. Adv Drug Deliv Rev Elsevier. 2004;56:1315–34.
Jiang Y, Zhang J, Meng F, Zhong Z. Apolipoprotein E peptide-directed chimeric polymersomes mediate an ultrahigh-efficiency targeted protein therapy for glioblastoma. ACS Nano. 2018;12:11070–9.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Alibolandi M, Ramezani M, Sadeghi F, Abnous K, Hadizadeh F. Epithelial cell adhesion molecule aptamer conjugated PEG-PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro. Int J Pharm. 2015;479:241–51.
Pepper MS. Role of the matrix metalloproteinase and plasminogen activator–plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. Am Heart Assoc. 2001;21:1104–17.
Street W. Cancer facts & figures 2019. Am Cancer Soc Atlanta, GA, USA. 2019
Chiang W-H, Huang W-C, Chang C-W, Shen M-Y, Shih Z-F, Huang Y-F, et al. Functionalized polymersomes with outlayered polyelectrolyte gels for potential tumor-targeted delivery of multimodal therapies and {MR} imaging. J Control Release. 2013;168:280–8.
Bakalova R, Lazarova D, Nikolova B, Atanasova S, Zlateva G, Zhelev Z, et al. Delivery of size-controlled long-circulating polymersomes in solid tumours, visualized by quantum dots and optical imaging {in vivo}. Biotechnol Biotechnol Equip. 2015;29:175–80.
Zavvar T, Babaei M, Abnous K, Taghdisi SM, Nekooei S, Ramezani M, et al. Synthesis of multimodal polymersomes for targeted drug delivery and MR/fluorescence imaging in metastatic breast cancer model. Int J Pharm Elsevier. 2020;578:119091.
Li Z, Wu L, Hu P, Han S, Zhang T, Fan H, et al. Soft nanomaterial-based targeting polymersomes for near-infrared fluorescence multispectral in vivo imaging. Nanoscale Royal Society of Chemistry. 2012;4:7097–105.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, A.K., Prasher, P., Aljabali, A.A. et al. Emerging era of “somes”: polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. and Transl. Res. 10, 1171–1190 (2020). https://doi.org/10.1007/s13346-020-00789-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00789-2