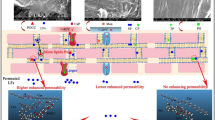
Abstract
Transdermal drug delivery is advantageous over other conventional drug administration routes. However, it can be inefficient because of the natural barrier of the stratum corneum which is the uppermost layer of the skin. A previous study verified that the treatment of magainin pore-forming peptide with N-lauroylsarcosine (NLS) on human skin can increase skin permeability by 47-fold. However, NLS is well known as a potential skin irritant. The irritation potential of NLS is known to decrease when mixed with sorbitan monolaurate (S20). Encouraged by these results, we combined S20 with magainin-NLS to enhance transdermal drug transport with less skin irritation. In this study, nine groups with magainin and NLS:S20 mixtures at different concentrations and weight fractions were screened to maximize their synergistic effect. To quantify the efficacy to toxicity ratio of each formulation, we defined the ratio as the “enhancement ratio/irritation potential (ER/IP).” The ER was observed by Franz cell diffusion of the target drug fluorescein, and the IP was measured by the cytotoxicity of the NIH/3T3 mouse fibroblast cell line. As a result, the magainin with the NLS:S20 mixture increased the permeability of porcine skin as well as decreased the toxicity. Among the various combinations, a formulation of 2% (w/v) NLS:S20 with a weight fraction of 0.6:0.4 had the largest ER/IP. ATR-FTIR spectroscopy of the formulations and skin was done to analyze the interactions in the formulations themselves and between the formulations and the skin. Both the intercellular lipidic route and transcellular route through the stratum corneum protein were involved in the delivery of fluorescein. This study turned pore-forming peptides into an efficient and safe penetration enhancer by combining them with other chemical penetration enhancers. Moreover, this discovery could be a possible method for enabling the transdermal delivery of macromolecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transdermal drug delivery offers numerous advantages over other drug administration routes [1]. It is painless and can be terminated rapidly by removal of the application and therefore offers better compatibility [2]. However, transdermal delivery still possesses some limitations due to the natural barrier function of the stratum corneum (SC) which is the uppermost layer of the skin. It forms a highly organized brick-and-mortar structure composed of corneocytes surrounded by a lipid matrix to protect the inner organs of the human body from foreign substances such as toxins or pathogens [3, 4]. Various methods have been developed to overcome this SC barrier using either physical or biochemical method.
Chemical penetration enhancers (CPEs) have been gaining interest among the chemical methods due to its direct effect on the SC barrier and simple application. A wide variety of CPEs have been discovered including azone derivatives, fatty acids, alcohols, esters, sulfoxides, pyrrolidones, glycols, surfactants, and terpenes. Several studies have been conducted so far to increase the permeation of the skin using chemical enhancers either alone or mixed together for their synergistic effects [5]. In addition to CPEs, skin permeability enhancement strategies using peptides have been investigated in recent years [6,7,8]. Skin-penetrating peptides (SPPs) including SPACE, DLP, or polyarginine have been shown to enhance the penetration of the hydrophobic macromolecule cyclosporine A [9, 10]. Previous studies showed that magainin pore-forming peptide also has the ability to increase the skin penetration of the fluorescein molecule 47-fold in the presence of N-lauroylsarcosine (NLS) and ethanol [11, 12].
Magainin is an antimicrobial peptide isolated from the skin of a frog, Xenopus laevis, and its amphipathic alpha helical structure has a critical role in its pore-forming activity [13, 14]. It is the only naturally occurring antimicrobial peptide which promotes skin permeation [12]. Because of its wide antimicrobial activity and low toxicity in mammals, it has great potential as a penetration enhancer [15, 16]. However, magainin can enhance drug permeation through the skin only when applied together with the anionic surfactant NLS dissolved in a 50% (v/v) phosphate buffered saline-ethanol (PBS-EtOH) solution [11]. NLS has been reported to be a skin irritant [17] which produces superoxide anions that can disrupt skin keratinocytes followed by skin erythema with a score of 2 [18, 19]. Because the ultimate utility of CPEs is strongly related to both their effectiveness and safety, the toxicity of NLS is a serious limitation of the magainin and NLS mixture.
In this research, we combined S20 with NLS and magainin to not only enhance transdermal transport but also prevent skin irritation. According to previous research [20], the binary mixture of NLS and the non-ionic surfactant sorbitan monolaurate (S20) can synergistically enhance permeability with reduced irritancy when compared to that of individual enhancers. Especially, NLS:S20 mixtures with a total concentration of 1% (w/v) and a weight ratio of 0.6:0.4 and 1.5% (w/v) with a weight ratio of 0.3:0.7 had the best synergistic interaction. Based on the compositions above and a report that 2% (w/v) NLS exhibits the maximum enhancement when treated with 1 mM magainin [11], we screened for the enhanced permeability of the target drug fluorescein and skin irritation using nine groups consisting of different concentrations and weight ratios of the NLS:S20 mixture with 1 mM magainin.
Herein, we report that the addition of S20 to magainin-NLS can both improve the permeability and reduce the toxicity on the skin. Additionally, we determined the most efficient composition of the NLS:S20 mixture from among different concentrations and weight ratios as a skin penetration enhancer. The efficiency was quantified by the enhancement ratio (ER) and the irritation potential (IP), while the ER/IP was considered as the parameter of for the efficacy to toxicity ratio. Additionally, as the first study to investigate the synergistic application of a pore-forming peptide with a binary CPE mixture, we did a mechanistic assay with attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) of the formulations and SC to identify the mechanism responsible for increasing the ER/IP value.
Materials and methods
Chemicals
Magainin (GIGKFLHSAKKFGKAFVGEIMNS) was chemically synthesized by Shanghai Top Peptide (Shanghai, China) at a purity of over 90%. Fluorescein, N-lauroylsarcosine (NLS), sorbitan monolaurate (S20), phosphate buffered saline (PBS), ethanol (EtOH), and trypsin were purchased from Sigma Chemicals (St. Louis, MO, USA).
Skin pretreatment
Full-thickness porcine skin (Heart Research Center of Chonnam National University, South Korea) was stored at − 80 °C and thawed at room temperature for 2 h prior to use. The hair was clipped first with a hair trimmer and then with a razor, and the skin was cut into 2 cm × 2 cm samples. The samples were washed by PBS and dried for about half an hour. Each skin sample was mounted on the Franz diffusion cell (FDC, Labfine, South Korea) between the donor and receiver chambers. Nine groups of formulations were prepared by dissolving 1 mM magainin and NLS with or without S20 in 50% (v/v) PBS-EtOH solution with different concentrations and weight ratios: (A) 1% (w/v) NLS; (B) 1% (w/v) NLS:S20, weight fraction of NLS, 0.6; (C) 1% (w/v) NLS:S20, weight fraction of NLS, 0.3; (D) 1.5% (w/v) NLS; (E) 1.5% (w/v) NLS:S20, weight fraction of NLS, 0.6; (F) 1.5% (w/v) NLS:S20, weight fraction of NLS, 0.3; (G) 2% (w/v) NLS; (H) 2% (w/v) NLS:S20, weight fraction of NLS, 0.6; and (I) 2% (w/v) NLS:S20, weight fraction of NLS, 0.3 (Table 1). Skin samples were pretreated with the formulations filling the donor chamber of the FDC with a total volume of 0.3 ml. The control group was pretreated with 50% (v/v) PBS-EtOH solution. The receiver chamber was filled with PBS solution through the sampling port. The pretreatment procedure was done for 12 h at 4 °C to prevent degradation of the skin samples.
Fluorescein penetration
After the 12-h skin pretreatment, FDCs were kept at 37 °C for an additional 3 h to re-equilibrate them. Then, both the donor chamber and receiver chamber were emptied. The receiver chamber was filled with fresh PBS, and the donor chamber was filled with 0.3 ml of 0.5 mM fluorescein (Sigma Aldrich, USA) dissolved in PBS. The receiver chamber was sampled every 3 h for 12 h by emptying and re-filling with fresh PBS. The fluorescence intensity of the target molecule was measured with a spectrofluorophotometer (Dong-il Shimadzu, South Korea) at a wavelength of 480 nm. The enhancement ratio (ER) was quantified by the cumulative fluorescence intensity of the skin sample pretreated with each formulation divided by that of the control. We defined the ER of each sample as follows:
Cell cytotoxicity assay
The irritation potential (IP) was confirmed by cell cytotoxicity assay of the NIH/3T3 mouse fibroblast cell line (Korean Collection for Type Cultures, South Korea). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma Aldrich, USA) with 10% heat-inactivated bovine calf serum (Gibco, USA) and 1% antibiotic antimycotic solution (Sigma Aldrich, USA) in a 75-cm2 cell culture flask at 37 °C. Cells were subcultured every 2–3 days and then seeded on a 96-well plate at a density of 7–8000 cells per well. After 24 h of incubation, old media were removed, and 100 μl of each formulation were applied to the cells for another 24 h. Cell viability was determined by the degree of change for yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-difenyltetrazolium bromide (MTT, Sigma) into purple formazan. After the incubation, 20 μl of the MTT solution were added to each well and incubated for 2 h. The incubated cells were lysed by adding 150 μl of dimethylsulfoxide and shaken for 15 min with the Multiskan GO device (Thermo Fisher Scientific, USA). The optical density was measured at a wavelength of 590 nm. Cell viability was obtained by dividing the optical density of the cells treated with each formulation with that of the control. The IP value was expressed as follows:
Attenuated total reflection Fourier-transform infrared spectroscopy of the formulations
The formulations with the different compositions were analyzed by attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy. One milliliter of each formulation containing magainin and NLS with or without S20 was prepared 1 h prior to the collection of the FTIR spectra and kept at room temperature. All spectra were obtained with the Nicolet iS50 FTIR spectrometer (Thermo Fisher Scientific Instrument, USA) at a resolution of 4 cm−1 and frequency range of 4000–400 cm−1 and finally averaged over 32 scans. The obtained spectra were smoothed, baseline corrected, and deconvoluted by the OMNIC software (Thermo Fisher Scientific Instrument, USA). The chemical environment change of the carboxylate group in the NLS due to the addition of S20 is represented by the carboxylate peak (1650 cm−1). The degree of the interaction was obtained by the change ratio of the peak area divided by the peak height of each formulation without and with S20 as follows:
Attenuated total reflection Fourier-transform infrared spectroscopy of the stratum corneum
Prior to the ATR-FTIR analysis of the skin lipids and proteins, a SC sheet was isolated from the epidermis (Medikinetics, South Korea) by trypsin digestion [21]. The epidermis was floated over 0.25% (w/v) trypsin solution at room temperature (25 °C) for 24 h. The isolated SC film was then rinsed with PBS solution and dried at room temperature for another 24 h. The ATR-FTIR spectra were first collected for each SC sheet as a control before treatment with the formulations. Then, the SC sheets were incubated with 500 μl of each formulation at 4 °C for 24 h. After the treatment, the SC sheets were washed with PBS solution and dried at room temperature for 24 h. The spectra of the post-treatment SC sheets were collected and compared with those of the untreated SC sheets to analyze the lipid bilayer and protein structure changes in the SC by the formulation. All spectra were obtained with a Nicolet iS50 FTIR spectrometer (Thermo Fisher Scientific Instrument, USA) at a resolution of 4 cm−1 and a frequency range of 4000–400 cm−1 and finally averaged over 32 scans. The obtained spectra were smoothed, baseline corrected, deconvoluted, and analyzed by the OMNIC software (Thermo Fisher Scientific Instrument, USA). The skin lipid fluidization was represented by the frequency shift of the asymmetric (2920 cm−1) and symmetric (2850 cm−1) C–H stretching peaks. Skin protein structure alteration was inferred by the frequency shift of the C=O stretching peak (1650 cm−1).
Results
Fluorescein permeation and ER
To confirm the skin penetration enhancement of the magainin peptide with the NLS:S20 mixture and to screen for the optimal composition, we measured the ER values of nine different formulations listed in Table 1. Porcine skin was used for the pretreatment, and the target drug fluorescein was permeated through the FDC. The fluorescence intensity of the drug delivered through the porcine skin samples was measured by a spectrofluorophotometer, and the cumulative results are shown in Fig. 1((a)). The difference among the amounts of fluorescein delivered by each group became significant after about 9 h of penetration. Group (H) had the largest increase in fluorescein permeation followed by Group (F).
(a) Cumulative fluorescein delivered in 3, 6, 9, and 12 h after the skin pretreatment with formulations (A) to (I). (b) ER value of each formulation. The asterisks (*) mean that the group has a statistically significant difference compared to the control (***P = < 0.001). The plus sign (+) means that the group has a statistically significant difference compared to the group with the same surfactant concentration (+++ P = < 0.001, + P = < 0.05). Each data point represents the mean ± std (n = 9)
The cumulative fluorescence intensity after 12 h was converted into the ER relative to that of the control by Eq. (1) in which the ER value of the control group was defined as 100%. As shown in Fig. 1((b)), all formulations showed enhanced permeation of fluorescein compared to the control group regardless of the presence of S20. White bars represent the formulations containing magainin and NLS with different compositions without S20, and the black bars indicate those with S20. Groups (A), (D), and (E) without S20 had ER values less than 1000%, and the values were proportional with their total concentration of surfactants. All cases except (C) showed the tendency that formulations with S20 had a larger ER value than those with the same concentration of NLS without S20. Noticeable ER values (***P < 0.001) of 1926.49 and 3087.30% were observed in the formulations (F) with magainin and 1.5% NLS:S20 with a weight ratio of 0.3:0.7 and (H) with 2% NLS:S20 and a weight ratio of 0.6:0.4, respectively. By adding S20 to groups with magainin and NLS only, both (F) and (H) had a permeability enhancement of about three or more folds (+++ P < 0.001). In contrast, (B) and (C) with a concentration of 1% had an insignificant effect on the ER compared to (A) without S20. Although the ER values seem to be far smaller compared to previous studies, these results are reasonable because full-thickness porcine skin was used here which is more than 60 times thicker compared to the human epidermis [22].
Cell cytotoxicity and IP
Because the safety of enhancers should be considered in addition to their potencies, the cell cytotoxicity was measured by performing the MTT assay on the NIH/3T3 mouse fibroblast cell line. This cell line was chosen because fibroblasts are a representative cell type in the skin dermis structure [23]. Among fibroblast cell lines, NIH/3T3 is widely used in studies, and because of early identification of risk factors in NIH/3T3, it has been suggested as an alternative for in vivo animal tests recently [24, 25]. The cell cytotoxicities from the treatments of the nine formulations were converted into the IP relative to the control according to Eq. (2). In agreement with our hypothesis, the IP values were largest in (D) and (G) for which the magainin was mixed only with the NLS as shown in Fig. 2. This correlation was observed between the concentrations of NLS and the IP values in groups only with magainin-NLS without S20, (A), (D), and (G), indicated by white bars. Their toxicity increased with the increase in NLS concentration. This result corresponds well with the irritancy of NLS caused by the superoxide anion mentioned in the introduction. In most of the cases except for formulation (C), the addition of S20 decreased the skin irritation (+++ P < 0.001), and this effect was greater for NLS:S20 concentrations of 1.5 and 2%. The toxicity of (D) and (G) without S20 was decreased by more than 6- and 11-fold in (F) and (H) which had the same concentration of NLS but also included S20 at weight ratios of 0.7 and 0.4, respectively. For formulations (D) to (I) with a concentration of 1.5 or 2%, the ER and IP values are negatively correlated to each other. Because toxicity is accompanied with permeability enhancement [26], it is worth noting that formulations (F) with 1 mM magainin and 1.5% NLS:S20 with a ratio of 0.3:0.7 and (H) with 2% NLS:S20 and a ratio of 0.6:0.4 had the largest ER value and simultaneously, the lowest toxicity among all the formulation groups. This will be discussed later in the next session.
Relationship between ER and IP
We calculated the ER/IP value to investigate the efficacy to toxicity ratio of each formulation group and determined the best composition of magainin and NLS:S20 which enhances the permeability with the least skin irritation. According to Table 2, (H) with magainin and 2% NLS:S20 with a weight ratio of 0.6:0.4 had the largest ER/IP value, 517.10, followed by (F) with 1.5% NLS:S20 with a weight ratio of 0.3:0.7. Both groups have ER values larger than 1000 and IP values less than 10; therefore, they both have great potential as penetration enhancers. Although the ER values of (D) and (G) were quite large, the ER/IP was even lower than that of the control because of their irritancy. In contrast, groups containing 1% surfactants had low toxicity; however, the ER/IP was insignificant because of their low potency. Moderate characteristics were observed for formulations (E) and (I) both with values over 50. The ER/IP value of control group was increased about 17-fold when compared with that of (D) and (F) and 42-fold when compared with that of (G) and (H). The addition of a specific weight ratio of S20 to the magainin-NLS mixture is responsible for the definite increase in utility for the NLS concentrations of 1.5 and 2%.
The ER and IP values are plotted for each formulation in Fig. 3 which is divided into four quadrants. Quadrant I represents potent but irritating enhancers, quadrant II potent and non-irritating enhancers, quadrant III non-irritating but weak enhancers, and quadrant IV irritating and weak enhancers. We focused on the movement of the groups without S20 (white dots) in an upper-left direction by the addition of S20 (black dots) on the plot. Groups (D) and (G) in quadrant IV moved to a great extent toward quadrant II in the presence of S20 which shows an increase in skin permeability and decrease in skin irritation. Group (H) is located in the uppermost and also leftmost region among all the mixtures on the plot. Therefore, it is regarded as the most effective composition when considering both potency and safety followed by (F) and (I).
Fourier-transform infrared spectroscopy of the formulations
To determine whether the chemical interaction between the NLS and S20 influences skin permeability when dissolved in 50% (v/v) PBS:EtOH with magainin, all the formulations were analyzed with ATR-FTIR spectroscopy. The chemical environment change of the carboxylate group in the NLS due to the S20 was quantified by the change ratio of the peak area to the peak height (A/H) at the C=O stretching peak (1650 cm−1) by Eq. (3) [20]. As seen in Table 3, the A/H ratio of the peak was changed due to the presence of S20. According to a previous study, this chemical environment change is attributed to the electrostatic interaction or hydrogen bond between the active carboxylate group in the NLS and the three hydroxyl groups in the S20 [20]. These interactions between these two surfactants still occurred when mixed with the magainin peptide.
A significant alteration (+ P = < 0.05) in the chemical environment was observed in formulation (F), three times larger than that of group (E) which had the same surfactant concentration but with a different weight ratio. This tendency was observed also in the ER value in these formulations. In a similar context, the area to height ratio change in formulations containing 2% NLS:S20 also affected the ER value in which a larger change led to more efficient permeation of fluorescein when compared with formulations (H) and (I). The ratio change was relatively insignificant in the groups containing a surfactant concentration of 1%, which also resulted in a low ER value. In this manner, we believe that the degree of interaction between the NLS and S20 with magainin is responsible for the ER value of each group.
ATR-FTIR spectroscopy of the stratum corneum
To determine the interaction of the formulations with the SC lipids and proteins, ATR-FTIR spectroscopy was measured. For the SC lipids, the frequency shift of the symmetric and asymmetric C–H stretching peaks at 2850 and 2920 cm−1 was used as a quantitative parameter. The peak spectra and peak wavelength of untreated and treated SC samples were collected shown in Fig. 4((a)). The peak frequency clearly increased after the treatment. For a precise analysis, four peaks constituting the C–H stretching region were used in the deconvolution shown by the dotted lines in Fig. 4((b)). The shifts in the wavelength from the untreated SC to the SC incubated with each formulation are listed in Table 4. All the formulations induced an increase in the frequency for both the symmetric and asymmetric peaks with the presence of S20 inducing a larger shift. This upfield shift of the C–H peak frequency indicates a change from a trans to a gauche conformation which represents the fluidization of the SC lipidic lamellae [27]. Consistent with the ER and ATR-FTIR analysis of the formulations, (F) and (H) had the largest degree of lipid bilayer fluidization which was larger for the asymmetric ones (***P < 0.001). They each showed a two- and sevenfold increase in the asymmetric peak frequency compared with (D) and (G) which have the same composition but also have S20 (+++ P < 0.001).
(a) ATR-FTIR spectroscopy of the symmetric (2850 cm−1) and asymmetric (2920 cm−1) C–H stretching peak of the untreated (dashed line) and treated (solid line) SC. Arrows indicate the upfield shift of the maximum absorbance peaks after incubation with the formulations. (b) Deconvolution of the spectroscopy at the C–H stretching peak. Original spectroscopy (solid line) was fitted (dashed line) by the sum of four deconvolution peaks (dotted line). (c) ATR-FTIR spectroscopy of the C=O stretching peak (1650 cm−1) of the untreated (dashed line) and treated (solid line) SC. Arrows indicate the upfield shift of the maximum absorbance peaks after incubation with the formulations
Such a trend was also observed in the SC protein structure. Structure alteration of SC proteins was estimated by the extent of changes in the C=O stretching peak at 1650 cm−1 shown in Fig. 4((c)). The amide band is representative of characteristics of keratin and ceramides located in the SC [28]. According to Table 4, all the formulations contributed to the increase in peak frequency, and it was even larger in the presence of S20. It has been suggested that the frequency increase in the protein range arises from an alteration in the alpha helix structure to other less rigid secondary protein structures including beta sheets and random coils. Such fluidizing effects in both the SC lipids and proteins seem to correlate well with the ER. In addition, it is clear that the formulations containing magainin-NLS:S20 have a greater structural alteration effect which can explain the superior ER values when compared with the formulations without S20. Therefore, we suggest these conformation changes induced by S20 act as an influential mechanism promoting the penetration of the target drug.
Discussion
Screening of the formulations by the ER/IP
Because the final utility of the penetration enhancer is dictated by both the potency and safety, we adopted the ER/IP value to quantify the effects of each formulation on porcine skin. The primary aim of this study was to confirm the hypothesis that the addition of S20 to magainin-NLS leads to skin permeability enhancement with reduced skin irritation. There was a substantial difference in the ER/IP between the formulations containing S20 compared to those without S20 except for (A)~(C), which were samples exposed to 1% NLS:S20. Due to the large molecular weight of the magainin peptide (2494 Da), the role of NLS dissolved in 50% (v/v) ethanol is to facilitate peptide penetration into the SC according to previous studies. This became obvious because there was only little permeation enhancement observed when the porcine skin samples were treated with magainin in the absence of NLS-ethanol [11]. In this manner, the NLS:S20 mixture in the formulations of the surfactants is assumed to promote the magainin interaction with the SC lipid bilayers and proteins more vigorously compared to NLS alone.
In addition to showing that the addition of S20 can increase the ER/IP, we further optimized the concentration and weight ratio of the NLS:S20 mixture. The concentration of magainin was fixed because the permeability reached maximum at 1 mM and decreased with increasing concentrations [29]. In the case of the two surfactants, concentrations of 1 and 1.5% (w/v) used in this study have been well reported to increase the efficacy to toxicity value, and 2% (w/v) was chosen because it is known to work well with 1 mM magainin. By adding S20, a dramatic increase in the ER/IP was observed in (H) containing magainin with 2% (w/v) NLS:S20, for which the weight ratio of the NLS was 0.6, followed by (F) containing magainin with 1.5% (w/v) NLS:S20, for which the weight ratio of the NLS was 0.3. As expected, the synergistic effect of NLS and S20 in certain compositions was still valid when applied together with 1 mM magainin peptide. Based on these results, we assume that 1% NLS:S20 is not sufficient to induce the penetration of magainin which is a large peptide.
Structural effect of formulation on ER and IP
According to the ATR-FTIR analysis of the formulations, a chemical environment change of the carboxylate group in the magainin-NLS appeared when S20 was added. This change ratio in the area to height suggests that NLS formed a mixed micellar aggregate structure with the S20 [30]. By itself, NLS acts as a strong extractor which deletes the lipid molecules in the SC lipid lamellar matrix, and S20 is a weak fluidizer which locates itself among the skin lipids. However, different from this characteristic, the NLS:S20 mixture has a strong fluidizing effect on skin lipid bilayers especially when dissolved simultaneously in a PBS-EtOH solution, in which the presence of ethanol is suggested to have impact on the formation of the mixed micellar structure. The role of ethanol was quite clear when comparing the IP of the solutions dissolved in 50% (v/v) PBS-ethanol and in PBS alone. The IP values of the formulations were dependent on the weight fraction of the NLS when the magainin-NLS:S20 was dissolved in pure PBS regardless of the S20. This can be explained by the absence of ethanol. The mixed micellar structure either did not form or, despite its formation, did not affect the SC. In contrast, the IP values were negatively correlated with the ER rather than with the amount of NLS for the formulations dissolved in PBS-ethanol. This result can be explained by the formation of mixed micelle aggregates which fluidize the lamella structure but do not extract the lipid molecules. Besides the novel fluidizing effect, the decrease in the IP corresponds well with two principles. The first one is that usually a monomer state has full surfactant properties, and the surfactants participating in the micelle structure are unlikely to have any irritancy potential [31]. Second, mixing of anionic and non-ionic surfactants results in lower toxicity compared to the simple addition of those of individual monomers with their toxicities [32].
Magainin is known to interact with the lipid bilayer causing internal stress and membrane tension to create pores [33]. The electrostatic interactions of the cationic part of the peptide and negatively charged lipids are a critical mechanism in its partitioning process [34]. The increased ER can be explained as follows: the pore-forming action of the magainin peptide could be more efficient when enough lipid molecules in the SC interact with its cationic, alpha helical structure rather than when the SC lipids are deleted. The ER can further be improved by changing the pH to maximize the electrostatic interaction between magainin and the target drug [2] or by using a drug with a lower molecular weight [29]. Additionally, ternary and quaternary combinations of surfactants could be used; however, skin toxicity should be taken into consideration.
Interaction with SC lipids and proteins
The chemical interaction of the formulations with the lipid matrix in the SC has a major role in enhancing skin permeability. ATR-FTIR spectrum was collected at the C–H stretching region (2850 and 2920 cm−1) to investigate the alteration in the lipid structure. The fluidization became greater with the addition of S20, and formulations (F) and (H) had a far larger effect on the SC lipid bilayers. This result is strongly associated with the transport of fluorescein. As mentioned above, the mixed micellar aggregates of the NLS:S20 have a strong fluidizing effect but not in their respective individual forms. Because a great portion of the skin lipids consists of cholesterol, the mixed micellar structure of NLS:S20 may disrupt its crystallinity and lead to the fluidization of the matrix.
Besides inducing disorder in the lipid bilayer, this micelle structure changed the SC protein microstructure to a less rigid conformation by changing the alpha helix to a beta sheet and other secondary structures. The peak frequency shift toward higher wavenumbers in the amide I band indicates that the keratin and ceramide present in SC proteins interacted more actively with the formulations containing S20. The conformation change of the SC lipids and proteins due to the magainin-NLS:S20 indicates that both the intercellular lipid pathway and transcellular pathway through corneocytes are involved in the delivery of fluorescein [9]. Indeed, the formulations containing both surfactants had a more intense effect on both routes compared to the formulations containing only magainin-NLS. However, the detailed structure should be further investigated by in silico modeling analysis of the interactions among the magainin peptide, surfactants, SC lipid bilayers, and SC protein molecules [10, 35].
Conclusion
We investigated both the potency and toxicity of nine formulations containing magainin pore-forming peptide and NLS:S20 mixtures with different compositions. They were quantitatively assessed by the ER/IP in this study to determine the final utility of each formulation. First, we confirmed that the addition of S20 has a clear effect by both increasing the ER and decreasing the IP through FDC permeation and cell cytotoxicity. Among all the groups, (F) with 1 mM magainin and 1.5% NLS:S20 with a weight fraction of 0.3:0.7 and (H) containing the same but with 2% NLS:S20 and a weight fraction of 0.6:0.4 had the best ER/IP values. The ATR-FTIR spectroscopy of the formulations and SC lipids and proteins supports the result of an increased ER/IP value. The chemical environment change in the carboxylate group of NLS due to S20 represents the formation of a mixed micellar structure. This structure greatly influenced the fluidization of the lipid bilayers and SC proteins which subsequently enhanced fluorescein transport and decreased skin irritation. However, the detailed mechanism of magainin and surfactants partitioning into the SC matrix needs to be further studied. We believe this is the first study to use the combination of a pore-forming peptide with more than one CPE to maximize the efficacy to toxicity ratio of permeation enhancers. This approach can contribute to the effective and safe delivery of macromolecules such as functional peptides, which has been a difficult problem in this area.
The asterisks (*) mean that the group has a statistically significant difference compared to the control (***P = < 0.001, *P = < 0.05). The plus sign (+) means that the group has a statistically significant difference compared to the group with the same surfactant concentration (+++ P = < 0.001, + P = < 0.05). Each data point represents the mean ± std (n = 3)
References
Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–24.
Kim Y-C, Late S, Banga AK, Ludovice PJ, Prausnitz MR. Biochemical enhancement of transdermal delivery with magainin peptide: modification of electrostatic interactions by changing pH. Int J Pharm. 2008;362:20–8.
Prausnitz MR, Elias PM, Franz TJ, Schmuth M, Tsai J-C, Menon GK, et al. Skin barrier and transdermal drug delivery. Dermatology. 2012;3:2065–73.
Wertz PW. Lipids and barrier function of the skin. Acta Derm Venereol Suppl. 2000;208:7–11.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–37.
Nasrollahi SA, Taghibiglou C, Azizi E, Farboud ES. Cell-penetrating peptides as a novel transdermal drug delivery system. Chem Biol Drug Design. 2012;80:639–46.
Singh G, Karande P. Peptide-mediated transdermal drug delivery. In: Dragicevic N, Maibach HI, editors. Percutaneous penetration enhancers chemical methods in penetration enhancement. Berlin Heidelberg: Springer; 2015. p. 353–61.
Kumar S, Narishetty ST, Tummala H. Peptides as skin penetration enhancers for low molecular. In: Dragicevic N, Maibach HI, editors. Percutaneous penetration enhancers chemical methods in penetration enhancement. Berlin Heidelberg: Springer; 2015. p. 337–52.
Kumar S, Zakrewsky M, Chen M, Menegatti S, Muraski JA, Mitragotri S. Peptides as skin penetration enhancers: mechanisms of action. J Control Release. 2015;199:168–78. https://doi.org/10.1016/j.jconrel.2014.12.006.
Menegatti S, Zakrewsky M, Kumar S, De Oliveira JS, Muraski JA, Mitragotri S. De novo design of skin-penetrating peptides for enhanced transdermal delivery of peptide drugs. Adv Healthc Mater. 2016;5:602–9.
Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by magainin pore-forming peptide. J Control Release. 2007;122:375–83.
Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by antimicrobial peptides. J Biomed Nanotechnol. 2010;6:612–20.
Kaushik S, Krishnan A, Prausnitz MR, Ludovice PJ. Magainin-mediated disruption of stratum corneum lipid vesicles. Pharm Res. 2001;18:894–6.
Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemist. 1996;35:13723–8.
Hall K, Lee T-H, Mechler AI, Swann MJ, Aguilar M-I. Real-time measurement of membrane conformational states induced by antimicrobial peptides: balance between recovery and lysis. Scient Rep. 2014;4
Sato H, Feix JB. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic alpha-helical antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1245–56. https://doi.org/10.1016/j.bbamem.2006.02.021.
Aioi A, Kuriyama K, Shimizu T, Yoshioka M, Uenoyama S. Effects of vitamin E and squalene on skin irritation of a transdermal absorption enhancer, lauroylsarcosine. Int J Pharm. 1993;93:1–6.
Aioi A, Shimizu T, Kuriyama K. Effect of squalene on superoxide anion generation induced by a skin irritant, lauroylsarcosine. Int J Pharm. 1995;113:159–64.
Shimizu T, Aioi A, Horiguchi T, Kuriyama K. Effect of vitamin E on keratinocyte-modulation induced by lauroylsarcosine. The Japanese Aust J Pharm. 1995;67:291–5.
Karande P, Jain A, Arora A, Ho MJ, Mitragotri S. Synergistic effects of chemical enhancers on skin permeability: a case study of sodium lauroylsarcosinate and sorbitan monolaurate. European J Pharm Sci. 2007;31:1–7.
Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:702–5.
Jung EC, Maibach HI. Animal models for percutaneous absorption. J Appl Toxicol. 2015;35:1–10.
Welss T, Basketter DA, Schröder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol in Vitro. 2004;18:231–43.
Qin G, Geng S, Wang L, Dai Y, Yang B, Wang J-Y. Charge influence of liposome on transdermal delivery efficacy. Soft Matter. 2013;9:5649–56.
Tomankova K, Kejlova K, Binder S, Daskova A, Zapletalova J, Bendova H, et al. In vitro cytotoxicity and phototoxicity study of cosmetics colorants. Toxicol in Vitro. 2011;25:1242–50.
Asbill CS, Michniak BB. Percutaneous penetration enhancers: local versus transdermal activity. Pharm Sci Technol Today. 2000;3:36–41.
Gennari CG, Franzè S, Pellegrino S, Corsini E, Vistoli G, Montanari L, et al. Skin penetrating peptide as a tool to enhance the permeation of heparin through human epidermis. Biomacromolecules. 2015;17:46–55.
Cilurzo F, Vistoli G, Selmin F, Gennari CG, Musazzi UM, Franzé S, et al. An insight into the skin penetration enhancement mechanism of N-methylpyrrolidone. Mol Pharm. 2014;11:1014–21.
Kim YC, Ludovice PJ, Prausnitz MR. Optimization of transdermal delivery using magainin pore-forming peptide. J PhyChem Solids. 2008;69:1560–3.
Yuan H, Zhao S, Cheng G, Zhang L, Miao X, Mao S, et al. Mixed micelles of Triton X-100 and cetyl trimethylammonium bromide in aqueous solution studied by 1H NMR. The J Phy Chem B. 2001;105:4611–5.
Lémery E, Briançon S, Chevalier Y, Bordes C, Oddos T, Gohier A, et al. Skin toxicity of surfactants: structure/toxicity relationships. Coll Surf A: Physicochemical Eng ASp. 2015;469:166–79.
Hall-Manning T, Holland G, Rennie G, Revell P, Hines J, Barratt M, et al. Skin irritation potential of mixed surfactant systems. Food and Chemical Toxicology. 1998;36:233–8.
Huang HW, Chen F-Y, Lee M-T. Molecular mechanism of peptide-induced pores in membranes. Phy Rev Lett. 2004;92:198304.
Matsuzaki K, Sugishita K-i, Harada M, Fujii N, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim Biophy Acta (BBA)-Biomembranes. 1997;1327:119–30.
Thind R, O'Neill D, Del Regno A, Notman R. Ethanol induces the formation of water-permeable defects in model bilayers of skin lipids. Chem Comm. 2015;51:5406–9.
Acknowledgements
Porcine skin was kindly donated by the Heart Research Center of Chonnam National University.
Funding
This work was supported financially by the Ministry of Science, ICT, and Future Planning (Project No. NRF-2014M3A9E4064580); Advanced Biomass R&D Center (ABC) of the Global Frontier Project funded by the Ministry of Science, ICT, and Future Planning (NRF-2015M3A6A2074238); and the KUSTAR-KAIST Institute at KAIST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, H., Park, J. & Kim, YC. Enhanced transdermal delivery with less irritation by magainin pore-forming peptide with a N-lauroylsarcosine and sorbitan monolaurate mixture. Drug Deliv. and Transl. Res. 8, 54–63 (2018). https://doi.org/10.1007/s13346-017-0433-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0433-0