Abstract
Wound healing is a physiological event that generates reconstitution and restoration of granulation tissue that ends with scar formation. As omega fatty acids are part of membrane phospholipids and participate in the inflammatory response, we investigated the effects of omega-3, omega-6, and omega-9 fatty acids in the form of oils on wound healing. Linseed (LO), evening primrose (EPO), and olive oils (OO) rich in omega-3, omega-6, and omega-9 fatty acids were formulated into emulsions and were topically applied on rats with excision wounds. All omega-3-, omega-6-, and omega-9-rich oil formulations were found to accelerate wound closure compared to untreated, with significant improvement (p < 0.05) being observed at day 14. EPO induced early deposition of collagen as evaluated by Masson trichrome staining that correlated well with the hydroxyproline content assay, with the highest level at days 3 and 7. Vascular endothelial growth factor (VEGF) showed greater amount of new microvasculature formed in the EPO-treated group, while moderate improvement occurs in the LO and OO groups. EPO increased both the expression of proinflammatory cytokines and growth factors in the early stage of healing and declined at the later stage of healing. LO modulates the proinflammatory cytokines and chemokine but did not affect the growth factors. In contrast, OO induced the expression of growth factors rather than proinflammatory cytokines. These data suggest that LO, EPO, and OO emulsions promote wound healing but they accomplish this by different mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound is defined as a cut or break in cellular, anatomical, or functional continuity of the living tissues [1]. Wound healing is a dynamic and complex biological repair process which occurs in overlapping phases, with coagulation of blood vessels and inflammatory phase involving recruitment of neutrophils and macrophages to the damaged area to clear the infection. Later, fibroblast and keratinocytes adjacent to the injury site start to migrate, proliferate, and differentiate to form new vessels to complete the epithelialization phase [2]. Synthesis and accumulation of collagen fibers will increase the tensile strength of the skin and to complete the restoration of tissue integrity. Alteration in any of these phases can lead to delay or failure to heal completely. Therefore, the main aim of wound treatment is to minimize the time for healing or avoid delayed wound healing that leads to chronic wounds.
The inflammatory reaction is crucial for the initiation and completion of tissue repair. This inflammation phase initiates immediately in response to injury with the release of arachidonic acid mediators, namely prostaglandins, leukotrienes, and thromboxanes. Fatty acids are the precursors for these mediators and are able to modulate numerous immune cells by regulating the migration of neutrophil and cytokine production at the wound site [3], vascular contraction, cellular activation, and death [4]. The immunomodulatory effects of fatty acids have been demonstrated [5, 6], such as α-linolenic acid (ALA) and oleic acid (OA) exhibited anti-inflammatory properties, while linoleic acid (LA) was reported to produce proinflammatory effects [3].
Since ancient times, naturally occurring animal fats and vegetable oils have widely been used as ingredients and components in many fields for therapeutic, nutritional, and esthetic applications. In cosmetology, they were used as emollient due to their characteristics of emolliency, moisturizing, grooming, and acting as solvents and vehicles to carry other agents. However, in the last half century, the odor problems derived from oils of animal origin had shifted attention towards vegetable oils with high safety profiles, less greasiness [7], wide availability, biodegradability, low cost, and low ecotoxicity [8]. Vegetable oils are predominantly constituted of triglycerides but also contain low proportions of other lipophilic substances such as mono- and diglycerides, phospholipids, and nonglycerolipids including sterols, tocopherols, free fatty acids, vitamins, pigments, proteins, phenolic compounds, and water [8] that determine their activity in cosmetic and pharmaceutical uses.
Unsaturated fatty acids such as linolenic (n-3), linoleic (n-6), and oleic (n-9) acids present in vegetables oils are widely utilized in the pharmaceutical industry by intravenous injection of free fatty acids [9], solely oil topical applications [10] or formulated as oil-in-water emulsions [11, 12]. The omega series on n-3, n-6, and n-9 play a significant role in the skin by reducing transepidermal water loss to improve skin hydration, activating regeneration of damaged lipid barrier of the epidermis, and stabilizing skin metabolism [13]. The ability of oils to control the invasion of microorganisms and avoid the development of infection during wound repair promotes healing and makes them attractive to be used therapeutically in topical wound treatment [5].
The potential biological effects of these omega fatty acids in wound healing have been investigated in various in vitro and in vivo models. In a study to assess the proliferative and restitution effects of fatty acids on the wound repair of intestinal epithelial cell line (IEC-6) cultures, Ruthig and Meckling-Gill [14] observed that supplementation of (n-3) fatty acids eicosapentanoic acid (EPA) and ALA, as well as the cis (n-6) fatty acids LA, γ-linolenic acid (GLA) and arachidonic acid (AA), enhanced cellular migration in the IEC-6, thus capable of improving reconstitution of epithelial integrity following mucosal injury. In contrast, docosahexaenoic acid (DHA) did not show similar effects and this suggests that EPA is an effective inhibitor of both cyclooxygenase and lipoxygenase while DHA inhibits only the cyclooxygenase pathway. Franco et al. [15] in a study on the effects of semisolid 1, 5, or 10% formulations of linseed oil (SSFLO) on skin wounds of rats found that topical administration of SSFLO (1 or 5%) achieved 100% re-epithelialization of treated animals, suggesting that the linseed oil at low concentrations have therapeutic potentials for the process of dermal repair. Despite promoting wound healing, some studies demonstrated contradicting evidence of fatty acids to cause delay in wound healing. A human trial supplemented orally with n-3 rich with EPA and DHA had demonstrated longer time for wound healing [16]. Thus, administration of ALA- or EPA/DHA-rich oils can influence the effects of fatty acids in inflammatory reactions and immune responses. Since the major n-3 fatty acid in linseed is ALA whereas fish oil is mainly composed of EPA and DHA, and the conversion of ALA to EPA is relatively to a small extent only, the efficacy of these fatty acids to modify eicosanoid production might be different [17]. Cardoso et al. [6] reported a similar result in evaluating the effect of topical administration of purified ALA, LA, and OA on the process of wound healing in rats, and they demonstrated that the groups treated topically with LA (n-6) and OA (n-9) acids showed significant reduction in wound area in contrast to the group treated with ALA (n-3) which exhibited delayed wound closure. This suggested a favorable effect of MUFA oleic and PUFA linoleic acids on the process of tissue repair. These studies thus suggest that the kind of fatty acids, types of model used, and route of fatty acid administration influence the immune responses.
Owing to the inconclusive and contradictive nature of available evidence, the aim of the present study is to investigate whether the fatty acids have beneficial or detrimental effects on wound healing. Three different vegetables oils—linseed oil (LO, n-3), evening primrose oil (EPO, n-6), and olive oil (OO, n-9)—were formulated into emulsions and were topically applied on an excision wound model in rats.
Materials and methods
Materials
Linseed oil (430021), evening primrose oil (E7134), olive oil (75343), phosphate buffered saline, hydroxypropylmethylcellulose (HPMC), and benzalkonium chloride were purchased from Sigma-Aldrich (USA). Span 80 was obtained from Merck (USA). Labrasol was kindly supplied by Gattefosse (Westwoold, NJ). Isoflurane was obtained from Unimed (Malaysia). Betadine (5% povidone-iodine as its active ingredient) was obtained from Mundipharm Pte Ltd. (Singapore). Rabbit polyclonal antibody of vascular endothelial growth factor A (VEGF-A, Cat: ab46154), collagen-1 (Cat: ab3471), phenylmethylsulfonyl fluoride (PMSF, Cat: ab141032), aprotinin (Cat: ab146286), and RIPA lysis buffer (Cat: ab156034) were purchased from Abcam (Cambridge, USA). Hydroxyproline assay kit was obtained from Biovision Inc. (Milpitas, CA, USA). Millipore’s®MAP rat cytokine/chemokine magnetic bead panel was supplied by Merck KGaA (Darmstadt, Germany). Punch biopsy was purchased from Premier Uni-Punch (USA). Wistar rats were obtained from the Laboratory Animal Resource Unit, Medical Centre, National University of Malaysia (Malaysia). All other chemicals and solvents were of reagent grade and were used without further purification.
Preparation of the LO, EPO, and OO emulsions
LO-, EPO-, and OO-based emulsions were prepared using high energy emulsification method. Briefly, LO, EPO, or OO oil; Span 80; and Labrasol comprised the oil phase, while distilled water served as the aqueous phase (Table 1). Both oil and aqueous phases were heated separately at 40 °C. When it has cooled down, the oil phase was added stepwise to the aqueous phase resulting in the formation of the primary emulsion. The resultant primary emulsions of LO, EPO, or OO were homogenized using Ultra-Turrax T25 (Janhke & Kunkel GmBh and Co KC, Staufen, Germany) at 10,000 rpm for 3 min. These emulsions were further homogenized using APV 2000 high-pressure homogenizer (APV system, Denmark) for seven cycles at 900 bar. Finally, 0.5% (w/w) of HPMC and 0.05% (w/w) benzalkonium chloride dissolved in a sufficient amount of heated distilled water were added to the obtained emulsion. The emulsions were stored in air-tight containers at 4 °C for further use.
Animals
Healthy adult male and female Wistar rats weighing 200–300 g (6–8 weeks old) were selected from the Laboratory Animal Resource Unit, Medical Centre, National University of Malaysia. Rodents were properly housed in clean, sterile, polypropylene cages with free access to a normal pellet diet and water ad libitum. They were acclimatized under standard laboratory conditions for 7 days of temperature 22 ± 1 °C with 12 h light/dark cycle. The animal study protocol was approved by the Animal Ethics Committee of Universiti Kebangsaan Malaysia (FF/2016/MOHD/18-MAY/751-OCT-2016-AUG-2017) and carried out according to the Guide for the Care and Use of Laboratory Animals, Universiti Kebangsaan Malaysia.
Excision wound model
Wound-healing activity of LO, EPO, and OO emulsions were tested on uninfected excisional wounds. The rats were divided into five groups, each group consisting of 24 rats. Half of these rats were studied for wound closure and rate of epithelialization, whereas the other half was used for biochemical and immunohistochemistry analysis. The wounding was created under general anesthesia by using open mask method with isoflurane. After shaving and sterilizing the skin with 70% alcohol, two full-thickness skin wound sections measuring 8 mm2 with about 1 mm depth were excised from the predetermined area by using a sterile punch-biopsy needle on the left and right dorsal of the midline. Bleeding was stopped using sterile cotton sticks.
Grouping and topical treatment
Rats were grouped into five groups as follows: (I) untreated (negative control), (II) Betadine (containing 5% (w/w) povidone-iodine as active ingredient, positive control), (III) LO emulsion, (IV) EPO emulsion, and (V) OO emulsion. All treatments were started immediately after the 8-mm2 wound creation. Formulations weighing 0.20 g per wound was applied topically once a day until the wound was completely healed. The rats were placed in their respective cages with one rat per cage.
Determination of wound closure percentage
The wound closure area (mm2) of each rat was measured on alternate days using a digital caliper. The wounds were photographed using a digital camera (Sony Cybershot, USA). The percent of wound healing values was determined by calculating the percentage of wound reduction from the original wound (Eq. 1).
Histological evaluation
Histological studies were performed on the rats. The rats from each group were euthanized on days 3, 7, and 14 using an overdose of isoflurane. Serial sections of 3 μm from every group were proceeded with hematoxylin and eosin (H&E) staining for general morphological observations, and Masson trichrome staining was performed for detection of collagen fibers [1]. H&E stains collagen fibers as pale pink, cytoplasm as purple, and nuclei as blue, and red blood cells appear cherry red. Masson trichrome stains collagen blue, while cytoplasm, red blood cells, and muscle are stained red. The intensity of blue color staining corresponds to the relative quantity of collagen fiber deposit which reflects the process of synthesis, degradation, and remodeling of the lesion [1]. The slides were examined and photographed under a light microscope equipped with a camera (BX41 Olympus, Tokyo, Japan).
Immunohistochemistry
Paraffin-embedded tissue sections of 3-μm serial sections from each skin block were placed on silanized-coated slides and left to dry at room temperature. The dried slides were treated with 3% peroxidase block (Dako, Carpinteria, CA, USA) for 10 min to inhibit endogenous peroxidase activity. The slides were immersed in a solution of citrate buffer, pH 6.0 (Dako, Carpinteria, CA, USA), and heated in a decloaking chamber at 90 °C for 30 min to retrieve masked antigens. After cooling down, the slides were incubated with the following antibodies: rabbit polyclonal antibody against VEGF-A (1:100) for 60 min or rabbit polyclonal to collagen-1 (1:300) for 45 min. Slides were then incubated with sufficient amount of streptavidin-HRP (Dako, Carpinteria, CA, USA) for 60 min (VEGF-A) and 45 min (collagen-1), respectively. All sections were visualized using DAB substrate chromogen solution (Dako, Carpinteria, CA, USA) for 10 min and counterstained with hematoxylin for 5 s. All of the washing processes in every step were done using Tris-buffered saline (Dako, Carpinteria, CA, USA). The stained sections were viewed under a bright-field microscope. Two independent observers blinded to the treatment regimen scored the stained slides. Histologic scores (H) for VEGF were calculated using the formula H = ΣPi, where i is the intensity of labeling with a value of 1, 2, or 3 (weak, moderate, or strong, respectively) and Pi is the percentage of staining cells for each intensity with P values of 1, 2, 3, 4, and 5 indicating < 15, 15–50, 50–85, > 85, and 100% positive-staining cells. Meanwhile, the staining for collagen-1 was graded as no staining (0), weak (1), moderate (2), and strong (3) [18, 19].
Hydroxyproline content
Hydroxyproline content of fresh rat skin samples was quantified by colorimetric assay using a commercial kit according to the manufacturer’s instructions.
Determination of cytokine concentrations in wound tissue
Wound lesions of 100 mg excised at days 3, 7, and 14 were homogenized with 1 mL of RIPA lysis buffer (0.22% beta glycerophosphate, 10% Tergitol-NP40, 0.18% sodium orthovanadate, 5% sodium deoxycholate, 0.38% EGTA, 1% SDS, 6.1% Tris, 0.29% EDTA, 8.8% sodium chloride, 1.12% sodium pyrophosphate decahydrate) supplemented with 1 mM of PMSF and 2 μg/mL of aprotinin. Samples were sonicated for 1 min and centrifuged at 12,000 rpm for 10 min at 4 °C. The concentrations of cytokines (interleukin (IL)-1b, epidermal growth factor (EGF), monocyte chemoattractant protein (MCP)-1, VEGF, and tumor necrosis factor (TNF)-α) in the supernatants were assessed by Millipore’s®MAP rat cytokine/chemokine magnetic bead panel by Merck KGaA (Darmstadt, Germany). Results were expressed as picograms per milliliter.
Statistical analysis
Data were presented as the mean value ± standard error of the mean. Statistical analyses were evaluated using GraphPad InStat version 3.05 (GraphPad Software, Inc). Two-way repeated measures analysis of variance, ANOVA, followed by Bonferroni’s post hoc test was used to identify the differences between the treated and control. Values of p < 0.05 were considered statistically significant.
Results
Wound closure and days of epithelialization
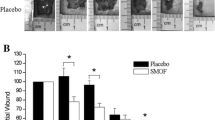
Macroscopic observation of wound closure is shown in Fig. 1a. Inflammation (redness and swelling) was immediately noticeable several hours after wound creation without any signs of infections. This indicated that the inflammatory phase began within a few minutes after the injury. The scab formation was observed in all groups as early as day 3. It was difficult to differentiate the changes by gross appearance in all groups from day 0 until day 7. However, by day 14, the rats topically applied with 5% povidone-iodine and oil emulsions demonstrated re-epithelialization in contrast to the untreated rats. Epithelialization time refers to the number of days taken by the wounds to appear completely closed with no moist granulation tissue and the wound was covered with new epithelium [1]. Wound treated with EPO emulsion was found to epithelialize the fastest followed by povidone-iodine, while the untreated group exhibited the slowest rate of epithelialization during the 14-day follow-up period.
Wound closure of untreated and topical application of omega-rich oil emulsions in full-thickness excision wound healing. a Representative images of the gross appearance of excisional wounds over time after treatment. b Quantitative data concerning the percentage of wound closure to the initial wound area at different time points after treatment. ap < 0.05 vs. the untreated group on the indicated day. Data are expressed as mean ± SEM (n = 12). Abbreviations: EPO, evening primrose oil; LO, linseed oil; OO, olive oil
The percentage of wound closure was determined by calculating the open wound area as a function of time. In the all groups investigated, the percentage of wound closure increased with time (Fig. 1b). On day 3, wound closure was accelerated in the treatment group, with the 5% povidone-iodine-treated group being the highest (23%) compared to the untreated group (15%). Similar results were demonstrated on day 7 as well wherein statistically significant difference was observed in the 5% povidone-iodine group (p < 0.05) reaching wound closure of 53% compared to the untreated group (41%). On day 14 of treatment, the EPO-treated group showed the highest wound closure reaching 69% followed by the 5% povidone-iodine-treated group (67%), the LO-treated group (66%), and the OO-treated group (65%) with significant difference (p < 0.05) in comparison to the untreated (54%). No significant difference in percentage of wound closure was observed among the omega-rich oil-treated groups.
Histopathological changes of wound healing
The granulation of tissues in the LO, EPO, and OO emulsion-treated groups showed marked improvement in comparison to the untreated group (Fig. 2). In the untreated group, a thick layer of necrotic area, edema, and epidermal destruction were seen on day 3 (Fig. 2a). Numerous inflammatory cells particularly lymphocytes infiltrated to the dermis by day 7 (as indicated by the arrow). Large numbers of new blood vessels were formed as well. The lymphocyte cells remained available at the wound area even after the healing progressed to day 14 (Fig. 2b, c). Meanwhile, in the 5% povidone-iodine-treated group, a thick layer of scab was clearly seen and new blood vessels began to form on day 3 (Fig. 2d). Lymphocytic infiltration was present in day 7 that was similar to the untreated group. However, after day 14, the granulation tissue was occupied by collagen fibers indicating the healed wound (Fig. 2f). In comparison to the untreated and 5% povidone-iodine groups, the omega-rich oil-treated groups showed an abundance of cell infiltration especially lymphocytes in the dermis as early as day 3. Among them, the EPO-treated group presented enormous numbers of new microvasculatures (Fig. 2g–m). These results suggest that the inflammatory phase in omega-rich oil-treated groups occurred immediately and was replaced by the proliferative phase as early as day 3. At day 7, these omega-rich oil-treated groups exhibited an accelerated replacement of necrotic area to granulation tissues. A new epithelium layer was also formed. However, in the EPO-treated group, the scab fell off earlier than the other groups and the underlying dermis was abundantly filled by fibroblasts. Epithelialization was the highest in the EPO-treated group (Fig. 2k), whereas the scab was still presented in the LO-treated group and the re-epithelialization was not completed in the OO-treated group.
Photomicrographs showing the histological analysis of wound healing at days 3, 7, and 14 after H&E staining at × 100 magnification (n = 4). In the untreated group, edema was visible at day 3 (a). In the 5% povidone-iodine group, blood vessels begin to form (appear as cherry red) (d) at day 3. High inflammatory cell infiltration (appear as blue color as shown by the arrow) in the LO-, EPO-, and OO-treated groups at day 3 (g, j, m) compared to day 7 for the untreated (b) and 5% povidone-iodine (e). The EPO-treated group also showed the highest fibroblast proliferation at day 7 (appear as purple (k)). Presence of organized collagen (appear as pale pink) in all the 5% povidone-iodine-treated and omega-rich oil-treated groups at day 14 (f, i, l, o). Abbreviations: BV, blood vessel; Co, collagen: Ep, epidermis; EPO, evening primrose oil; Fb, fibroblast; GT, granulation tissue; IC, inflammatory cells; LO, linseed oil; Oe, edema; OO, olive oil
By day 14, all of the treatment groups including the 5% povidone-iodine group showed complete re-epithelialization. There was an increase in keratin formation and more organized collagen deposition in the 5% povidone-iodine-treated as well as the omega-rich oil-treated groups in contrast to the numerous scattered inflammatory cells and relatively disorganized collagen fibers in the untreated group reflecting to the active fibroproliferative phase. As the wound healed, the blood vessels also will disappear as seen in the 5% povidone-iodine- and EPO-treated groups. Meanwhile, blood vessels were still present in the LO- and OO-treated groups. Thus, from the histological analysis findings, it can be seen that the treatment groups speed up the healing process as compared to the untreated group, with the highest acceleration occurring in the EPO-treated group.
Masson trichrome staining
The detection of collagen formation was done by histological examination using Masson trichrome staining (Fig. 3). Increased collagen fibers were observed in all groups over time. At day 3, the epidermis at the wound site was destroyed in all the groups. The underlying dermis especially in the untreated group showed necrosis and was occupied by many inflammatory cells (appear as red color, Fig. 3a). In contrast, a slight deposition of very fine collagen fibers (blue coloration) was observed in the 5% povidone-iodine-treated and omega-rich oil-treated groups (Fig. 3d–m). Among these treatment groups, collagen was highly expressed in the EPO-treated group on day 3 and more prominent on day 7 (Fig. 3k). Intense blue coloration of collagen in the EPO group indicated that the EPO-treated group was favored by fibroblasts to synthesize the collagen fibers. The LO-treated group demonstrated higher collagen deposition as well on day 7 (Fig. 3h). At day 14, collagen synthesis by fibroblast still actively occurred in the untreated group, indicating that the collagen deposition rate was slower in this group (Fig. 3c). In contrast, complete restructuring of collagen was significantly seen on day 14 of postwounding in all treated groups. At this time point, the blue color of collagen was intense, and collagen deposition in the dermis was more organized and compact in the treated as well as positive control groups (Fig. 3f, i, l, o).
Level of VEGF expression
The angiogenesis process is closely associated with wound healing and was evaluated using the expression level of the VEGF. The scores of positive immunostained VEGF expression were tabulated in Table 2, while its intensity is shown in Fig. 4. As new blood vessels were formed, the intensity of the endothelial cells lining the blood vessels becomes strong and its ability to take up the staining was reduced as the wound healed; thus, its intensity was weak. The score was determined by calculating positive immunostained new blood vessels over the total number of blood vessels. On day 3, the treatment groups comprised of the 5% povidone-iodine-treated and omega-rich oil-treated groups demonstrated marked expression with significant difference (p < 0.05) in the EPO-treated group (71.9%) compared to the untreated (42.6%). Then, when the healing reached day 7, the level of VEGF expression was gradually decreased in the treatment groups of 5% povidone-iodine (28.7%), LO (27.1%), and OO (29.6%) and was significantly different (p < 0.05) to the untreated group (54.4%) which showed an increased level of VEGF. The VEGF expression then declined as the wound healed on day 14. No significant difference was observed between the 5% povidone-iodine-treated and omega-rich oil-treated groups or within the omega-rich oil-treated groups.
a–o Photomicrograph from immunostained slides with VEGF antibody in the untreated and treated groups on days 3, 7, and 14 of samples at × 100 magnification (n = 4). The arrow shows a positive VEGF immunostaining in the endothelial cells lining the blood vessels. Abbreviations: EPO, evening primrose oil; LO, linseed oil; OO, olive oil
Level of collagen I expression
Immunohistochemistry images of collagen-1 obtained from the untreated and treated samples with oil emulsions as well as 5% povidone-iodine as shown in Fig. 5 were in agreement with the Masson trichrome staining results. Weak immunostained areas of collagen-1 were observed in the untreated group at days 3 and 7 (Fig. 5a, b) and similarly in the 5% povidone-iodine-treated group (Fig. 5d, e). It reflects the low synthesis of collagen in both of these groups in the first 7 days after injury. The intensity tended to moderate when the healing approached day 14 (Fig. 5c, f). In contrast to the untreated and 5% povidone-iodine, prominent expression of collagen-1 in the LO-, EPO-, and OO-treated groups was observed as early as day 3 (Fig. 5g–m) and the intensity was increased with time. This indicated that the omega-rich oils contributed to the healing process by increasing collagen synthesis to replace the damaged collagen in injured tissue. By day 14, an intense staining was clearly seen in the LO- and EPO-treated groups as compared to others (Fig. 5i, l, as indicated by the arrow).
a–o Photomicrograph from immunostained slides with collagen-1 antibody in the untreated and treated groups on days 3, 7, and 14 of samples at × 100 magnification (n = 4). The brown color shows positive collagen-1 immunostaining. Abbreviations: Co, collagen-1; EPO, evening primrose oil; LO, linseed oil; OO, olive oil
Hydroxyproline content
The hydroxyproline contents of wound healing on different days are presented in Fig. 6. Collagen breakdown resulted in the liberation of free hydroxyproline which can be a good index for measuring collagen turnover in the granulation tissue [20]. Among all of the groups, the untreated group had the lowest amount of hydroxyproline (0.03 ± 0.01 μg/μL) in comparison to the 5% povidone-iodine group which showed the highest amount of hydroxyproline (0.11 ± 0.01 μg/μL) followed by the EPO group (0.09 ± 0.01 μg/μL) on day 3. Similarly, on day 7, the hydroxyproline level in both of the 5% povidone-iodine and EPO groups continued to increase to 0.14 ± 0.01 and 0.12 ± 0.01 μg/μL, respectively, and was significantly different (p < 0.05) compared to the untreated (0.06 ± 0.01 μg/μL). Meanwhile, the hydroxyproline content in the OO-treated group increased gradually (0.08 ± 0.01 μg/μL) and was statistically different in comparison to the 5% povidone-iodine group (p < 0.05) on the same indicated day. The hydroxyproline level reached the peak of 0.16 ± 0.01 μg/μL in the 5% povidone-iodine group at day 14. The production of hydroxyproline also was elevated in LO (0.11 ± 0.01 μg/μL), EPO (0.13 ± 0.01 μg/μL), and OO (0.12 ± 0.01 μg/μL) but were not significantly different compared to the untreated (0.09 ± 0.01 μg/μL). There was no significant difference between the 5% povidone-iodine and omega-rich oil emulsion and among the oils as well on the predetermined time point.
Quantitative data concerning the hydroxyproline content (μg/μL) at different time points after treatment. ap < 0.05 vs. the untreated group, bp < 0.05 vs. 5% povidone-iodine on the indicated day. Data are expressed as mean ± SEM (n = 4). Abbreviations: EPO, evening primrose oil; LO, linseed oil; OO, olive oil
Level of cytokine concentrations in wound tissue
Figure 7 summarizes the cytokine and growth factor concentrations of rats left untreated and receiving 5% povidone-iodine, LO, EPO, and OO emulsions at 3, 7, and 14 days of posttreatment. The omega-rich oil-treated groups altered proinflammatory cytokines in a time-dependent manner. At day 3, a significant increase of IL-1β (p < 0.05) was observed in all of the three omega-rich oil groups compared to the untreated group with maximum expression in the EPO group. Although the 5% povidone-iodine group had a slight increase compared to the untreated, it was not significant. Then, the IL-1β level rapidly declined in all of the treatment groups but rose up in the untreated group. By day 14, both of the untreated and treated groups demonstrated very low IL-1β expression which dropped down almost to zero (Fig. 7a).
Quantitative data concerning the concentration of cytokines and growth factor expression (pg/mL) at different time points (3, 7, and 14 days) after treatment. a IL-1β. b IL-6. c TNF-α. d MCP-1. e EGF. f VEGF. ap < 0.05 vs. the untreated group, bp < 0.05 vs. 5% povidone-iodine, cp < 0.05 vs. LO, dp < 0.05 vs. EPO on the indicated day. Data are expressed as mean ± SEM (n = 4). Abbreviations: EPO, evening primrose oil; LO, linseed oil; OO, olive oil
Figure 7b shows significant elevation of IL-6 expression in the treatment groups within the first 3 days (p < 0.05) compared to the untreated, and the greatest concentration of IL-6 was found in the EPO group which was significantly different to the 5% povidone-iodine as well (p < 0.05). Surprisingly, the expression of this cytokine was not affected in the OO-treated group, and there was a significant difference (p < 0.05) when compared to the 5% povidone-iodine, LO, and EPO groups. Similar to IL-1β, the untreated group had a favorable effect to release IL-6 cytokines by day 7, whereas the concentration of IL-6 declined steadily in both the 5% povidone-iodine and EPO groups while speedily downregulated in the LO-treated group (p < 0.05). Enormous depletion of IL-6 concentration by day 14 was observed in all of the groups.
Figure 7c shows the TNF-α expression level in the untreated and treated groups over time of healing. A significant rise of TNF-α was promoted by the 5% povidone-iodine as well as EPO groups (p < 0.05), although it remained low in the other groups on day 3. Then, as healing progressed to day 7, a marked increase of TNF-α was exhibited in the untreated group, whereas it suddenly diminished in the treatment groups except in the EPO group (p < 0.05).
On day 3, only the 5% povidone-iodine group showed a significant increment of MCP-1 (p < 0.05) as compared to the untreated. Although increased, MCP-1 expression was insignificant in the LO-treated group. Unexpectedly, the EPO group did not affect MCP-1 production and release as indicated by a low level of MCP-1 in this group and significant difference (p < 0.05) compared to the 5% povidone-iodine and LO groups. By day 7, the MCP-1 concentrations were significantly reduced (p < 0.05) in the treatment groups, while its expression was highest in the untreated group (Fig. 7d).
The concentration of EGF was measured in the tissue homogenate of the untreated and treated rats on days 3, 7, and 14 as well (Fig. 7e). The rats receiving treatment of EPO and OO emulsions expressed EGF in the greatest amount at day 3 and were statistically significant compared to the untreated, 5% povidone-iodine, and LO groups (p < 0.05). The EGF levels then dropped by days 7 and 14. The level of EGF in the untreated group was low in the early and mid-stages of healing, which then increased at the end of 14 days of treatment.
VEGF plays a vital role in the process of new blood vessel formation in wound healing. Thus, its expression level was also determined and the result is shown in Fig. 7f. The highest peak of VEGF expression was found in the EPO-treated group, which was statistically significant compared to the untreated, 5% povidone-iodine, and LO groups (p < 0.05) on day 3. Similarly, the 5% povidone and OO-treated groups had high VEGF concentrations, and these were significantly different compared to the untreated group (p < 0.05). By day 7, there was a gradual diminishing of VEGF concentration in the EPO group in contrast to the significantly more rapid reduction in the 5% povidone-iodine and LO groups (p < 0.05). The OO group was also significantly different compared to the 5% povidone-iodine group (p < 0.05).
Discussion
It has been documented that fatty acids particularly α-linolenic, linoleic, and oleic acids exert beneficial health effects by modulating the signaling pathways regulating inflammatory response, cell differentiation, and proliferation. In this study, we determined the effects of unsaturated fatty acids of n-3, n-6, and n-9 fatty acids on wound healing in the form of oil. The vegetable oils were chosen over animal oils for the present study based on published reports that vegetable oils such as linseed oil [21,22,23,24] and olive oil [25,26,27,28,29] showed wound-healing improvement rather than fish oil [30]. Thus, linseed, evening primrose, and olive oils were used as a base and active ingredient in the formulation development.
Selection of an appropriate composition of oil phase and surfactant is crucial when designing a formulation. Surfactant or a blend of surfactants is required to reduce surface tension and to stabilize droplets against recoalescence [31]. From our earlier screening, a Box-Behnken design using response surface methodology was used in order to obtain an optimal formulation. A total of 17 different combinations of oil, surfactant, and co-surfactant compositions generated from the Design-Expert software were carried out, and the formulations that yield a mean particle size below 500 nm, PDI value less than 0.4, and stable formulations for over 7 days were selected for the final formulation as shown in Table 1. The fatty acid components in oils were also investigated through gas chromatography with flame ionization detector (GC-FID) to determine the fatty acid contents. Our findings revealed that LO contained predominantly 53% of α-linolenic acid, 21% of oleic acid, and 16% of linoleic acid, whereas EPO was rich in n-6 fatty acid of linoleic acid (75%) and OO had 81% oleic acid content (data not shown). Numerous studies have shown that the fatty acids can modify the production and activity of various components of the immune system by altering the membrane fluidity [32], lipid peroxidation [33], prostaglandin production [34], and regulation of gene expression [35]. Thus, manipulating the availability of these fatty acids either through dietary or topical application shifts the fatty acid compositions and influences the local production of eicosanoid, thereby noninvasively assisting in wound healing.
In this study, we analyzed the process of cutaneous wound healing by several parameters such as wound closure, histological features, and protein expression levels as summarized in Table 3.
Collectively, our findings showed that the topical application of 5% povidone-iodine and omega-rich oils of LO, EPO, and OO in emulsion accelerated wound healing in comparison to the untreated. Wound closure occurs as a result of a combination of wound contraction and re-epithelialization that reflects the advancement of the epithelium over the granulation tissue [6]. According to statistical analysis, a significant increase (p < 0.05) of wound closure was observed in the 5% povidone-iodine group at day 7 compared to the untreated, but this was insignificant when compared to the omega-rich oil groups. All wounds are colonized by microorganisms. The presence of some microorganisms may help in healing, and conversely, contamination with pathogenic microorganisms can cause infection and sepsis resulting in prolonged inflammation together with defective remodeling of the extracellular matrix and, subsequently, failure of re-epithelialization [36]. Microbial colonies may produce biofilm that will reduce the metabolic state and enhance resistance to antibiotics and evasion of host defense mechanism [36]. Application of povidone-iodine in wound management as antiseptic can prevent and combat infection through its microbicidal properties. The bactericidal activity of iodine even at low concentration (approximately one part per million, 1 ppm) is able to penetrate and attack nucleotides fatty/amino acids in bacterial cell membranes and cytosolic enzymes involved in the respiratory chain causing them to become denatured and deactivated [37]. Povidone-iodine may exert antimicrobial activity and prevent formation of biofilm during the crucial stage of inflammation, which accelerated the healing process in the present study. The findings were in accordance with a previous study done by Wang et al. [38] assessing the effects of 0.5% povidone-iodine on acute skin wounds. They demonstrated that topical application of 0.5% povidone-iodine promotes acute skin wound healing through increased expression of transforming growth factor-β (TGF-β) leading to enhanced formation of granulation tissue, neovascularization, phenotypic switching of fibroblast to myofibroblast, and re-epithelialization. Similarly, 5% povidone-iodine cream has been shown to be effective and well tolerated in an uncontrolled pediatrical trial [39] and was superior to silver sulfadiazine in terms of application and wound healing [40].
Topical application of omega-rich oils provided a better advancement in wound closure than the untreated. The LO-treated group had faster wound closure that was statistically significant at day 14. Our finding was consistent with a previous study that showed that the rate of wound contraction was significantly higher and shortened healing period on burn wounds in rabbits treated with linseed oil [22] compared to untreated, Vaseline gel, or Cicatryl-Bio ointment. The biological effects of LO on fibroblast cells in vitro were tested by Lewinska et al. [23], and they demonstrated that this oil ameliorated the process of wound healing by enhanced migration of fibroblasts to the wounding area. One of the limitations of the present study is that we did not perform a phytochemical analysis to determine the bioactive components in each of these omega-rich oils. However, several studies have reported that abundant bioactive terpenoids in LO may promote wound healing through its astringent activity which is responsible for wound contraction and increased epithelialization rate. Flavonoids found in LO stimulate fibroblast cell proliferation and differentiation into specialized myofibroblast in the granulated tissue that was consistent with our results as shown in Fig. 2h. Moreover, flavonoids play a role in reducing lipid peroxidation by preventing the onset of cell necrosis and inducing angiogenesis. The presence of tocopherols, β-carotene, and phenolic compound prevents and protects the cell against oxidative damage from free radicals. In addition, LO also contains high amounts of calcium that may regulate the inflammatory cell infiltration [22]. Furthermore, our fatty acid composition in LO revealed the presence of a high amount of polyunsaturated fatty acids mainly ALA and OA which was similarly found in a study by Bardaa et al. [21]. ALA and OA exert inflammatory activities and stimulated recruitment of inflammatory cells at the wound site. Thus, all of these bioactive components that exist in LO could explain how it accelerated the wound-healing process.
Similar to LO, rats treated topically with EPO emulsion had faster wound closure by day 14. EPO is rich with LA. Rodrigues et al. [5] reported that an unesterified linoleic acid demonstrated faster wound closure, specifically significant on day 7 of postwounding in comparison to the untreated. In addition, Park et al. [41] also showed that supplementation of 1% conjugated linoleic acid in mice feed modulates the oxidative stress by reducing the reactive oxygen species level and downregulating the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in inflammatory response. Apart from LA, the chemical composition of the EPO herbs mainly contains macrocyclic ellagitannin oenothein B. This component isolated from the aerial part of EPO herbs exhibited strong inhibition against reactive oxygen species production in human neutrophil cells. Moreover, macrocyclic ellagitannin oenothein B also was reported as a strong hyaluronidase inhibitor. Hyaluronidase is an enzyme associated with the degradation of hyaluronan, and by inhibiting the hyaluronidase, EPO increases the integrity of tissue during the progression of inflammation [42]. Thus, EPO contributed to the speeding up of wound healing by promoting the initial proinflammatory phase due to the effect of fatty acids and then impeding a prolonged inflammation phase due to the antioxidative properties of the bioactive components inside the oil.
Wound closure occurred faster in the OO-treated group by day 14 as well. Similar results were found in full-thickness burn wounds of rats. Rats treated topically with olive or sea buckthorn/olive oil mixture revealed increased re-epithelialization with continuous basement membrane and mature granulation tissue [26]. Clinical studies evaluating the effect of olive oil taken orally on thermally injured patients with hospital stays showed that oral administration of olive oil accelerates wound healing and decreases the duration of hospitalization [28]. Its richness with almost 80% of MUFA oleic acid has anti-inflammatory effects that could be responsible for modulating wound inflammation and accelerate its healing. Olive oil also contains antioxidant components such as hydrocarbons, polyphenols, tocopherols, sterols, and triterpenoids [43, 44]. Oleuropein and hydroxytyrosol are the most prevalent compounds in high phenolic olive oil that can offset the deleterious effects of extreme inflammatory responses by inhibiting the respiratory burst of neutrophils, reducing oxidation load to surrounding keratinocytes, and promoting connective tissue formation. Both of these components also reduce the level of acute inflammation by obstructing the production of IL-1β and TNF-α [45]. Excessive reactive oxygen species production hampers wound healing; thus, hydrophilic phenols that are the most abundant antioxidants of olive oil that have scavenging abilities contribute to its therapeutic effects [46].
To understand the molecular mechanisms through which the topical application of omega-rich oils modulates wound closure, we investigated the expression of several cytokines. IL-1β, IL-6, and TNF-α are representatives of proinflammatory cytokines. Our data have shown that the level of IL-1β was upregulated at day 3 with high expression (p < 0.05) demonstrated in the EPO group compared to the untreated and the 5% povidone-iodine groups. The IL-1β level then declined when the healing approached day 7, whereas it was increased in the untreated group. IL-1β is produced by neutrophils, monocytes, macrophages, and keratinocytes, and it will be released immediately upon wounding to activate the endothelial cells [47]. Oleic and linoleic acids were shown to increase the secretion of IL-1β by neutrophils [48]. In the expression of adhesion molecules, the leukocytes integrins and endothelials selectins contribute to the accumulation of phagocytes in the inflamed site [49].
Much like IL-1β, IL-6 was expressed (p < 0.05) in the 5% povidone-iodine-, LO-, and EPO-treated groups at day 3 and was downregulated by day 7. Yoshida et al. [50] had reported that both oleic and linoleic acids could significantly increase the secretion of IL-6 in rat intestinal epithelial cells (IEC); however, in our study, no changes of IL-6 level in the OO-treated group were seen at day 3 and its level was increased slightly on day 7. The different effects of these fatty acids on the expression of specific mediators appear to be dependent on cell type. Increased expression of IL-6 after wounding is important for keratinocyte proliferation and neutrophil chemoattractant [51]. The migration of neutrophils during the early phase of wound healing is to engulf and clear the pathogens entrapped in the clot at the time of injury [52]. Gallucci et al. [53] in their study done on IL-6 knockout model to elucidate the importance of IL-6 on wound healing showed that the IL-6 knockout animal took up to three times longer to heal than the control. This indicated that a lack of IL-6 expression caused delayed granulation tissue formation and re-epithelialization. Thus, IL-6 presence is crucial to initiate the healing response, both via mitogenic effects on wound edge keratinocytes and chemoattractive effect on neutrophils.
Similarly, TNF-α elevates their expression during the inflammatory phase of wound healing, stimulated by epidermal barrier disruption to alert the surrounding cells. TNF-α induces the production of fibroblast growth factor which indirectly promotes re-epithelialization [51]. The present study has shown that TNF-α expression was statistically increased in the 5% povidone-iodine and EPO groups but insignificantly in the LO group. Then, the TNF-α level was decreased by day 7 and onwards. Bigliardi et al. [36] have stated that povidone-iodine not only has broad spectrum antibacterial effects, but also counteracts inflammation elicited by host response. Povidone-iodine enhances healing signals from proinflammatory cytokines by activating monocytes, T lymphocytes, and macrophages and has inhibitory effect on human inflammatory effector cells and mediates inflammation such as TNF-α and β-galactosidase. This might explain the increased level of proinflammatory cytokines during the early phase of healing which then diminished later in the povidone-iodine-treated group. The low level of TNF-α in the LO and OO groups could be due to the anti-inflammatory effects of n-3 ALA and n-9 OA. Simopoulos [54] stated that both omega-3 and omega-6 can influence gene expression. A study done by Verlengia et al. [55] found decreasing immunoregulatory cytokine production of IL-10, TNF-α, and INF-γ in the presence of 12.5 μM of EPA and DHA. Other studies have shown that TNF-α or IL-6 production was increased or not modified in mice, suggesting that diets containing olive oil suppressed cytokine production [56, 57]. Transient release of TNF-α in the early stage promoted wound healing by indirectly stimulating and increasing macrophage-produced growth factors which was well correlated with our histological analysis that showed high amounts of neutrophil influx at the wounded area by the third day of postwounding in the omega-rich oil groups particularly in the EPO-treated group. Thus, TNF-α is influenced by n-6 rather than n-3 and n-9 rich oils. High levels of TNF-α expression, as we can see in the untreated group at day 7 postwounding, could have delayed the wound healing.
Other than proinflammatory cytokines, chemokines also participate in the wound-healing process. Chemokines stimulate the inflammatory cell migration and regulate re-epithelialization, tissue remodeling, and angiogenesis [58]. Thus, one of the CC families of chemokines which is MCP-1 expression level was determined. A sustained high level of MCP-1 resulted from prolonged existence of neutrophils and macrophages in the wound site can contribute to an excessive inflammatory response, leading to chronic wounds. On the other hand, the lack of MCP-1 significantly delays wound healing, particularly with re-epithelialization, angiogenesis, and collagen synthesis as seen in mouse models [59]. In the present study, high levels of MCP-1 were observed in the LO and OO groups but were insignificant compared to the untreated though no changes were seen in the EPO group at day 3. The MCP-1 levels then diminished by day 7, suggesting that LO and OO were affecting the expression of MCP-1 rather than EPO. MCP-1 is induced by keratinocytes upon wounding and acts as a chemoattractant for monocytes/macrophages, T cells, and mast cells [51]. The presence of MCP-1 coincides with maximum cell infiltration in the LO group in the early phase of wounding observed by histological studies, suggesting that they may mediate the recruitment of inflammatory cells. In contrast, the level of MCP-1 in the untreated group remained high at day 7, which correlated well with many inflammatory cells appearing on day 7 and onwards. Upregulated expression of IL-1β, IL-6, and TNF-α in the inflammation phase and diminishing in the resolution phase hastens wound closure in the omega-rich oil groups.
Recruitment of inflammatory cells in response to proinflammatory cytokines will release growth factors such as EGF. This EGF is also secreted by platelets. High expression of EGF gives a positive effect on wound repair, and this is in agreement with our data which exhibited significantly increased (p < 0.05) EGF levels in the EPO and OO groups at day 3 and reduced by day 7, whereas no changes were seen in the LO group. EGF upregulation after injury significantly accelerated re-epithelialization and increased tensile strength which has been demonstrated in an in vitro study to be via increased expression of keratin K6 and K16 mechanism [60], and the subsequent EGF ligand shedding afterward is essential for keratinocyte migration in promoting re-epithelialization [61]. However, high expression of EGF at the later phase of wounding slows down the repair process as seen in the untreated group of our study at day 14.
A hypoxic condition is a stimulus for angiogenesis. In this study, the angiogenesis process was assessed via immunohistochemistry and ELISA through an angiogenic marker, VEGF. VEGF is an angiogenic peptide produced by endothelial cells, macrophages, fibroblast, smooth muscle cells, and keratinocytes to promote early events of angiogenesis, particularly endothelial cell migration and proliferation [19, 51]. Galiano et al. [62] showed that exogenous supplementation of 20 μg of VEGF every day in wounded diabetic mice produced increased epithelialization, increased matrix deposition, and enhanced cellular proliferation that accelerated repair. Similar observations were demonstrated in our study where topical application of EPO significantly increased (p < 0.05) the expression of VEGF at day 3 that was reduced by day 7 compared to the untreated, 5% povidone-iodine, and LO groups. This result suggests that EPO promotes angiogenesis which later contributes to faster healing. The OO treatment group also showed similar observations. Oleuropein is a component in OO that has been shown to increase VEGF expression during in vivo wound-healing studies; thus, olive phenols may serve as suitable proangiogenic compounds associated with wound healing [45]. In contrast, VEGF production was not influenced by topical application of LO.
Activation and increased expression of proinflammatory cytokines, chemokines, and growth factors indirectly stimulate cells such as fibroblast to migrate, differentiate, and secrete collagen at the wound site. Collagen is secreted by fibroblast and it is required to support and strengthen the extracellular matrix of a wound. Hydroxyproline is a major component of collagen and was used as a biochemical marker for determination of collagen content. Type 1 collagen is essential during the maturation phase and is prevalent in healed wounds, while type III collagen is needed for creating the provisional matrix during the proliferative phase. Topical application of LO, EPO, and OO altered the deposition of collagen as detected through Masson trichrome staining where the EPO-treated group demonstrated a high amount of collagen fiber deposition by as early as day 3 and prominent in day 7 onwards. This finding correlated well with the hydroxyproline content and EGF and VEGF expression. Significantly greater expression of these mediators corresponds to the rapid new cell formation and collagen turnover. Previous studies showed that linoleic acid increased the release of cytokines and stimulated the proliferation and differentiation of endothelial cells, keratinocytes, and fibroblast, which all accelerate the production of collagen and subsequently promote the repair process [63, 64]. The high amount of collagen formation that was observed in the LO group corroborated with a previous study that showed an increase in collagen deposition in animals supplemented with n-3 PUFA of linseed and fish oils [65]. PUFA together with a high amount of flavonoids present in LO alters collagen formation by stimulating fibroblast cell proliferation, promoting the viability of collagen fibrils, and strengthening the collagen fibers. A number of amino acids such as glutamine, valine, leucine, and arginine present in LO may contribute to restoring dermal collagen protein synthesis and promote wound healing [22].
Conclusion
Topical application of 5% povidone-iodine has antimicrobial properties to prevent contamination of microbial infection in the wound site. As the wound area is clean from any pathogenic microbials, the immune system has a proper healing process in an orderly manner. LO was found to induce the expression of proinflammatory cytokines and chemoattractant for monocytes/macrophages, subsequently increasing inflammatory cell infiltration. The early release of these proinflammatory cytokines during the inflammation phase and reduction at the next phase together with increased cell infiltration and collagen deposition could explain the faster healing in wounded rats treated with LO emulsion. Meanwhile, the topical application of EPO initially increased the proinflammatory cytokines and growth factors to actively increase cell migration, proliferation, angiogenesis, and collagen deposition and finally accelerated wound closure. In contrast, topical application with OO emulsion increased the EGF and VEGF levels rather than proinflammatory cytokines to initiate the response upon wounding. Compared to the 5% povidone-iodine and omega-rich oil groups, the EPO emulsion was found to be superior in promoting wound healing.
References
Lai HY, Lim YY, Kim KH. Potential dermal wound healing agent in Blechnum orientale Linn. BMC Complement Altern Med. 2011;11(1):62.
Dhiyaaldeen SM, Alshawsh M a, Salama SM, Alwajeeh NSI, Batran R Al, Ismail S, et al. Potential activity of 3-(2-chlorophenyl)-1-phenyl-propenonein accelerating wound healing in rats. Biomed Res Int. 2014;10 pages.
Neves J, Ract R, Andreia F, De MS, Gomes H, Bortolon JR, et al. Production of vegetable oil blends and structured lipids and their effect on wound healing. Brazilian J Pharm Sci. 2015;51(2):415–27.
Ferreira AM, de Souza BMV, Rigotti MA. The use of fatty acids in wound care: an integrative review of the Brazilian literature. Rev Esc Enferm USP. 2012;46(3):745–53.
Rodrigues HG, Vinolo MA, Magdalon J, Vitzel K, Nachbar RT, Pessoa AF, et al. Oral administration of oleic or linoleic acid accelerates the inflammatory phase of wound healing. J Invest Dermatol. 2012;132:208–15.
Cardoso CRB, Souza MA, Ferro EAV, Favoreto S, Pena JDO. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12(2):235–43.
Berdick M. The role of fats and oils in cosmetics. J Am Oil Chem Soc. 1972;49(7):406–8.
Yara-Varón E, Li Y, Balcells M, Canela-Garayoa R, Fabiano-Tixier A-S, Chemat F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules. 2017;22(9)
King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26(17):4672–80.
Jafari HRN, Taghavi MM, Shariati M, Vazeirnejad R, Rezvani ME. Both omega-3 and omega-6 polyunsaturated fatty acids stimulate foot wound healing in chronic diabetic rat. African J Pharm Pharmacol. 2011;5(14):1713–7.
Goyal A, Sharma V, Upadhyay N, Singh a K, Arora S, Lal D, et al. Development of stable flaxseed oil emulsions as a potential delivery system of ω-3 fatty acids. J Food Sci Technol. 2015;52(7):4256–65.
Waraho T, McClements DJ, EA D. Impact of free fatty acid concentration and structure on lipid oxidation in oil-in-water emulsions. Food Chem. 2011;129(3):854–9.
Zielińska A, Nowak I. Fatty acids in vegetable oils and their importance in cosmetic industry. Chem Aust. 2014;68(2):103–10.
Ruthig DJ, Meckling-Gill KA. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6. J Nutr. 1999;129(10):1791–8.
Franco EDS, Maria C, de Aquino F, de Medeiros PL, Evêncio LB, Bernadete M, et al. Effect of a semisolid formulation of Linum usitatissimum L. (linseed) oil on the repair of skin wounds. Evidence-based Complement Altern Med. 2012;7 pages.
McDaniel J, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16(3):337–45.
Nettleton JA. Omega-3 fatty acids: comparison of plant and seafood sources in human nutrition. J Am Diet Assoc. 1991;91(3):331–7.
Koca Kutlu A, Ceçen D, Gürgen SG, Sayın O, Cetin F. A comparison study of growth factor expression following treatment with transcutaneous electrical nerve stimulation, saline solution, povidone-iodine, and lavender oil in wounds healing. Evid Based Complement Alternat Med. 2013;9 pages.
Perini JA, Angeli-Gamba T, Alessandra-Perini J, Ferreira LC, Nasciutti LE, Machado DE. Topical application of Acheflan on rat skin injury accelerates wound healing: a histopathological, immunohistochemical and biochemical study. BMC Complement Altern Med. 2015;15(1):203.
Gangwar M, Gautam MK, Ghildiyal S, Nath G, Goel RK. Mallotus philippinensis Muell. Arg fruit glandular hairs extract promotes wound healing on different wound model in rats. BMC Complement Altern Med. 2015;1(123):1–9.
Bardaa S, Moalla D, Ben Khedir S, Rebai T, Sahnoun Z. The evaluation of the healing proprieties of pumpkin and linseed oils on deep second-degree burns in rats. Pharm Biol. 2016;54(4):581–7.
Beroual K, Agabou A, Abdeldjelil M, Boutaghane N, Haouam S, Hamdi-pacha Y, et al. Evaluation of crude flaxseed (Linum usitatissimum L.) oil in burn wound healing in New Zealand rabbits. Afr J Tradit Complement Altern Med. 2017;14(3):280–6.
Lewinska A, Zebrowski J, Duda M, Gorka A, Wnuk M. Fatty acid profile and biological activities of linseed and rapeseed oils. Molecules. 2015;20(12):22872–80.
Farahpour MR, Taghikhani H, Habibi M, Amin M. Wound healing activity of flaxseed Linum usitatissimum L. in rats. African J Pharm Pharmacol. 2011;5(21):2386–9.
Nasiri M, Fayazi S, Jahani S, Yazdanpanah L, Haghighizadeh MH. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14(1):1–10.
Edraki M, Akbarzadeh A, Hosseinzadeh A, Tanideh N, Salehi A, Koohi-Hosseinabadi O. Healing effect of sea buckthorn, olive oil, and their mixture on full-thickness burn wounds. Adv Ski Wound Care. 2014;27(7):317–23.
Sakazaki F, Kataoka H, Okuno T, Ueno H, Semma M, Ichikawa A, et al. Ozonated olive oil enhances the growth of granulation tissue in a mouse model of pressure ulcer. Ozone Sci Eng. 2007;29(6):503–7.
Najmi M, Shariatpanahi ZV, Tolouei M, Amiri Z. Effect of oral olive oil on healing of 10–20% total body surface area burn wounds in hospitalized patients. Burns. 2015;41(3):493–6.
Shamaki BU, Yusuf A, Balla HJ, Halima IG, Sherifat OB, Abdulrahman FI, et al. Evaluation of chemical composition and the comparative wound healing effect of natural honey and olive oil in rabbits. Infin Commun Appl Sci. 2014;2(2):149–69.
Rosa A dos S, Bandeira LG, Monte-Alto-Costa A, Romana-Souza B. Supplementation with olive oil, but not fish oil, improves cutaneous wound healing in stressed mice. Wound Repair Regen. 2014;22(4):537–47.
Mahdi ES, Noor AM, Sakeena MH, Abdullah GZ, Abdulkarim MF, Sattar MA. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int J Nanomedicine. 2011;6:2499–512.
Choi J-H, Yu BP. Brain synaptosomal aging: free radicals and membrane fluidity. Free Radic Biol Med. 1995;18(2):133–9.
Orengo IF, Black HS, Kettler AH, Wolf JE. Influence of dietary menhaden oil upon carcinogenesis and various cutaneous responses to ultraviolet radiation. Photochem Photobiol. 1989;49(1):71–7.
Kinsella JE, Broughton KS, Whelan JW. Dietary unsaturated fatty acids: interactions and possible needs in relation to eicosanoid synthesis. J Nutr Biochem. 1990;1(3):123–41.
Kumagai T, Kawamoto Y, Nakamura Y, Hatayama I, Satoh K, Osawa T, et al. 4-Hydroxy-2-nonenal, the end product of lipid peroxidation, is a specific inducer of cyclooxygenase-2 gene expression. Biochem Biophys Res Commun. 2000;273:437–41.
Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260–8.
Goldenheim PD. An appraisal of povidone-iodine and wound healing. Postgr Med J. 1993;3:97–105.
Wang L, Qin W, Zhou Y, Chen B, Zhao X, Zhao H, et al. Transforming growth factor β plays an important role in enhancing wound healing by topical application of povidone-iodine. Sci Rep. 2017;7(1):1–8.
de Wet PM, Rode H, Matley P, Brown R. A clinical assessment of the pharmacodynamics of 5% povidone iodine cream in burned children. Dermatology. 1997;195:155.
de Kock M, van der Merwe AE, Swarts C. A comparative study of povidone iodine cream and silver sulphadiazine in the to pical treatment of burns. In: Selwyn S (ed), Proceedings of the First Asian/Pacific Congress on Antisepsis, Royal Society of Medicine Services Ltd. 1988.
Park N-Y, Valacchi G, Lim Y. Effect of dietary conjugated linoleic acid supplementation on early inflammatory responses during cutaneous wound healing. Mediat Inflamm. 2010;2010:2–9.
Granica S, Czerwińska ME, Piwowarski JP, Ziaja M, Kiss AK. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J Agric Food Chem. 2013;61(4):801–10.
Perona JS, Cabello-Moruno R, Ruiz-Gutierrez V. The role of virgin olive oil components in the modulation of endothelial function. J Nutr Biochem. 2006;17(7):429–45.
Puertollano MA, Puertollano E, Álvarez de Cienfuegos G, de Pablo MA. Significance of olive oil in the host immune resistance to infection. Br J Nutr. 2007;98(1):54–8.
Ray NB, Lam NT, Luc R, Bonvino NP, Karagiannis TC. Cellular and molecular effects of bioactive phenolic compounds in olives and olive oil. In: AOCS Press. 2015. p. 53–92.
Lin T-K, Zhong L, Santiago J. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19(1):70.
Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203(1–3):93–8.
Smith AN, Muffley LA, Bell AN, Numhom S, Hocking AM. Unsaturated fatty acids induce mesenchymal stem cells to increase secretion of angiogenic mediators. J Cell Physiol. 2012;227(9):3225–33.
Pereira LM, Hatanaka E, Martins EF, Oliveira F, Liberti EA, Farsky SH, et al. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem Funct. 2008;26:197–204.
Yoshida H, Miura S, Kishikawa H, Hirokawa M, Nakamizo H, Nakatsumi RC, et al. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr. 2001;131(11):2943–50.
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601.
Mohamad N, Mohd Amin MCI, Pandey M, Ahmad N, Rajab NF. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: accelerated burn wound healing in an animal model. Carbohydr Polym. 2014;114:312–20.
Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. Fed Am Soc Exp Biol. 2000;14(15):2525–31.
Simopoulos P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60(9):502–7.
Verlengia R, Gorjão R, Kanunfre CC, Bordin S, De Lima TM, Fernandes Martins E, et al. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids. 2004;39(9):857–64.
Yaqoob P, Calder P. Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell Immunol. 1995;163(1):120–8.
de Pablo MA, Ortega E, Gallego AM, Alvarez C, Pancorbo PL, de Cienfuegos GA. The effect of dietary fatty acid manipulation on phagocytic activity and cytokine production by peritoneal cells from Balb/c mice. J Nutr Sci Vitaminol. 1998;44:57–67.
Raja, Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;1(12):2849–68.
Low QEH, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, et al. Wound healing in MIP-1α−/− and MCP-1−/− mice. Am J Pathol. 2001;159(2):457–63.
Jiang CK, Magnaldo T, Ohtsuki M, Freedberg IM, Bernerd F, Blumenberg M. Epidermal growth factor and transforming growth factor alpha specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. In: Proceedings of the National Academy of Sciences of the United States of America. 1993. p. 6786–90.
Ando Y, Jensen PJ. Epidermal growth factor and insulin like growth factor I enhance keratinocyte migration. J Invest Dermatol. 1993;100:633–9.
Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164(6):1935–47.
Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149(2):755–61.
Detmers PA, Lo SK, Olsen-Egbert E, Walz A, Baggiolini M, Cohn ZA, et al. Neutrophils-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171(4):1155–62.
Otranto M, Do Nascimento AP, Monte-Alto-Costa A. Effects of supplementation with different edible oils on cutaneous wound healing. Wound Repair Regen. 2010;18(6):629–36.
Funding
This study was supported by a research grant from the Ministry of Higher Education, Malaysia (ERGS/1/2013/SKK02/UKM/02/3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This manuscript submitted for publication must comply with the current laws of Malaysia.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Ishak, W.M.W., Katas, H., Yuen, N.P. et al. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv. and Transl. Res. 9, 418–433 (2019). https://doi.org/10.1007/s13346-018-0522-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0522-8